Abstract
The clonal plant Schoenoplectus americanus shows variable belowground clonal architecture as a result of producing two types of ramets: those with very long rhizomes (long rhizome ramet, LRR) and those with very short ones (short rhizome ramet, SRR). In a previous study we demonstrated that the two types of ramets are functionally specialised. The production of SRRs results in the formation of consolidated clonal patches with densely packed shoots, while the production of LRRs results in a more diffuse network of connected rhizomes with widely spaced shoots. We hypothesised that the two types of ramets would be produced at different times during the growing season because of their functional differences. The production of LRRs throughout the growing season would enable the species to continuously explore new habitats while the production of SRRs early in the growing season would enable the species to occupy and consolidate resources in available open patches. We evaluated this hypothesis through field observations in different communities with S. americanus and indeed found that SRRs were produced early in the growing season while LRRs tended to be produced over an extended period of time. Plants in high-quality environments (i.e. higher light conditions) produced more SRRs, and these were formed early in the growing season. In contrast, plants in low-quality environments produced more LRRs, and these were formed continuously over the growing season. We also observed that the shoot longevity was greater for SRR. In high-quality patches, the production of the lower cost SRRs results in a more rapid occupancy of open spaces; in lower quality patches, the production of LRRs throughout the growing season enables plants to explore the immediate environment for higher quality patches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clonal growth is one of the most successful propagation strategies in the plant world. By repeatedly producing genetically identical ramets, clonal plants develop a variety of architectural forms that result from the formation of spacers (rhizomes, stolons or roots) of different lengths, different numbers of branches and different branching angles, all of which result in the placement of ramets in space and time. The complexity of clonal architectures differs among plant species or within the same species growing in different environments (Bell 1980, 1984; Lovett Doust 1981; de Kroon and Knops 1990; Hutchings and de Kroon 1994; Ikegami 2004; Ikegami et al. 2006). Clonal plants may locally change the architectural elements of their morphology, such as by producing shorter spacers to occupy local resource patches or by producing longer spacers to place new ramets in areas where resources may be greater or competition may be less (Slade and Hutchings 1987a, b, c; Dong and de Kroon 1994; de Kroon and Hutchings 1995; Dong 1996; Ikegami et al. 2007). Shoots placed at the end of short or long spacers thus allow plants to more effectively respond to changing environmental conditions, and because environmental conditions change in space and time, there should also be differences in the timing of short and long spacer ramet production. The production of short spacers would allow clonal plants to occupy available high resource patches faster, while the production of shorter rhizomes, with a resultant higher density of shoots, would potentially keep other species out of the patches (e.g. a consolidation strategy). In contrast, when ramets are in low resource or highly competitive microhabitats, it would be beneficial to the plant to continuously produce ramets at the end of longer spacers, thus increasing the probability of placing ramets in higher quality environments (i.e. an exploration strategy). Although many studies have examined clonal architectures, no study has focused on ramet phenology in relation to clonal architectures.
In a related study, we previously demonstrated that the clonal sedge, Schoenoplectus americanus, maintained architectural plasticity by producing two types of ramets: those with long rhizomes (long rhizome ramet, LRR) and those with short rhizomes (short rhizome ramet, SRR) (Fig. 1). Subsequent research on this species demonstrated that S. americanus growing in environments with high resource levels produced more biomass and SRRs with a larger number of branches compared to plants growing in low resource environments (Ikegami 2004; Ikegami et al. 2007). These results suggested that S. americanus occupies and exploits resources in favourable patches by producing SRRs and explores other favourable patches by producing LRRs in spatially heterogeneous ecosystems. They further indicated that S. americanus varied its clonal architecture from a Phalanx strategy, with the aim of consolidating favourable environmental patches, to a Guerrilla strategy, with the aim of escaping/exploring different environmental patches, in the sense of Lovett Doust (1981). We also found that LRRs tended to continuously produce LRRs, while SRRs tended to produce relatively few SRRs following the spring phenophase (Ikegami 2004; Ikegami et al. 2007), suggesting that LRRs and SRRs could have differences in appearance patterns over a growing season. By repeatedly producing LRRs throughout the growing season, plants increase the probability that they will reach higher quality habitats or escape low-quality patches. Short rhizome ramets, in contrast, only produce new ramets once or twice a year and are better able to continuously occupy higher quality habitats at lower costs.
Here, we report the results of a field-based study in which we tested the hypothesis that the two types of ramets had different phenology patterns. We predicted that LRRs would appear throughout a growing season while SRRs would only be produced early in the growing season. We also hypothesised that plants in different environments would have different patterns of shoot phenology that would be related to patterns of rhizome production. We predicted that plants growing in lower quality patches would have a lower SRR ratio (i.e. higher LRR ratio) and that aboveground shoots would be produced throughout the growing season. In contrast, plants in higher quality patches would have a higher SRR ratio, and most of the aboveground shoots would be produced early in the growing season.
Plant and communities
Schoenoplectus americanus (Pers.) Volk. ex Schinz & R. Keller, a member of the sedge family Cyperaceae, occurs in different plant communities in tidal wetlands that range from brackish to fresh water along the East Coast of the USA (McCormick and Somes 1982; Drake 1984; Ikegami et al. 2006). A common synonym of this species that appears in the wetland literature is Scirpus olneyi A. Gray.
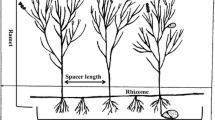
The aboveground part of each ramet consists of a vegetative or reproductive shoot that is annual. Shoots are erect, sharply triangular and needle-like, with rudimentary leaves. The belowground parts of a ramet consist of roots, a tuber and a rhizome. The node of each underground ramet is a tuber from which long or short internodes emerge. A daughter tuber with a measurable rhizome is defined as a “long rhizome ramet” (Fig. 1a), and a daughter tuber with an unmeasurable rhizome (maximum of a few millimeters) attached to the parent tuber is defined as a “short rhizome ramet” (Fig. 1b). Hereafter in this study, we use the word “a rhizome” to indicate “a tuber with a rhizome”.
The research was conducted in three physically separated tidal wetlands (locally known as Hog Island Marsh, Corn Island Marsh and Kirkpatrick Marsh, respectively) at the Smithsonian Environmental Research Centre (SERC, 38°53′ N, 76°33′ W) in Maryland, USA. The three brackish wetlands are physically separated by at least 500 m, and each is about 2–5 ha. Schoenoplectus americanus occurs in several plant communities, and we conducted observations in three of these: (1) Schoenoplectus High marsh community (HIGH), (2) Schoenoplectus Shaded marsh community (SHADE) and (3) Spartina community (SPARTINA) (Ikegami 2004; Ikegami et al. 2006). Schoenoplectus americanus is a dominant species in the HIGH and SHADE communities, and it invades the SPARTINA community from the edge (McCormick and Somes 1982). Each community has specific characteristics, as described below (Ikegami 2004; Ikegami et al. 2006):
In the HIGH community, light availability declined from the top to the bottom of the canopy due to high shoot densities of S. americanus. Living and partially decomposed shoots, roots and rhizomes of Spartina patens, Distichlis spicata and S. americanus formed a hard, compact organic substrate. In the SHADE community, the only common species were S. americanus and Phragmites communis (McCormick and Somes 1982). This community occurred around the margins of each wetland, thereby forming the boundary with the adjacent upland. Because of its proximity to the adjacent upland, overhanging tree branches resulted in low light availability during the growing season. The substrate in the SHADE community was composed of highly decomposed organic matter, resulting in a very soft substrate. In the SPARTINA community, S. patens was the dominant species, and S. americanus appeared to invade the community from the margins. Light availability in the SPARTINA community was high for most of the growing season because the shoots of S. patens become horizontal shortly after they were mature. The substrate in the SPARTINA community was a highly organic peat that was composed of a dense mat of living and dead rhizomes and roots of S. patens.
Methods
To evaluate the phenology of S. americanus ramets, we randomly selected several plots in the three study sites on 3 April 2000. Since no plant had appeared in some of the plots in the SHADE community on 17 April, we abandoned those plots and decided to use a total of 31 plots as observation plots (25 × 25 cm) in the HIGH, SHADE and SPARTINA communities. To compensate for the much lower densities of ramets in the SHADE and SPARTINA communities than in the HIGH community, we assigned a larger number of observation plots to the former communities. The distribution of plots among sites and communities is shown in Table 1. All new shoots in each plot were tagged with numbered plastic labels at 2-week intervals during the observation period. The status (living or dead, sexual or asexual) of all tagged shoots was evaluated bi-weekly until 28 November, when the first killing frost occurred. The phenology observation period was therefore 238 days. Phenology observations were not made on 4 and 18 September; we therefore had to estimate the number of new shoots and the number of dead shoots for those two dates based on shoot heights and colour. Shoots that were higher than 30 cm were assumed to have appeared between 4 and 18 September, and shoots that were <30 cm were assumed to have appeared between 18 September and 2 October. Shoots that were completely yellow on October 2 were assumed to have died before 4 September, and shoots that still had some green colour were assigned to the cohort that died by 18 September.
In late November and December 2000 we excavated 20 samples from the phenology plots (Table 1) to characterise clonal architectures. The samples were 25 × 25 cm, and they were excavated to a depth of 20 cm, which was deep enough to collect all living S. americanus ramets. The samples were washed in the laboratory to remove loose organic material, and living roots and rhizomes were carefully extracted. For each ramet, we recorded the type (LRR or SRR), the age (current year or previous year), the number of daughter ramets (branching) and the rhizome length (included the tuber length). The rhizome systems were individually weighed after drying for 72 h at 68°C in a Grieve forced air oven.
We used the shoot phenology data to estimate the date of appearance of each current-year rhizome. The date of appearance of each rhizome was assumed to be the date of appearance of its aboveground shoot. Some number tags were found on the ground or were lost during the observation period, mainly due to the die-back of shoots immediately after tags had been left on the ground. We treated those shoots as missing shoots.
In August 2001, we conducted a separate study to compare shoot morphology and biomass allocation patterns in the three communities. We excavated ten plots (10 × 10 × 20 cm) in each community: four plots from Hog Island marsh, three plots from the Corn Island marsh and three plots from the Kirkpatrick marsh (Table 1). For up to four shoots in each plot, we measured shoot height, the width of the broadest side of the triangular shoot at about 10 cm above the soil surface and the hypotenuse of the “triangle” at that point. We used the shoot dimension data to calculate the green area (GA) of the shoots, which was calculated as the total surface area of the triangular pyramidal shoot. We also determined the weight of each shoot and the weights of the current-year and previous-year rhizomes. Dry weights were determined after 72 h of drying at 68°C. Shoot weight and area data were used to calculate the specific green area (SGA), as GA divided by shoot weight.
Data analysis
From the bi-weekly monitoring of the plots we were able to calculate a rate of appearance of new shoots and a rate of shoot mortality. To statistically compare the phenology data among the three communities, we separated the growing season into three 70-day periods. The spring phase was between 3 April and 12 June; the summer phase, between 3 June and 21 August; the autumn phase, between 22 August and 30 October. Although we continued the observations until 28 November, we ended the autumn phase on October 30 because the last new shoots were observed on that date. We calculated averages during each phase in each community for: (1) new shoot appearance ratio, (2) dead shoot appearance ratio, (3) green shoot ratio, (4) flowering shoot appearance ratio, (5) new SRR appearance ratio, (6) new LRR appearance ratio, (7) rhizome length ratio and (8) rhizome weight ratio. We also calculated the ratio of surviving shoots and missing shoots at the end of the observation period. The ratios for each plot and each phase were calculated as described here below, and the definitions are given in Table 2.
Each appearance ratio is the number of each item (i.e. new shoots, dead shoots, flowering shoots, SRR and LRR) that appeared in a plot during a phase divided by the total number of the item that appeared throughout the growing season. The surviving shoot ratio and missing shoot ratio are the ratio of the number of green shoots present and the ratio of the number of missing shoots present, respectively, at the end of the growing season to the total number of shoots that appeared during the growing season. The average green shoot ratio in a phase is the mean value of the green shoot ratios on all observation days during a phase in each plot, and the green shoot ratio on an observation day is calculated as the number of green shoot in each plot on an observation day divided by the total number of shoots that appeared during the growing season in that plot. The rhizome length ratio and the rhizome weight ratio in a phase are calculated as the increase in total rhizome length and total rhizome weight, respectively, that appear in a phase divided by the total rhizome length and total rhizome weight produced during the growing season.
To compare shoot longevity, rhizome weight and rhizome weight/length between SRRs and LRRs in the three communities, we used the phenology data from the period between 3 April and 15 May. We used 15 May as the cut-off date for the evaluation of shoot longevity because shoots that appeared after 15 May tended to survive until they were killed by frosts and, therefore, they could not be used for longevity calculations. At the same time, we used rhizome weight data during this period for comparison, since the mean rhizome weight and length also differed between the early and late parts of the growing season. Shoot longevity was defined as the number of days between the day of shoot appearance and the day of shoot death. If a shoot was green until the day of excavation, then the date of shoot death was set on the last observation day. Since the last observation day was after the first major frost, we assumed the each shoot was dead at this time regardless of its colour. The ratio of rhizome weight to rhizome length was used to evaluate the biomass investment to an increase in rhizome length in each community. Mean branching numbers and rhizome length of SRRs and LRRs that appeared in each community in the three phases were also calculated to compare seasonal differences in clonal architectures.
We used two-way ANOVA to test for differences in each appearance ratio (i.e. new shoot, dead shoot, SRR, LRR), average green shoot ratios, rhizome length ratios, rhizome weight ratios, branching numbers and the average rhizome length of LRRs and SRRs among three phases and three communities. After testing for statistical significance by two-way-ANOVA, we used one-way ANOVA among three communities and three phases followed by the Bonferroni–Dunn test for multiple comparisons. The t test was used to test for differences in shoot longevity between SRR and LRR in three communities, for differences in the flowering shoot ratio between phases and between communities and for differences in the branching number per ramet between LRRs and SRRs. We used one-way ANOVA to test for differences in number of current-year shoots, shoot height, SGA, total biomass, the ratio of living aboveground to living belowground biomass, the ratio of current-year belowground biomass to all living belowground biomass and the SRR ratio among three communities as well as for the branching number of rhizomes, length of rhizomes, shoot longevity, rhizome weight and the ratio of rhizome weight to rhizome length of LRRs and SRRs among three communities. All statistical tests were done in Stat-View ver. 5.21. (SAS Institute, 1998).
Results
Shoot morphology
The mean number of current-year shoots was significantly larger in the HIGH community than in the other two communities (Table 3). Plants in the HIGH community had a significantly larger biomass than those in the SHADE and SPARTINA communities (Table 3). Shoots were taller in the SHADE community and shortest in the SPARTINA community. The SGA values were highest in the SHADE community, but the means were not significantly different from values in the SPARTINA and HIGH communities (Table 3). The living belowground biomass of plants in the HIGH community was twice that of the aboveground biomass. In contrast, plants in the other two communities had more aboveground biomass than belowground biomass (Table 3). Plants in the SHADE community had the highest ratio of aboveground to belowground biomass, but the means were not significantly different from those of the SPARTINA community (Table 3). Current-year belowground biomass accounted for only 11% of all living belowground mass in the HIGH community, while in the other communities, current-year belowground biomass accounted for more than 60% of the total living belowground biomass (Table 3).
Rhizome morphology
Sixty-two percent of all ramets were SRRs in the HIGH community compared to 28% in the SPARTINA community (Table 4). The LRRs tended to branch more frequently than SRRs, but the differences between the two types of ramets were only significant in the HIGH community. In the SHADE community, both SRRs and LRRs branched more frequently than their counterparts in the other two communities (Table 4). The lengths of LRRs were greatest in the SHADE community and shortest in the HIGH community (Table 4). The average LRR rhizome weight did not differ statistically among communities, while the average SRR rhizome weight was greatest in the SPARTINA community (Table 5). Plants in the SPARTINA community had significantly higher SRR weight/length ratios, and plants in the SHADE community had significantly lower LRR weight/length ratios (Table 5).
Shoot phenology
Plants in the three communities had similar shoot appearance patterns. Most shoots appeared in the spring phase, and only a few appeared in the autumn phase. There were, however, some differences in the proportion of shoot appearances in each phase (Fig. 2a). Plants in the HIGH community produced more than 80% of their shoots during the spring phase, a value that was significantly higher than that observed for the SPARTINA community (Fig. 2a). In contrast, in the autumn phase, plants in the HIGH community produced about 1.4% of all shoots, while plants in the SPARTINA community produced about 11% (Fig. 2a). Plants in the SHADE community were intermediate between the HIGH and SPARTINA communities, with plants producing many shoots during the spring and summer phases and few in the autumn. There was, however, no significant difference between the HIGH and SPARTINA communities.
(a) Average new shoot appearance ratio (±SE), (b) average dead shoot appearance ratio (±SE), (c) average green shoot ratio (±SE), (d) average flowering shoot appearance ratio (±SE) of observation plots in each phase in the three communities. The category Surviving in b indicates the green shoot ratio at the end of the observation season. Different lower-case letters above the bars indicate significant differences among phases in each community. Different upper-case letters above the graphs indicate significant differences among communities in each phase. Significance levels are P < 0.0167 in a and c and P < 0.0083 among phases, and P < 0.0167 among communities in b, all by one-way ANOVA followed by the Bonferroni–Dunn test; P < 0.05 in d by the t test. The results and F value of the two-way ANOVA are given in Table 6. HIGH Schoenoplectus High marsh community, SHADE Schoenoplectus Shaded marsh community, SPARTINA Spartina community
Patterns of shoot mortality differed among the three communities (Fig. 2b). In the HIGH community, most shoots (61% of all shoots) died in autumn (Fig. 2b) compared to earlier shoot senescence in the SHADE and SPARTINA communities; in the latter two communities, shoots began to die earlier in the growing season and the largest number died in the summer phase (Fig. 2b). In the HIGH community, the average green shoot ratio was 80% through the summer phase and 20% in the autumn phase (Fig. 2c), and few shoots were green at the end of the growing season (Fig. 2b). In contrast, plants in the SHADE and SPARTINA communities had lower green shoot ratios (less than 60%) during the growing season and the number of green shoots gradually declined, but more than 10% of the shoots were still green at the end of the growing season (Fig. 2b, c). On average, 1.8% of the shoots in the HIGH community, 13.5% of the shoots in the SHADE community and 18.3 % of the shoots in the SPARTINA community were green at the time of the last sampling (Fig. 2b). We missed 1.8% of the shoots in the HIGH community, 17.6% of the shoots in the SHADE community and 6.2% of the shoots in the SPARTINA community during the observation period.
Flowering shoots appeared mostly in the spring phase, and only a few appeared in the summer phase (P < 0.001 in the HIGH and P < 0.05 in the SPARTINA community, respectively). Plants in the HIGH community had a higher ratio of flowering shoots than those in the SPARTINA community (70 and 12%, respectively; P < 0.001; Fig. 2d). No flowering shoots were observed in the SHADE community. Shoot longevity was highest in the HIGH community and least in the SPARTINA community (Table 5). Shoots that originated from SRRs had a higher longevity than shoots from LRRs in the HIGH and SPARTINA communities (Table 5).The results and F value of the two-way ANOVA are given in Table 6.
Rhizome phenology
In each community, most SRRs appeared in the spring phase (Fig. 3a), while LRRs appeared mainly in the spring and summer phases (Fig. 3b). Plants in the SHADE and SPARTINA communities produced LRRs in the autumn, while plants in the HIGH community stopped producing LRRs in the summer phase. In the SPARTINA community, plants showed a significantly higher ratio of LRR appearance in the autumn phase, with 14% of LRRs appearing in the autumn phase. In the HIGH community, this was only 2% (Fig. 3b). Based on total rhizome length and weight ratio, plants in the HIGH community produced most of the rhizome growth in the spring and summer phases (Figs. 4a, b). In the SHADE and SPARTINA communities, plants continued to produce relatively constant amounts of rhizome material, invested both in rhizome length and biomass, right into the autumn phase (Figs. 4a, b).
The average new SRR appearance ratio (±SE) (a) and average new LRR appearance ratio (±SE) (b) on observation plots in each phase in the three communities. Different lower-case letters above the bars indicate significant differences among phases in each community. Different upper-case letters above the graphs indicate significant differences among communities in each phase. Significance levels are P < 0.0167 by Bonferroni–Dunn test. The results and F value of two-way ANOVA are given in Table 6. HIGH Schoenoplectus High marsh community, SHADE Schoenoplectus Shaded marsh community, SPARTINA Spartina community
The average total belowground ramet length ratio (±SE) (a) and the average total belowground ramet weight ratio (±SE) (b) on observation plots in each phase in three communities. Different lower-case letters above the bars indicate significant differences among phases in each community. Different upper-case letters above the graphs indicate significant differences per phase among communities in each phase. Significance levels are P < 0.0167 by the Bonferroni–Dunn test. The results and F value of two-way ANOVA are given in Table 6. HIGH Schoenoplectus High marsh community, SHADE Schoenoplectus Shaded marsh community, SPARTINA Spartina community
Current-year SRRs did not show differences among communities and phases (Fig. 5a). On the other hand, current-year LRRs in the SHADE community tended to branch more frequently at later growing phases, while LRRs in other two communities did not (Fig. 5b). The mean length of rhizomes tended to be longer at later phases, both in SRRs and LRRs, except in SRRs in the SHADE community (Fig. 5c, d).
The average branching number of SRRs (±SE) (a), the average branching number of LRRs (±SE) (b), the average rhizome length of SRRs (±SE) (c) and the average rhizome length of LRRs (±SE) (d) in each phase in three communities. Different lower-case letters above the bars indicate significant differences among phases in each community. Different upper-case letters above the graphs indicate significant differences among communities in each phase. Significance levels are P < 0.05 in a by the t test and P < 0.0167 in b, c and d among communities by the Bonferroni–Dunn test. Since fewer number of SRRs appeared in the Autumn phase and those SRRs did not branch, no statistical comparison was made (n = 1 in the SPARTINA community). The results and F value of two-way ANOVA are given in Table 6. HIGH Schoenoplectus High marsh community, SHADED Schoenoplectus Shaded marsh community, SPARTINA: Spartina community
Figure 6 shows the total weight of SRRs (a), LRRs (b) and the sum of SRRs and LRRs weights (c) at each observation day. In the HIGH community, plants showed two peaks (Fig. 6c). The first peak was on the 15th and 29th observation days, due to SRRs (Fig. 6a), and the second was on the 70th and 84th days, due to LRRs (Fig. 6b). In the other two communities plants did not show clear peaks.
The total weight of SRR ramets (a), LRR weight (b) and weight of all ramets (c) of all observation plots at each observation day in three communities. HIGH (filled triangle) Schoenoplectus High marsh community, SHADED (open circle) Schoenoplectus Shaded marsh community, SPARTINA (filled square) Spartina community
Discussion
Two of our hypotheses on shoot and ramet appearances were confirmed by the field observations. Although plants in every community produced the greatest numbers of shoots in the spring phase, plants in the HIGH community produced most of their shoots in the spring and summer phases, while plants in the SPARTINA community produced new shoots until the end of the growing season, and plants in the SHADE community were intermediate (Fig. 2). The two types of ramets also showed different phenological patterns. Short rhizome ramets appeared mainly in the spring phase, while LRRs appeared in the spring and summer phases (Fig. 3). Thus, as we hypothesised, plants in the HIGH community, where plants showed the highest SRR ratio (Table 4), produced most of their shoots from SRRs in the spring phase, and plants in the SPARTINA community, where plants showed the lowest SRR ratio (Table 4), kept producing new shoots from LRRs until the end of the growing season, while plants in the SHADE community were intermediate between the two (Table 4, Fig. 2).
Kikuzawa (1983, 2003) categorised leaf expansion patterns into three types: “the simultaneous type”, “the successive type” and “the intermediate type” (Kikuzawa 1983; Kikuzawa 2003). According to this classification, plants in the HIGH community were of the simultaneous type, plants in the SPARTINA community of the successive type and plants in the SHADE community were of the intermediate type. Kikuzawa (2003) suggested that the successive leafing is suitable for open habitats while simultaneous leafing is suitable for light-limiting habitats (Kikuzawa 2003). Iwasa and Cohen (1989) studied the optimal growth schedule of a perennial plant with a theoretical model and predicted that simultaneous leafing is suitable under conditions of low productivity (e.g. light-limiting environments) when plants are small and also under stable conditions when plants are mature (Iwasa and Cohen 1989). Kikuzawa's (2003) and Iwasa and Cohen's (1989) hypotheses can well explain our results from the HIGH community (mature plants with stable condition) and SPARTINA community (open habitat), but not our results from the SHADE community. We anticipated that plants in the SHADE community would show simultaneous leafing due to the light-limiting environment, but our field observations showed that plants in this community showed intermediate leafing and successive ramet production from rhizomes that grew in length and weight (Figs. 4, 6).
Shoot and ramet phenology patterns of S. americanus are, however, explained by the foraging behaviour of clonal plants. Although the spacer length of several clonal plants tends to be insensitive to environmental conditions, some clonal plants can escape from poor to better environmental patches by producing longer spacer ramets, while non-clonal plants cannot (Slade and Hutchings 1987a, b, c; Dong and de Kroon 1994; de Kroon and Hutchings 1995; Dong 1996). Our work with S. americanus has shown that the production of LRRs results in a higher probability that new shoots will be placed in higher quality environments (Ikegami et al. 2007). The weight and length growth of ramets in this study supports the results of the modelling effort. Plants in the SHADE and SPARTINA communities produced new rhizomes in the autumn (Fig. 4a, b), although the number of ramets produced in the autumn was significantly lower than the number produced in the spring and summer phases (Fig. 3a, b). We interpret this result to mean that S. americanus in the SHADE and SPARTINA communities continues to explore new localities throughout the growing season with fewer but heavier and longer LRRs (Fig. 5c, d). Thus, an extended period of production of LRRs is interpreted here as an effective foraging behaviour that results in successive or intermediate leafing. Foraging theory can be used to explain the phenology of SRRs as well. One of our studies (Ikegami et al. 2007) showed that current-year SRRs in the HIGH community tended to produce new SRRs during the following growing season. For a plant to continue to occupy higher quality patches where the parent ramets already were present during the previous growing season, it is important to produce new ramets (shoots) before those from other plants invade that habitat. Short rhizome ramets are more suitable than LRRs for this purpose due to their short spacer length and, thereby, less cost and less time needed to be produced (Tables 4, 5). Consequently, plants produce most of their SRRs early in the growing season. Since most patches are occupied after the spring phase, plants in the HIGH community cease producing new ramets at this time and do not explore new localities intensively; instead, they exploit the local habitat by producing SRRs (Figs. 3, 4), and their shoots persist longer (Table 5).
While shoot appearance showed rather similar patterns among communities, shoot mortality showed clear differences. Most shoots in the HIGH community senesced in the autumn phase, while shoots in the SPARTINA and SHADE communities had the highest mortality in the summer phase (Fig. 2b). In general, shoots produced on SRRs tended to show greater longevity than those produced on LRRs (Table 5). As a result, throughout the growing season,plants in the HIGH community consistently had a higher percentage of green shoots (more than 80% in the summer phases), while plants in the other two communities had lower ratios (<60%) (Fig. 2c). According to Koike (1988), leaf longevity is determined by the balance between the production costs of a leaf and the maximum photosynthesis efficiency of the leaf. In this study, SGA may be used as a proxy for the production costs of shoots. The SGA was highest in the SHADE community and lowest in the SPARTINA community (Table 3). In the HIGH community, occupancy of a patch that is occupied by shoots produced on SRRs results in minimising the costs of shoot production. Accordingly, the shoots from SRRs have a greater longevity in this community (Table 5). On the other hand, lower shoot longevity in the SPARTINA community can be explained as the result of the costs of replacing shoots that are stressed and potentially damaged by the intense light conditions, in order to maintain high photosynthetic gains (Koike 1988; Reich et al. 1995). Light availability is highly limited in the SHADE community; for this reason, plants produced shoots with a high SGA (less production cost per green area) but with a lower longevity. In light-limited habitats, the exploration strategy would potentially result in the placement of new shoots under higher light conditions. This strategy could explain the lower shoot longevity of LRRs compared to that of SRRs in the HIGH and SPARTINA communities, and the lower SRR longevity in the SHADE and SPARTINA communities compared to that of SRRs in the HIGH community.
Summary and perspective
Two contrasting strategies related to foraging are suggested by the results of this study. In the HIGH community, the plants clearly showed two peaks of ramet production (Fig. 6c). The first peak was due to SRR production (Fig. 6a), and the second peak was due to the production of LRRs (Fig. 6b). These results imply that the plants may benefit from the production of a large number of SRRs early in the growing season by using resources stored over winter. A high shoot density may result in increased photosynthetic gains of the genet, and the plants can use these gains to produce LRRs that may reach higher quality habitats and thus increase resource capture. The larger photosynthetic gains and greater storage of carbon resources can explain the large number of flowering shoots in the HIGH community (Fig. 2d). Once open patches are occupied by ramets early in the growing season, genets cease producing new ramets. On the other hand, plants in the other two communities also showed peaks of shoot production with an intervening period of low ramet production. In the same communities, however, plants produced about the same amount of ramets throughout the growing season. These results imply that plants in some habitats produce LRRs throughout the growing season, probably by using resources obtained in the same growing season, and that this gives the plants a higher chance to reach higher quality environments.
Our field study shows that S. americanus has plasticity in ramet phenology that results in either the consolidation of shoots in higher light habitats or a continuous spatial dynamics of shoots in low light habitats, the latter being interpreted as a foraging strategy. This plasticity is manifested through the production of different types of spacers and that the production of spacers in different habitats follows different phenological patterns. In higher light environments, individuals produce ramets with short spacers early in the growing season or they escape to/explore different environments by continuously producing ramets with longer spacers until the end of the growing season. These results are suggestive of the Phalanx and Guerrilla strategies that have been described for clonal plants (Lovett Doust 1981). Our previous studies demonstrated that S. americanus had both Phalanx and Guerrilla strategies within relatively small-scale environments (Ikegami et al 2007). We also showed that resource allocation patterns differed between the Phalanx and Guerrilla strategies. Plants with the Phalanx strategy had larger and, on average, older rhizomes than plants with the Guerrilla strategy. This result suggested that plants with the Phalanx strategy tended to allocate more carbon resources for storage, while plants with the Guerrilla strategy tended to allocate more carbon resources for exploration (Ikegami et al 2007). In this study, we showed that both strategies had different phenological patterns in the production of two types of ramets with different timing. These observations suggest that plants with the Phalanx strategy utilise carbon resources early in the growing season and start storing carbon resources to old ramets thereafter, while plants with the Guerrilla strategy utilise carbon resources as long as possible throughout the growing season (Fig. 6). These resource allocation patterns seem to be adequate considering the functions of SRRs (consolidation) and LRRs (exploration).
To date, studies of foraging strategies have mainly focussed on architectural elements rather than the phenology of ramets or resource allocation in a growing season or over growing seasons. As we showed in this study, however, architectural elements can be strongly linked to phenology and resource allocation over time. To further confirm our explanation hypothesis, it is necessary to trace the fate of fixed carbon in plants under different environments with different clonal architectures. For this purpose, S. americanus could be suitable material.
References
Bell AD (1980) Adaptive architecture in rhizomatous plants. Bot J Linn Soc 80:125–160. doi:10.1111/j.1095-8339.1980.tb01662.x
Bell AD (1984) Dynamic morphology: a contribution to plant population ecology. In: Dirzo R, Sarukhan J (eds) Perspectives on plant population ecology. Sinauer, Sunderland, pp 48–65
de Kroon H, Hutchings MJ (1995) Morphological plasticity in clonal plants: the foraging concept reconsidered. J Ecol 83:143–152. doi:10.2307/2261158
de Kroon H, Knops J (1990) Habitat exploration through morphological plasticity in two chalk grassland perennials. Oikos 59:39–49. doi:10.2307/3545120
Dong M (1996) Clonal growth in plants in relation to resource heterogeneity: Foraging behavior. Acta Bot Sin 38:828–835
Dong M, de Kroon H (1994) Plasticity in morphology and biomass allocation in Cynodon dactylon, a grass species forming stolons and rhizomes. Oikos 70:99–106. doi:10.2307/3545704
Drake BG (1984) Light response characteristics of net carbon dioxide exchange in brackish wetland plant communities. Oecologia 63:264–270. doi:10.1007/BF00379887
Hutchings MJ, de Kroon H (1994) Foraging in plants: the role of morphological plasticity in resource acquisition. Adv Ecol Res 25:159–238. doi:10.1016/S0065-2504(08)60215-9
Ikegami M (2004) Functional specialization of Ramets in a clonal plant network. PhD thesis. Utrecht University, Utrecht
Ikegami M, Whigham DF, Werger MJA (2006) Scirpus olneyi A. Gray shows phenotypical differentiation in a salt marsh of the east coast of the U.S.A. Pol Bot Stud 22:241–249
Ikegami M, Whigham DF, Werger MJA (2007) Responses of rhizome length and ramet production to resource availability in the clonal sedge Scirpus olneyi A. Gray Plant Ecol 189:247–259. doi:10.1007/s11258-006-9181-9
Iwasa Y, Cohen D (1989) Optimal growth schedule of a perennial plant. Am Nat 133:480–505. doi:10.1086/284931
Kikuzawa K (1983) Leaf survival of woody plants in deciduous broad-leaved forests. I. Tall trees Japan. Can J Bot 61:2133–2139
Kikuzawa K (2003) Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Funct Ecol 17:29–38. doi:10.1046/j.1365-2435.2003.00707.x
Koike T (1988) Leaf structure and photosynthetic performance in deciduous broadleaved trees in relation to forest succession. Plant Species Biol 3:77–88. doi:10.1111/j.1442-1984.1988.tb00173.x
Lovett Doust L (1981) Population dynamics and local specialization in a clonal perennial (Ranunculus repens): 1. The dynamics of ramets in contrasting habitats. J Ecol 69:743–756. doi:10.2307/2259633
McCormick J, Somes HAJ (1982) The coastal wetlands of Maryland. Maryland Department of Natural Resources, Baltimore
Reich PB, Koike T, Gower ST, Schattle A (1995) Causes and consequences of variation in conifer leaf life-span. In: Smith WK, Hinkley TM (eds) Ecophysiology of coniferous forests. Academic, San Diego, pp 225–254
SAS Institute (1998) Statview reference. SAS Institute, Cary, NC
Slade AJ, Hutchings MJ (1987a) Clonal integration and plasticity in foraging behaviour in Glechoma hederacea. J Ecol 75:1023–1036. doi:10.2307/2260311
Slade AJ, Hutchings MJ (1987b) The effects of light intensity on foraging in the clonal herb Glechoma hederacea. J Ecol 75:639–650. doi:10.2307/2260196
Slade AJ, Hutchings MJ (1987c) The effects of nutrient availability on foraging in the clonal herb Glechoma hederacea. J Ecol 75:95–112. doi:10.2307/2260538
Acknowledgements
This study was financially supported by a grant to MJAW from the Andrew W. Mellon Foundation through the Smithsonian Institution and by the Faculty of Biology at Utrecht University. We would like to thank Jay O’Neil for his technical help in the fieldwork. We thank Editor in Chief Prof. Arnold Gerard van der Valk, and two anonymous reviewers for their help in improving the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ikegami, M., Whigham, D.F. & Werger, M.J.A. Ramet phenology and clonal architectures of the clonal sedge Schoenoplectus americanus (Pers.) Volk. ex Schinz & R. Keller. Plant Ecol 200, 287–301 (2009). https://doi.org/10.1007/s11258-008-9453-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-008-9453-7