Abstract
Spatial associations of tree saplings with spiny or toxic plants in grazed woodlands are generally explained by associational resistance, i.e., protection against grazing via a well-defended neighbor. In this study, we tested whether directed seed dispersal and post-dispersal seed removal by wood mice are additional explanations for the observed spatial association between thorny shrubs and trees, hence before associational resistance. We performed three studies in grazed woodlands in the Netherlands to test this idea. Our first seed dispersal experiment with tagged acorns showed indeed that wood mice disperse acorns directed towards shrubs. The majority of these dispersed acorns were, however, consumed. Our second experiment revealed that post-dispersal removal of cached acorns was higher under shrubs than in grassland and under trees, but also indicated the importance of within-shrub position: shrub centers were faster depleted than shrub edges. Also the number of freshly emerged seedlings and older saplings differed between micro-habitats, being higher under trees and at shrub inner-edges than at shrub outer-edges, shrub centers, and in grassland. The spatial associations with shrubs got stronger with the age of saplings, which probably reflect accumulated grazing effects over time, being higher in the open than in shrubs. We conclude that directed acorn dispersal and post-dispersal acorn removal by wood mice are two additional explaining mechanisms for the spatial associations between thorny shrubs and trees in grazed woodlands, before associational resistance. Our findings change the view that large herbivores are the sole main drivers behind tree recruitment patterns in grazed woodlands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grazed woodlands are ecosystems consisting of a mosaic of grassland patches, thickets of spiny shrubs, and isolated or clustered trees, often growing within these shrub thickets (Bakker et al. 2004). Within this high structural diversity, a rich biodiversity in both plant and animal species can develop (Olff et al. 1999; Vera 2000). Several studies show that low-intensity grazing plays an essential role in maintaining the rich structure and associated biodiversity in these systems (Olff et al. 1999; Bakker et al. 2004; Bakker et al. 2006; Smit et al. 2006a). Introducing large herbivores is therefore an increasingly used method throughout Europe since the last two decades (Van Uytvanck et al. 2008a). However, little is known about the long-term dynamics and key processes operating in grazed woodlands. This type of knowledge is currently highly needed for the development of effective long-term management strategies for both ancient and newly created grazed woodlands.
Olff et al. (1999) proposed that the diverse mosaic landscape in grazed woodlands is best explained by a long-term cyclical succession with mosaics shifting in space and time from grassland to shrubs to trees and back to grassland again: spiny shrubs facilitate the establishment of palatable trees, the trees outcompete the shrubs by shading, and grasslands reappears when the tree dies. Grazers are hypothesized to play a key role in this succession by damaging palatable tree saplings within grassland but not within spiny shrubs. Several empirical studies have indeed shown that unpalatable species—spiny or toxic plants—protect tree saplings from ungulate damage (Smit et al. 2006a; Van Uytvanck et al. 2008b), resulting in spatial associations between young palatable trees (e.g., pedunculate oak Quercus robur) and spiny or toxic plants (e.g., spiny blackthorn Prunus spinosa) (Bakker et al. 2004; Smit et al. 2005; Van Uytvanck et al. 2008a). This process in which an unpalatable species facilitates a palatable species by reducing the amount of herbivory is called associational resistance (Hamback et al. 2000; Milchunas and Noy-Meir 2002) and is considered an important factor in the proposed cyclic succession in grazed woodlands (Olff et al. 1999).
Thus far, studies on grazing and spatial associations in grazed woodlands have focused on the sapling stage (≥1 year) of trees, while seed and seedling stage (<1 year) are rarely studied (but, see Smit et al. 2008, 2009). However, the seed and seedling stages are considered as most vulnerable, hence most relevant for plant dynamics (Gómez 2004; Arrieta and Suárez 2005). The high spatial variety in microhabitats in grazed woodlands, with alternating patches of grassland, shrubs, and trees, creates high variability in conditions for tree recruitment. For example, the differences in light intensities (Arrieta and Suárez 2005) and grazing pressure (Bakker et al. 2004) between microhabitats largely affect the establishment success during the seedling and the sapling stage. However, also processes at the seed stage, such as seed dispersal and seed predation, may vary between these different microhabitats, and so strongly affect emergence and survival.
Wood mice (Apodemus sylvaticus) and Eurasian jays (Garrulus glandarius) play an important role as dispersers of acorns of pedunculate oak in woodlands (Kollmann and Schill 1996; Den Ouden et al. 2005; Vander Wall et al. 2005). Both animals bury large amounts of acorns during the autumn as food supply for the winter. This burial increases seedling establishment for two main reasons. First, the wetter and colder conditions underground are favorable for seedling germination and second, buried acorns are hidden from other predating animals (Gómez 2004). Jays prefer to bury their acorns in open microhabitats such as grasslands (Bossema 1979; Kollmann and Schill 1996; Den Ouden et al. 2005) while mice prefer denser vegetation types to cache their winter supply (Den Ouden et al. 2005; Iida 2006; Takahashi et al. 2006, 2007), possibly to avoid exposure to predators (Kollmann 1995; Manson and Stiles 1998) or because mice use vegetation structures as memory points for their caches (Den Ouden et al. 2005). Such directed dispersal toward shrubs could be an important explanation for the observed spatial associations between oaks and blackthorn shrubs, besides associational resistance. On the other hand, mice act as predators of cached acorns, leaving only a small fraction of seeds to survive post-dispersal (Li and Zhang 2003; Iida 2006). Various seed tagging experiments show that not only dispersal, but also removal of seeds is higher under dense vegetation such as shrubs (Iida 2006; Smit et al. 2006b; Pérez-Ramos and Marañón 2008), which is again mostly attributed to reduced predation risk (Manson and Stiles 1998). Those studies that measured high post-dispersal predation of acorns (Li and Zhang 2003; Iida 2006), therefore questioned the effectiveness of wood mice as dispersers. In contrast, many other studies underline the importance of seed dispersal by wood mice for tree regeneration despite high predation rates, as even a tiny fraction of acorns that escapes from post-dispersal seed predation may be sufficient for a substantial tree recruitment over time (Gómez 2004; Den Ouden et al. 2005; Vander Wall et al. 2005; Gómez et al. 2008; Smit et al. 2009).
The aim of this research was to investigate oak regeneration in grazed woodlands with the focus on the seed and seedling stages, to test whether dispersal and predation behavior of wood mice are an additional explanation for the spatial association between oaks and blackthorn shrubs, besides associational resistance. We investigated these processes in the main microhabitats (grassland, shrubs, and under oak trees) in a grazed woodland ecosystem in the Netherlands and hypothesized that: (1) acorn dispersal by wood mice is directed toward shrubs, (2) post-dispersal removal of cached acorns is higher under shrubs than in open microhabitats, and (3) recently emerged oak seedlings are spatially associated with shrubs; grazing on saplings in open accessible microhabitats (grassland or under trees) strengthens this spatial association in subsequent years.
Methods
Study site
We performed three studies in an ancient grazed woodland ecosystem, Junner Koeland (52°2′N, 6°29′E), a nature reserve of ca. 100 ha in the east of the Netherlands. The site is situated on a riverine floodplain along the Overijsselse Vecht and consists of a mosaic of short grazed grasslands (dominated by Festuca Rubra and Agrostis capilaris), thickets of dense shrubs (dominated by blackthorn), and isolated or small groups of trees (dominated by pedunculate oak). The oaks are spatially associated with blackthorn shrubs (Bakker et al. 2004); dense thickets of shrubs surround these trees, with palatable grasses re-appearing under the older trees. The Junner Koeland area is one of the few remaining examples of the natural vegetation that was once present along most large rivers in temperate Western European lowlands before canalization and intense agriculture (Bakker et al. 2004). The site has been grazed by cattle from medieval times onwards while no fertilizer or additional management has been applied for decades, which makes it very suitable for studying natural processes in grazed environments. Our studies took place between autumn 2007 and spring 2009 during which the site was year-round grazed by 15 horses and by varying numbers of cattle, from 20 individuals in winter to 60 individuals in summer (pers. obs.). Natural herbivores occurring at the site are European rabbit (Oryctolagus caniculus), roe deer (Capreolus capreolus), brown hare (Lepus europaeus), common vole (Microtus arvalis), bank vole (Clethrionomys glareolus), and wood mouse.
Acorn dispersal experiment
To test the acorn dispersal and predation behavior of wood mice, we performed a cafeteria style experiment during the autumn of 2007. We collected acorns from 21 mature, fruiting oaks and performed a floating test to dismiss non viable or insect-infected acorns (Gribko and Jones 1995). We selected 156 viable acorns with a mean weight of 6.27 ± 1.32 g (1 sd, range: 3.50–10.53 g) and a mean length of 2.82 ± 0.21 cm (1 sd, range: 2.40–3.50 cm). We tagged the acorns by inserting a little neodymium magnet inside a small hand drilled hole in the blunt end of the acorns. In addition, the acorns got tiny aluminum tags with unique numbers and were closed with crafting wax. These numbers were also written on the outside of the acorns with a permanent marker for identification. Previous studies showed no effect of the added weight from the magnet on dispersal distances (Iida 2006; Takahashi et al. 2007).
On October 5th 2007, we placed the 156 tagged acorns in four equal-sided triangular 90 × 90 × 90 cm exclosures of concrete steel (mesh width 8 cm), with at least 50 m between individual exclosures. Each exclosure was placed in grassland under the canopy of a seed baring mature oak at ca. 2 m from the edge of a blackthorn shrub thicket (distance measured from lower ramets, not overhanging taller canopy). Hence, mice were given the choice to pick up acorns and disperse them to either grassland or shrubs. The exclosures prevented other animals than wood mice (notably horses, roe deer, and jays) to remove the acorns from the plots. The top of the pyramid was covered with a mesh (1-cm width) to prevent that falling acorns would end up in our plots. The 39 tagged acorns per plot were uniformly distributed, hence at a seed density of 96.3 acorns/m2, which is relatively high compared to natural seed densities observed (range: 12.7–80.2 acorns/m2, measured under 21 mature oaks in the site) but was needed to increase the chance that mice were attracted to our plots. After placing the acorns, we checked the plots biweekly for acorn removal. By the time all acorns were removed from the plots (between 2 and 5 weeks), we relocated the acorns using a magnet detector (Fisher M-Scope Ferro Probe FX 3) in a 20-m radius circle around each supply plot. For every relocated acorn, we recorded the distance and direction from the supply point, the state of the dispersed acorn (predated = only magnet found with remnants of acorn; intact = dispersed but no further damage), and the microhabitat of retrieval (shrubs or grassland). Furthermore, we mapped the contours of these microhabitats and the exact retrieval locations around each supply point in 64 5 × 5-m grid cells and digitized these maps using Cartalinx (1.2) and Idrisi Kilimanjaro (14.0).
We tested the differences in acorn dispersal between the two microhabitats (shrubs and grassland) with a Pearson Chi-square goodness of fit test. We pooled the data over all the four plots as the number of observations for each microhabitat was too low to perform Chi-square tests for each plot separately. Expected values (number of acorns per microhabitat) were calculated by assuming that for undirected (random) dispersal the amount of acorns per microhabitat is proportional to the area that it covers (Gómez et al. 2008). Significant deviations from expected values would indicate directed dispersal towards a particular microhabitat. To control for distance dependent dispersal (with potentially more dispersal close to the supply point), we grouped all the dispersed acorns into two distance classes (<5 m or 5–20 m; more classes resulted in too little observations per class) and repeated the Chi-square test for each distance class separately. Different results between the two distance classes would indicate distance dependent dispersal.
Furthermore, we used a Chi-square test for Independency (with Yates continuity correction) to test for differences in acorn predation (acorns eaten vs. intact) between the two microhabitats. Possible differences in acorn dispersal distance between the four plots and microhabitats were tested using Kruskal–Wallis tests.
Post-dispersal removal experiment
We selected 20 transects, each starting at the base of a tree and running through shrubs to end in grassland. Each transect was divided into four microhabitats: under trees, middle of shrubs, outer-edge of shrubs, and grassland (Fig. 1). We selected 80 viable acorns following the same procedure as before and buried one acorn per microhabitat on all transects on October 23rd, 2007. Single acorns were buried horizontally at a depth of 2 cm to mimic a mouse-made cache (Den Ouden et al. 2005; Smit et al. 2009). To monitor the rates of seed removal in the different microhabitats, we checked the presence of the acorns at 3, 10, 85, 108, 135, and 198 days after burial.
We performed a Life-Table analysis (a.k.a. survival analysis or time-to-event analysis) with pair-wise Gehan–Wilcoxon comparisons to evaluate the rates of post-dispersal removal in the four microhabitats. In this analysis, we used the amount of removed acorns per microhabitat (%) as the dependent variable and time (days since burial) as the independent variable.
Survey of seedlings and saplings
We performed a survey on 24 transects (4-m wide, 20–30-m long) to observe the distribution of recently emerged seedlings (<1 year) over different microhabitats, and to compare it with the distribution of saplings (≥1 year). Similar to the preceding acorn removal experiment, transects started at the base of a tree and ran through shrubs to end in grassland (Fig. 1). Individual transects were at least 10 m apart to avoid interdependence. For this survey, we divided the transects into five microhabitats: under trees, inner-edge of shrubs, middle of shrubs, outer-edge of shrubs, and grassland (Fig. 1). This allowed us to check for possible differences between the inner-edge and outer-edge of shrubs, e.g., caused by distance effects from the tree. We counted seedlings and saplings by carefully inspecting smaller 1 × 1 m quadrates on the 4-m wide transects. For each observed seedling or sapling, we recorded the surrounding microhabitat (see five categories above), age [estimated by counting bud scars (Dobrowolska 2006)], distance from the stem of the tree (m), height of the seedling or sapling (cm), and the presence of grazing marks. We calculated the area of coverage of every microhabitat by measuring their proportions on the transects. We used Pearson Chi-square goodness of fit tests to test for differences in spatial distribution of seedlings and saplings over the five microhabitats. The expected values for seedlings or saplings for each microhabitat were proportional to the observed area of each microhabitat. We thus tested whether seedlings and saplings were homogeneously distributed over the landscape (observed numbers proportional to the area of a microhabitat) or whether seedlings and saplings are spatially associated with a particular microhabitat (observed numbers higher than expected).
To test if the spatial association between oak recruits and shrubs increases with aging of the recruits—due to accumulation of grazing events—we compared the spatial distribution of recently emerged seedlings (<1 year) with that of older saplings in a step-wise manner. For this test, we only distinguished two classes of microhabitats, shrubs and open, due to limited observations (increase test-power). For the class “shrubs,” we pooled the microhabitats inner-edge of shrubs, middle of shrubs, and outer-edge of shrubs (Fig. 1), assuming that the protection against large grazers was similar in these three microhabitats. For the class “open,” we pooled the microhabitats under trees and grassland (Fig. 1), assuming similar grazing effects in both microhabitats (we frequently observed grazing cattle and horses in both grassland and under the trees that were easily reached through large gaps between shrub thickets). We performed three Pearson Chi-square goodness of fit tests, each time excluding younger saplings in a step-wise manner (>1, 2, and 3 year) and comparing the spatial distribution of the saplings over “shrubs” and “open” with that of seedlings (<1 year) as reference level. Hence, the expected values for “shrubs” and “open” were equal to the distribution of seedlings (<1 year) as we assume that grazing had not yet affected the recently emerged seedling distribution at the time of our experiment (early spring).
To further investigate the impact of grazing, we compared heights of saplings of equal age between the classes “shrubs” and “open” with a Mann–Whitney U-test. Also, we tested whether the number of saplings with grazing marks differed between microhabitats, using a Chi-square test for independency (with Yates continuity correction).
Results
Acorn dispersal experiment
Of the 156 dispersed acorns, 85 (54.5%) were relocated. Mean acorn dispersal distance for all the plots pooled was 7.9 m (median: 7.7 m, range: 0.1–19.7 m). The dispersal distribution curve was skewed slightly positive (skewness = 0.35). The acorn dispersal distances did not differ significantly between the four plots (χ 2 = 0.14, P < 0.993, df = 3).
Out of the 85 acorns, 76 acorns (89%) were found back within shrubs and 9 acorns (11%) were found back within grassland (Fig. 2, Table 1). For all plots pooled, the area covered by grass was 75%, and the area covered by shrubs was 25% (Fig. 2, Table 1). The observed distribution of acorns over the two microhabitats differed significantly from the expected values based on the areas of the microhabitats (Table 1): the dispersal toward shrubs was higher than expected, while the dispersal toward grassland was lower. This pattern remained for both distance classes (0–5 m and 5–20 m), indicating that wood mice disperse acorns directed toward shrub vegetation, independent of the distance from the supply point. In line with this, acorn dispersal distance did not differ between the microhabitats grassland and shrubs (χ 2 = 0.747, P < 0.387, df = 1).
Locations of retrieved dispersed acorns (small dots; squared intact acorn, other dots acorn predated) in the four plots in grassland (white) and blackthorn shrub (grey), with original supply position at the centre of the plots (crossing) in grassland, 2 m away from the edge of a shrub. The inner and outer circles represent a 5-m radius and a 20-m radius dispersal distance from the original supply position, respectively
All the 76 retrieved acorns dispersed to shrubs were predated, while of the nine acorns dispersed to grassland only three were predated and six were still intact. This difference in post-dispersal predation between shrub and grassland was significant (χ 2 = 44.827, P < 0.001, df = 1), indicating that such predation is non-random: acorns dispersed toward shrubs have a higher predation risk than acorns dispersed toward grassland.
Post-dispersal removal experiment
Of all the 80 experimentally cached acorns, 46 acorns (57.5%) were removed at the end of the monitoring period (198 days). The post-dispersal removal was the highest in the middle of shrubs and at the outer-edge of shrubs, with, respectively, 90 and 75% of the acorns removed, while under trees only 35% of the acorns were removed and in grassland only 30% (Fig. 3).
The post-dispersal removal rates differed significantly between the four microhabitats (pair-wise Gehan–Wilcoxon comparisons). The post-dispersal removal rate was significantly higher in the middle of shrubs than at the outer-edge of shrubs (D = 5.604, P < 0.018, df = 1), higher at the outer-edge of shrubs than under trees (D = 10.687, P < 0.001, df = 1), and in grassland (D = 11.463, P < 0.001, df = 1), and not different between grassland and under trees (D = 0.54, P = 0.817, df = 1).
Most of the post-dispersal removal in the middle and outer-edge of shrubs took place within 40 days from the start of the experiment (Fig. 3). Contrarily, there was no predation at all during the first month in grassland and under trees. The median survival time in days was 1.82 for middle of shrub, 46 for outer-edge of shrubs, and 160 for both grassland and under trees. Clearly, the amount of removal of cached acorns is much higher in densely vegetated areas (shrubs) than in open areas (grassland and under trees).
Survey of seedlings and saplings
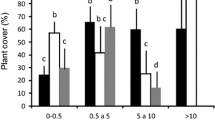
On the 24 transects, we counted 121 young oaks, including 46 seedlings (<1 year) and 75 saplings varying from 1 to 6-year old (28 × 1, 27 × 2, 10 × 3, 6 × 4, 3 × 5, and 1 × 6 year). Seedlings measured 7.6 ± 2.7 cm (±1 sd, range: 1.2–13.5 cm) and saplings measured 13.7 ± 8.9 cm (±1 sd, range: 4.5–46.8 cm). Most of the individuals were found in the inner-edge of shrubs and under trees (Fig. 4, Table 2). Both the distributions of seedlings and saplings over the five microhabitats differed significantly from the expected values based on microhabitat coverage (Table 2), and were found more than expected under trees and in the inner-edge of shrubs. Conversely, seedlings were observed slightly less than expected in the outer-edge of shrubs and much less than expected in the middle of shrubs and in grassland (Table 2). This outcome indicates that seedlings and saplings are spatially associated with the microhabitats under trees and inner-edge of shrubs (Fig. 4, Table 2).
The distribution of recently emerged seedlings over “shrubs” and “open” did not differ from the distribution of saplings ≥1 year, nor from saplings ≥2 year, while the distribution of saplings ≥3 year showed a trend, with more saplings than expected within shrubs (Table 3).
Mean heights of 1-year-old saplings did not differ between “shrubs” and “open” (U = 74.5, P < 0.449, N = 27; Fig. 5), while mean heights of 2-year-old saplings only showed a trend (U = 54.5, P < 0.083, N = 27; Fig. 5) with lower heights in “shrubs” than in “open.” The number of saplings >3 year was too low to further test for differences in height between microhabitats.
Of all saplings, 46% showed signs of grazing damage (54% undamaged). The number of damaged saplings did not differ between “shrubs” and “open” (χ 2 = 1.455, P < 0.228, df = 1). Recruits were the youngest under trees (0.91 year) and the oldest in the middle of shrubs (2.00 year), but ages were not different between the five microhabitats (H = 7.558, P < 0.109, df = 4).
Discussion
Acorn dispersal experiment
Results from our dispersal experiment confirm the hypothesis that wood mice disperse acorns directed toward shrubs in grazed woodlands. Directed dispersal toward dense vegetation has been observed in other ecosystem types (Den Ouden et al. 2005; Iida 2006; Takahashi et al. 2007) and is generally explained by the preference of mice to forage in densely vegetated microhabitats to avoid exposure to predators (Kollmann 1995; Manson and Stiles 1998). Recent mouse trappings at our study site indeed showed higher mice abundance within shrubs compared to grassland (Smit, unpublished data). Our results are, however, in contrast with Gómez et al. (2008) who reported less than expected dispersal toward shrubs in a Mediterranean (not-cattle grazed) oak woodland. This difference may be explained by the shorter mean dispersal distance as observed in their study (3.6 vs. 7.9 m in our study), preventing acorns to reach microhabitats other than where initially placed in. The relatively low recovery rate of our tagged acorns (54.5%) was similar to other studies (Li and Zhang 2003; Iida 2006) and is unlikely to have caused biased results in terms of microhabitat, as most acorns were found under shrubs where the retrieval with the magnet detector was the most difficult.
The survival of the dispersed seeds was, however, particularly low in shrubs. This is in accordance with several studies performed in other ecosystems. The reported predation of unburied acorns under shrubs was similarly high (Li and Zhang 2003; Pons and Pausas 2007; Pérez-Ramos and Marañón 2008), probably due to predator avoidance and associated higher mice densities under shrubs. Also other seed tracking experiments with rodents as dispersal agent found similar low acorn survival varying from 0.4 to 1.4% (Takahashi et al. 2006; Gómez et al. 2008). On the one hand, such low survival percentages lead to the questioning of the effectiveness of wood mice as dispersal agent. On the other hand, even very low survival percentages are important for plant recruitment (Levey and Byrne 1993; Vander Wall et al. 2005) and may add up to a considerable recruitment over time, especially for long-lived trees such as Quercus robur (Takahashi et al. 2006; Gómez et al. 2008).
Post-dispersal removal experiment
Our results confirm the hypothesis that post-dispersal removal of cached acorns is higher in shrubs than under trees and in grassland. Our outcome is in accordance with Gómez (2004) who also found higher post-dispersal removal of artificially cached acorns under shrubs than in other habitats in Mediterranean oak woodlands. Our study additionally shows that post-dispersal seed removal varies largely between the different positions within shrubs, being lower at the edges than at the centers of the shrubs. The most likely reason for this is again that mice prefer foraging in the middle of shrubs where the cover is denser (pers. obs.) and thus offer more protection against predators than at the edges of shrubs.
We note that our observed overall cache removal (57%) may be different in other years given the fluctuations in the number of seeds produced and densities of rodents. The predator satiation hypothesis (Janzen 1971) states that synchronized large seed crops (i.e., masting) decrease the percentage seed predation. Indeed various studies have shown lower acorn predation in high-seed years (reviewed in Kelly and Sork 2002), and there is evidence that the acorn seed crop affects the mice population during the next spring and summer (McShea 2000). Given the fact that there was no masting event during our studies, we may expect higher post-dispersal survival in years with a high crop yield, with large effects for the spatial patterning of oak recruitment in grazed woodlands.
Survey of seedlings and saplings
We found more than expected recruits in the inner-edge of shrubs and low numbers in grassland which supports our hypothesized spatial association between recently emerged oak seedlings and shrubs. Furthermore, the number of seedlings was high under trees and low in the middle and outer-edge of shrubs, which is in line with Gómez et al. (2008) that similarly show higher acorn survival under oaks (0.7%) and shrubs (0.4%) than in grassland (0.001%). What is new in our study is that seedling numbers vary largely between the different positions within shrubs, with high numbers at the inner-edge, but low numbers in the middle and at the outer-edge of shrubs. We think that this has first to do with the relatively short dispersal distance and high availability of acorns close to the tree; arrival of acorns will be higher under the tree and at the inner-edge of shrubs as a consequence of this distance effect. Second, high predation rates in the middle of shrubs, as observed in our post-dispersal removal experiment, may explain the low amount of seedlings observed. Third, light conditions are less favorable in the middle of shrubs, which reduce the growth and survival of the light-demanding oak seedlings (Bakker et al. 2004). The low amount of seedlings in the outer-edge of shrubs could be explained by the effects of distance in combination with predator avoidance behavior, as mentioned before.
Our findings confirm the hypothesis that the spatial association between shrubs and saplings strengthens over time, despite the relatively low number of observations. The difference in the distribution of seedlings and saplings over the microhabitats shrubs and open increased as we step-wise excluded younger saplings, with a trend showing for saplings ≥3 year (Table 3). In addition, 2-year-old saplings were smaller (P < 0.1) in open microhabitats than in shrubs (Fig. 5), while we recorded no differences in heights of 1-year-old saplings between open and shrubs. Thus, differences in saplings between open and shrub habitats increase with age, which is indicative for the hypothesized cumulative grazing-effect, leading to stronger spatial associations between oaks and shrubs over time. Our findings are in accordance with results of van Uytvanck et al. (2008a) that show that saplings are significantly taller in shrubs than in open grassland in a wood-pasture ecosystem in Flanders. Moreover, they found that the percentage of saplings with browsing damage was lower in shrubs than in open grassland. We did not find such differences in percentage of saplings with grazing marks between the microhabitats. This may have to do with the fact that we were unable to differentiate between damage by cattle grazing and rabbit or roe deer browsing. Yet, in contrast with larger cattle and horses, roe deer and particularly rabbits are able to browse tree saplings situated within the protective range of shrubs (Bakker et al. 2004), which may explain the observed grazing marks on saplings within shrubs. We acknowledge that such removal of sapling-tissue via grazing occasionally complicated the age estimation (counting of bud scars, Dobrowolska 2006), leading to potential underestimation of the age of some saplings, but we believe this would not alter our results.
Synthesis
We aimed to test if acorn dispersal and post-dispersal acorn removal by wood mice can explain the spatial association between oak trees and blackthorn shrubs, besides associational resistance. Our acorn dispersal experiment indeed clearly showed that wood mice transport acorns directed toward shrubs, although most of these acorns were consumed. Post-dispersal removal of cached acorns was higher in shrubs than under trees or in grassland, but numbers were higher in the middle of shrubs than at shrub edges. This pattern was also subscribed by the observed lower numbers of recently emerged seedlings in the middle and outer-edge of shrubs, while the number of recently emerged seedlings was the highest at the inner-edge of shrubs. Much of these patterns are probably best explained by the combination of higher mice activity in the middle of shrubs due to reduced predation risk, and higher acorn input close to the tree (distance effect). Our study shows that not only acorn dispersal to and subsequent seedling survival in shrubs is important for successful oak recruitment, but also the position within shrubs is a crucial factor. It was already noted that shrub edges, particularly the outer-edges, are probably the most suitable positions for oak saplings due to an optimal balance between protection from large herbivores and light availability (Bakker et al. 2004; van Uytvanck et al. 2008a). With our study, we now give evidence that these observed spatial associations between oak saplings and shrubs may not just be caused by associational resistance during the sapling phase, but may also be caused by seed handling behavior by wood mice in the preceding stage. Hence, while thus far particularly large herbivores were held responsible for the observed patterns of tree recruitment in grazed woodlands (Olff et al. 1999; Vera 2000) our findings demonstrate the importance of wood mice for initiating the spatial associations between oaks and spiny shrubs.
References
Arrieta S, Suárez F (2005) Spatial patterns of seedling emergence and survival as a critical phase in holly (Ilex aquifolium L.) woodland recruitment in Central Spain. For Ecol Manage 205:267–282
Bakker ES, Olff H, Vandenberghe C, De Maeyer K, Smit R, Gleichman JM, Vera FWM (2004) Ecological anachronisms in the recruitment of temperate light-demanding tree species in wooded pastures. J Appl Ecol 41:571–582
Bakker ES, Ritchie ME, Olff H, Milchunas DG, Knops JMH (2006) Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol Lett 9:780–788
Bossema I (1979) Jays and oaks: an eco-ethological study of a symbiosis. Behaviour 70:1–117
Den Ouden J, Jansen PA, Smit R (2005) Jays, mice and oaks: predation and dispersal of Quercus robur and Q. petraea in north-western Europe. In: Forget PM et al (eds) Seed fate. CAB Int, Wallingford, pp 223–239
Dobrowolska D (2006) Oak natural regeneration and conversion processes in mixed Scots pine stands. Forestry 79:503–513
Gómez JM (2004) Importance of microhabitat and acorn burial on Quercus ilex early recruitment: non-additive effects on multiple demographic processes. Plant Ecol 172:287–297
Gómez JM, Puerta-Piñero C, Schupp EW (2008) Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 155:529–537
Gribko LS, Jones WE (1995) Test of float method of assessing northern red oak seed condition. Tree Planter’s Notes 46:143–147
Hamback PA, Agren J, Ericson L (2000) Associational resistance: insect damage to purple loosestrife reduced in thickets of sweet gale. Ecology 81:1784–1794
Iida S (2006) Dispersal patterns of Quercus serrata acorns by wood mice in and around canopy gaps in a temperate forest. For Ecol Manage 227:71–78
Janzen DH (1971) Seed predation by animals. Ann Rev Ecol Syst 2:465–492
Kelly D, Sork VL (2002) Mast seedling in perennial plants: why, how, where? Annu Rev Ecol Syst 33:427–447
Kollmann J (1995) Regeneration window for fleshy-fruited plants during scrub development on abandoned grassland. Ecoscience 2:213–222
Kollmann J, Schill HP (1996) Spatial patterns of dispersal, seed predation and germination during colonization of abandoned grassland by Quercus petraea and Corylus avellana. Vegetatio 125:193–205
Levey DJ, Byrne MM (1993) Complex ant-plant interactions: rain forest ants as secondary dispersers and post-dispersal seed predators. Ecology 74:1802–1812
Li HJ, Zhang ZB (2003) Effect of rodents on acorn dispersal and survival of the Liaodong oak (Quercus liaotungensis Koidz.). For Ecol Manage 176:387–396
Manson RH, Stiles EW (1998) Links between microhabitat preferences and seed predation by small mammals in old fields. Oikos 82:37–50
McShea WJ (2000) The influence of acorn crops on annual variation in rodent and bird populations. Ecology 81:228–238
Milchunas DG, Noy-Meir I (2002) Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99:113–130
Olff H, Vera FWM, Bokdam J, Bakker ES, Gleichman JM, De Maeyer K, Smit R (1999) Shifting mosaics in grazed woodlands driven by the alternation of plant facilitation and competition. Plant Biol 1:127–137
Pérez-Ramos IM, Marañón T (2008) Factors affecting post-dispersal seed predation in two coexisting oak species: microhabitat, burial and exclusion of large herbivores. For Ecol Manage 255:3506–3514
Pons J, Pausas JG (2007) Rodent acorn selection in a Mediterranean oak landscape. Ecol Res 22:535–541
Smit C, Béguin D, Buttler A, Müller-Schärer H (2005) Safe sites for tree regeneration in wooded pastures: a case of associational resistance? J Veg Sci 16:209–214
Smit C, Den Ouden J, Müller-Schärer H (2006a) Unpalatable plants facilitate tree sapling survival in wooded pastures. J Appl Ecol 43:305–312
Smit C, Gusberti M, Müller-Schärer H (2006b) Safe for saplings; safe for seeds? For Ecol Manage 237:471–477
Smit C, Den Ouden J, Díaz M (2008) Facilitation of Quercus ilex recruitment by shrubs in Mediterranean open woodlands. J Veg Sci 19:193–200
Smit C, Díaz M, Jansen P (2009) Establishment limitation of holm oak (Quercus ilex subsp. ballota Desf. Samp.) in a Mediterranean savanna—Forest ecosystem. Ann For Sci 66:511–518
Takahashi K, Sato K, Washitani I (2006) The role of the wood mouse in Quercus serrata acorn dispersal in abandoned cut-over land. For Ecol Manage 229:120–127
Takahashi K, Sato K, Washitani I (2007) Acorn dispersal and predation patterns of four tree species by wood mice in abandoned cut-over land. For Ecol Manage 250:187–195
Van Uytvanck J, Decleer K, Hoffmann M (2008a) Establishment patterns of woody species in low intensity-grazed pastures after the cessation of intensive agricultural use. For Ecol Manage 256:106–113
Van Uytvanck J, Maes D, Vandenhaute D, Hoffmann M (2008b) Restoration of wood pasture on former agricultural land: the importance of safe sites and time gaps before grazing for tree seedlings. Biol Conserv 141:78–88
Vander Wall SB, Kuhn KM, Beck MJ (2005) Seed removal, seed predation, and secondary dispersal. Ecology 86:801–806
Vera FWM (2000) Grazing ecology and forest history. CAB International, Oxon
Acknowledgments
We would like to thank Staatsbosbeheer (National Forest Service) for permission to perform these studies at Junner Koeland, C. Zweep for preparing and labor-intensive retrieval of the tagged acorns, and J. den Ouden for lending us the equipment and for helpful comments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Smit, C., Verwijmeren, M. Tree-shrub associations in grazed woodlands: first rodents, then cattle?. Plant Ecol 212, 483–493 (2011). https://doi.org/10.1007/s11258-010-9839-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-010-9839-1