Abstract
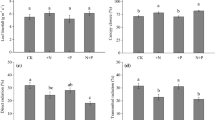
The combustion of fossil fuels and associated warmer temperatures are causing a global increase in the availability of soil nutrients such as nitrogen. This will have pronounced effects on plants at northern latitudes that are adapted to low nutrient conditions. An experiment in northern Canada set up in 1990 has investigated the effects of long-term nutrient enrichment (fertilizer addition) and mammalian herbivore exclusion (fencing) on an understory plant community. We used this experiment to assess how 22 years of fertilization has affected investment in sexual reproduction in four herbaceous understory species. We measured reproductive output at the plot level (proportion of plants flowering) for four species (Mertensia paniculata, Epilobium angustifolium, Achillea millefolium and Festuca altaica) and at the individual plant level (biomass allocation to flower parts) for M. paniculata and E. angustifolium. Fertilization increased the probability of flowering for A. millefolium and resulted in a higher allocation of biomass to flower parts for E. angustifolium. Sexual reproduction in M. paniculata and F. altaica was largely unaffected by increased nutrient supply, and, as expected, herbivore exclusion had almost no effect on any species. Whereas plants in northern ecosystems currently reproduce mainly through clonal growth, rapidly changing environmental conditions and warmer temperatures will likely result in increased benefits of sexual reproduction. This could give a competitive advantage to species such as A. millefolium and E. angustifolium that increase investment in sexual reproduction when released from nutrient limitation.



Similar content being viewed by others
References
Aerts R, Cornelissen JHC, Dorrepaal E (2006) Plant performance in a warmer world: general responses of plants from cold, northern biomes and the importance of winter and spring events. Plant Ecol 182:63–77
Bai W, Sun X, Wang Z, Li L (2009) Nitrogen addition and rhizome severing modify clonal growth and reproductive modes of Leymus chinensis population. Plant Ecol 205:13–21
Burkle LA, Irwin RE (2009) The effects of nutrient addition on floral characters and pollination in two subalpine plants, Ipomopsis aggregata and Linum lewisii. Plant Ecol 203:83–98
Canada Environment (2012) Canadian climate normals 1971–2000 for burwash. National Climate Data and Information Archive, Yukon Territory
DeKoning PK (2011) Consequences and recovery after nutrient enrichment and herbivore reduction in the boreal forest understory. Dissertation, University of British Columbia
Dormann C, Woodin S (2002) Climate change in the arctic: using plant functional types in a meta-analysis of field experiments. Funct Ecol 16:4–17
Fremlin KM, McLaren JR, DeSandoli L, Turkington R (2011) The effects of fertilization and herbivory on the phenology of the understory vegetation of the boreal forest in Northwestern Canada. Arct Antarct Alp Res 43:389–396
Fu L, Wang S, Liu Z, Nijs I, Ma K, Li Z (2010) Effects of resource availability on the trade-off between seed and vegetative reproduction. J Plant Ecol 3:251–258
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Gardner SN, Mangel M (1999) Modeling investments in seeds, clonal offspring, and translocation in a clonal plant. Ecol 80:1202–1220
Goldberg DE, Barton AM (1992) Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. Am Nat 139:771–801
Gough L, Wookey P, Shaver G (2002) Dry heath arctic tundra responses to long-term nutrient and light manipulation. Arct Antarct Alp Res 34(2):211–218
Grainger TN, Turkington R (2012) Germinability of Epilobium angustifoium seeds from plants treated annually with fertilizer for 22 years. Davidsonia 22:2–7
Grainger TN, Turkington R (2013) Mechanisms for success after long-term nutrient enrichment in a boreal forest understory. PLoS One 8:e61229
Harper J (1967) A Darwinian approach to plant ecology. J Anim Ecol 36:495–518
Harper JL, Ogden J (1970) The reproductive strategy of higher plants: I. The concept of strategy with special reference to Senecio vulgaris L. J Ecol 58:681–698
Hautier Y, Randin CF, Stöcklin J, Guisan A (2009) Changes in reproductive investment with altitude in an alpine plant. J Plant Ecol 2:125–134
Hedhly A, Hormaza JI, Herrero M (2009) Global warming and sexual plant reproduction. Trends Plant Sci 14:30–36
Huang YHY, Zhao XZX, Zhou DZD, Luo YLY, Mao WMW (2009) Allometry of Corispermum macrocarpum in response to soil nutrient, water, and population density. Botany 88:13–19
John E, Turkington R (1995) Herbaceous vegetation in the understorey of the boreal forest: does nutrient supply or snowshoe hare herbivory regulate species composition and abundance? J Ecol 83:581–590
Kawano S, Nagai Y (2005) Regulatory mechanisms of reproductive effort in plants 1. Plasticity in reproductive energy allocation and propagule output of Helianthus annuus L. (Compositae) cultivated at varying densities and nitrogen levels. Plant Species Biol 1:1–18
Kellner O (1993) Effects on associated flora of sylvicultural nitrogen fertilization repeated at long intervals. J Appl Ecol 30:563–574
Klady RA, Herny GHR, Lemay V (2011) Changes in high arctic tundra plant reproduction in response to long-term experimental warming. Glob Change Biol 17:1611–1624
Krebs CJ, Boonstra R, Boutin S, Sinclair ARE (2001) What drives the 10-year cycle of snowshoe hares? Bioscience 51:25–35
Krebs CJ, O’Donoghue M, Loewen V, Jung T, Gilbert S, Taylor S, Larocque L, Boonstra R, Boutin S and Kenney A (2012) The Community Ecological Monitoring Program Annual Report 2012
Liu F, Chen J-M, Wang Q-F (2009) Trade-offs between sexual and asexual reproduction in a monoecious species Sagittaria pygmaea (Alismataceae): the effect of different nutrient levels. Plant Syst Evol 277:61–65
Loehle C (1987) Partitioning of reproductive effort in clonal plants: a benefit-cost model. Oikos 49:199–208
Moulton CA, Gough L (2011) Effects of soil nutrient availability on the role of sexual reproduction in an Alaskan tundra plant community. Arct Antarct Alp Res 43:612–620
Nams VO, Folkard NFG, Smith JNM (1993) Effects of nitrogen fertilization on several woody and nonwoody boreal forest species. Can J Bot 71:93–97
Niu K, Luo Y, Choler P, Du G (2008) The role of biomass allocation strategy in diversity loss due to fertilization. Basic Appl Ecol 9:485–493
Pachauri RK and Reisinger A (2007) Climate change 2007: synthesis report: part of the working group III contribution to the fourth assessment report of the intergovernmental panel on climate change
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rustad L, Campbell J, Marion G, Norby R, Mitchell M, Hartley A, Cornelissen J, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Sakai S (1995) Optimal resource allocation to vegetative and sexual reproduction of a plant growing in a spatially varying environment. J Theor Biol 175:271–282
Samson DA, Werk KS (1986) Size-dependent effects in the analysis of reproductive effort in plants. Am Nat 127:667–680
Smith MD, Knapp AK, Collins SL (2009) A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecol 90:3279–3289
Steltzer H, Hufbauer RA, Welker JM, Casalis M, Sullivan PF, Chimner R (2008) Frequent sexual reproduction and high intraspecific variation in Salix arctica: Implications for a terrestrial feedback to climate change in the high arctic. J Geophys Res. doi:10.1029/2007JG000503
Sugiyama S, Bazzaz F (2002) Size dependence of reproductive allocation: the influence of resource availability, competition and genetic identity. Funct Ecol 12:280–288
Treberg MA, Turkington R (2010) Density dependence in an experimental boreal forest understory community. Botany 88:753–764
Turkington R, John E, Watson S, Seccombe-Hett P (2002) The effects of fertilization and herbivory on the herbaceous vegetation of the boreal forest in north-western Canada: a 10-year study. J Ecol 90:325–337
Veresoglou D, Fitter A (1984) Spatial and temporal patterns of growth and nutrient uptake of five co-existing grasses. J Ecol 72:259–272
Weiner J (2004) Allocation, plasticity and allometry in plants. Perspect Plant Ecol Evol Syst 6:207–215
Zhang D, Armitage A, Affolter J, Dirr M (1996) Environmental control of flowering and growth of Achillea millefolium L. summer pastels’. HortScience 31:364–365
Acknowledgments
Funding for this research was provided by the Natural Science and Engineering Research Council of Canada, the Association of Canadian Universities for Northern Studies and the Northern Scientific Training Program. We would also like to thank the staff at Kluane Lake Research Station, Douglas Curley, De Wet van Niekerk and Jennie McLaren for their invaluable assistance. We are grateful to the Kluane and Champagne-Aishihik First Nations for allowing us to do research on their traditional lands.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grainger, T.N., Turkington, R. Long-term nutrient enrichment differentially affects investment in sexual reproduction in four boreal forest understory species. Plant Ecol 214, 1017–1026 (2013). https://doi.org/10.1007/s11258-013-0227-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-013-0227-5