Abstract
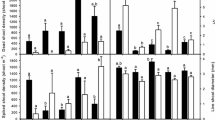
Morphological plasticity could facilitate invasions of wetland plants into areas that experience increased durations of flooding and eutrophication. We explored canopy plasticity of Phalaris arundinacea, an aggressive invader of wetlands, as it differentially invaded wet prairie mesocosms under 3 flooding durations and 3 levels of nutrient addition. Phalaris grew as a sward with intermittent and early-season flooding but shifted to tussocks under constant flooding. These two growth forms differed by >20% in several canopy ratios. Clones that formed tussocks produced 45% more shoots per unit biomass (P = 0.007) and a 25% higher ratio of total shoot length to biomass (P = 0.04). Lighter-weight shoots supported 33% fewer leaves and, consequently, had 35% less leaf area per shoot height (P < 0.002). Tussocks developed a continuous mat of adventitious roots, with root mats reaching 20.9 ± 0.6 cm in diameter and 4.7 ± 0.3 cm in height over two growing seasons. While forming tussocks, Phalaris tolerated longer durations of flooding and more than doubled its aboveground biomass. Invasions occurred rapidly, with Phalaris exceeding 75% canopy cover and accounting for 66% of the total aboveground biomass under constant flooding. Early-season flooding increased the lateral spread of individual shoots. High nutrient addition produced shoots that were 27% taller and 50% heavier (P < 0.02), with 81% more leaf area (P < 0.0003) than shoots that received no nutrients. Consequently, under early-season flooding with high nutrient additions, Phalaris was primed to invade, nearly doubling its proportion of the total aboveground biomass and exceeding 50% canopy cover during year two.





Similar content being viewed by others
References
Armstrong W, Brandle R, Jackson M (1994) Mechanisms of flood tolerance in plants. Acta Bot Neerl 43:307–358
Baker JM (1965) Characteristics and modes in origin of weeds. In: Baker JM, Stebbins GL (eds) The genetics of colonizing species. Academic Press Inc., New York, pp 147–168
Blom C (1999) Adaptations to flooding stress: from plant community to molecule. Plant Biol 1:261–273
Caldwell MM (1987) Plant architecture and resource competition. In: Schulze ED, Zwolfer H (eds) Potentials and limitations of ecosystem analysis. Springer-Verlag, Berlin Heidleberg, pp 164–177
Conchou O, Fustec E (1988) Influence of hydrological fluctuations on the growth and nutrient dynamics of Phalaris arundinacea L in a riparian environment. Plant Soil 112:53–60
Conchou O, Pautou G (1987) Modes of colonization of an heterogeneous alluvial area on the edge of the Garonne River by Phalaris arundinacea L. Regul Rivers 1:37–48
Coops H, van den Brink FWB, van der Velde G (1996) Growth and morphological responses of four helophyte species in an experimental water-depth gradient. Aquat Bot 54:11–24
Curtis JT (1959) Vegetation of Wisconsin. University of Wisconsin Press, Madison
De Kroon H, Hutchings MJ (1995) Morphological plasticity in clonal plants: the foraging concept reconsidered. J Ecol 83:143–152
Dekker J (2003) The foxtail (Setaria) species-group. Weed Sci 51:641–656
Dietz H, Kohler A, Ullmann I (2002) Regeneration growth of the invasive clonal forb Rorippa austriaca (Brassicaceae) in relation to fertilization and interspecific competition. Plant Ecol 158:171–182
Edwards A, Lee D, Richards J (2003) Responses to a fluctuating environment: effects of water depth on growth and biomass allocation in Eleocharis cellulosa Torr. (Cyperaceae). Can J Bot 81:964–975
Galatowitsch SM, van der Valk AG (1994) Restoring prairie wetlands: an ecological approach. Iowa State University Press, Ames
Galatowitsch SM, Anderson NO, Ascher PD (1999) Invasiveness in wetland plants in temperate North America. Wetlands 19:733–745
Garbey C, Thiebaut G, Muller S (2004) Morphological plasticity of a spreading aquatic macrophyte, Ranunculus peltatus, in response to environmental variables. Plant Ecol 173:125–137
Givnish TJ (2002) Ecological constraints on the evolution of plasticity in plants. Evol Ecol 16:213–242
Grace JB (1989) Effects of water depths on Typha latifolia and Typha domingensis. Am J Bot 76:762–768
Green EK, Galatowitsch SM (2001) Differences in wetland plant community establishment with additions of nitrate-N and invasive species (Phalaris arundinacea and Typha × glauca). Can J Bot 79:170–178
Hermanutz LA, Weaver SE (1996) Agroecotypes or phenotypic plasticity? Comparison of agrestal and ruderal populations of the weed Solanum ptycanthum. Oecologia 105:271–280
Huber-Sannwald E, Pyke DA, Caldwell MM, Durham S (1998) Effects of nutrient patches and root systems on the clonal plasticity of a rhizomatous grass. Ecology 79:2267–2280
Hutchings MJ, Wijesinghe DK (1997) Patchy habitats, division of labour and growth dividends in clonal plants. Trends Ecol Evol 12:390–394
Jackson MB, Drew MC (1984) Effects of flooding on growth and metabolism of herbaceous plants. In: Kozlowski TT (ed) Flooding and plant growth. Academic Press, Inc., Orlando, Florida, pp 47–128
Kercher SM, Zedler JB (2004a) Flood tolerance of wetland angiosperms: a comparison of invasive and noninvasive species. Aquat Bot 80:89–102
Kercher SM, Zedler JB (2004b) Multiple disturbances accelerate invasion of reed canary grass (Phalaris arundinacea L) in a mesocosm study. Oecologia 138:465–464
Kirkman LK, Sharitz RR (1993) Growth in controlled water regimes of three grasses common in freshwater wetlands of the southeastern USA. Aquat Bot 44:345–359
Knops J, Reinhart K (2000) Specific leaf area along a nitrogen fertilization gradient. Am Midland Nat 144:265–272
Lavergne S, Molofsky J (2004) Reed canary grass (Phalaris arundinacea) as a biological model in the study of plant invasions. Crit Rev Plant Sci 23:415–429
Lefor MW (1987) Phalaris arundinacea L (reed canary grass – gramineae) as a hydrophyte in Essex, Connecticut, USA. Environ Manage 11:771–773
Lepik M, Liira J, Zobel K (2005) High shoot plasticity favors plant coexistence in herbaceous vegetation. Oecologia 145:465–474
Liefers VJ, Shay JM (1981) The effects of water level on the growth and reproduction of Scirpus maritimus var. paludosus. Can J Bot 59:118–121
Lindig-Cisneros R, Zedler JB (2002) Phalaris arundinacea seedling establishment: effects of canopy complexity in fen, mesocosm, and restoration experiments. Can J Bot 80:617–624
Maurer DA, Zedler JB (2002) Differential invasion of a wetland grass explained by tests of nutrients and light availability on establishment and clonal growth. Oecologia 131:279–288
Miller R (2001) How stormwater runoff alters wetland hydrology, impacts native and invasive plants, and challenges transboundary planning and management. Master’s Thesis, University of Wisconsin, Madison, WI, USA
Miller RC, Zedler JB (2003) Responses of native and invasive wetland plants to hydroperiod and water depth. Plant Ecol 167:57–69
Parker I, Rodriguez J, Loik M (2003) An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conserv Biol 17:59–73
R Development Core Team (2003) R: a language and environment for statistical computing, Version 2.0.1. Vienna, Austria
Rogers W, Siemann E (2003) Effects of simulated herbivory and resources on Chinese tallow tree (Sapium sebiferum, Euphorbiaceae) invasion of native coastal prairie. Am J Bot 90:243–249
Schippers P, Snoeijing I, Kropff MJ (1999) Competition under high and low nutrient levels among three grassland species occupying different positions in a successional sequence. New Phytol 143:547–559
Schweitzer J, Larson K (1999) Greater morphological plasticity of exotic honeysuckle species may make them better invaders than native species. J Torrey Bot Soc 126:15–23
Sexton J, McKay J, Sala A (2002) Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol Appl 12:1656–1660
Sultan S (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5:537–542
Tilman D (1988) Plant strategies and the dynamics and structure of plant communities. Princeton University Press, Princeton
Valverde T, Pisanty I (1999) Growth and vegetative spread of Schizachyrium scoparium var. littoralis (Poaceae) in sand dune microhabitats along a successional gradient. Can J Bot 77:219–229
van de Vijver CADM, Boot RGA, Poorter H, Lambers H (1993) Phenotypic plasticity in response to nitrate supply of an inherently fast-growing species from a fertile habitat and an inherently slow-growing species from an infertile habitat. Oecologia 96:548–554
Vartapetian B, Jackson M (1997) Plant adaptations to anaerobic stress. Ann Bot 79:3–20
Veltman RLD (2002) Effect of stormwater flood pulsing on wetland vegetation. Master’s Thesis, University of Wisconsin, Madison, WI, USA
Vretare V, Weisner S, Strand J, Graneli W (2001) Phenotypic plasticity in Phragmites australis as a functional response to water depth. Aquat Bot 69:127–145
Weisner S, Miao S (2004) Use of morphological variability in Cladium jamaicense and Typha domingensis to understand vegetation changes in an Everglades marsh. Aquat Bot 78:319–335
Werner KJ, Zedler JB (2002) How sedge meadow soils, microtopography, and vegetation respond to sedimentation. Wetlands 22:451–466
Wetzel PR, van der Valk AG (1998) Effects of nutrients and soil moisture on competition between Carex stricta, Phalaris arundinacea and Typha latifolia. Plant Ecol 138:179–190
Williams DG, Mack RN, Black RA (1995) Ecophysiology of introduced Pennisetum setaceum on Hawaii: the role of phenotypic plasticity. Ecology 75:1569–1580
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23:431–452
Acknowledgements
This research was supported by EPA-STAR (Science to Achieve Results) Grant number R-82801001-0 (Drs. Richard Lathrop and Ken Potter, principal investigators). We thank the UW Arboretum Leopold Endowment for providing the mesocosm facility and funding for Herr-Turoff, and the UW Arboretum, Biotron, and Walnut Street Greenhouse staff for logistical support. We also thank Suzanne Kercher, Aaron Boers, Charles Campbell, David and Lester Edinger, Kara Jorstad, Laura Ladwig, Roberto Lindig-Cisneros, Debbie Maurer, Kevin McCartney, Rebecca Miller, Hem Morzaria-Luna, and Katy Wallace for assistance in setting up and maintaining the mesocosms and collecting data, Dr. Bret Larget for statistical advice, and Dr. Thomas Givnish for lending the ceptometer. Our manuscript was improved from the comments of two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herr-Turoff, A., Zedler, J.B. Does morphological plasticity of the Phalaris arundinacea canopy increase invasiveness?. Plant Ecol 193, 265–277 (2007). https://doi.org/10.1007/s11258-007-9264-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9264-2