Abstract
Chronic kidney disease (CKD) represents a significant global health challenge, characterized by kidney damage and decreased function. Its prevalence has steadily increased, necessitating a comprehensive understanding of its epidemiology, risk factors, and management strategies. While traditional prognostic markers such as estimated glomerular filtration rate (eGFR) and albuminuria provide valuable insights, they may not fully capture the complexity of CKD progression and associated cardiovascular (CV) risks.
This paper reviews the current state of renal and CV risk prediction in CKD, highlighting the limitations of traditional models and the potential for integrating artificial intelligence (AI) techniques. AI, particularly machine learning (ML) and deep learning (DL), offers a promising avenue for enhancing risk prediction by analyzing vast and diverse patient data, including genetic markers, biomarkers, and imaging. By identifying intricate patterns and relationships within datasets, AI algorithms can generate more comprehensive risk profiles, enabling personalized and nuanced risk assessments.
Despite its potential, the integration of AI into clinical practice faces challenges such as the opacity of some algorithms and concerns regarding data quality, privacy, and bias. Efforts towards explainable AI (XAI) and rigorous data governance are essential to ensure transparency, interpretability, and trustworthiness in AI-driven predictions.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is defined by the presence of kidney damage, primarily marked by albuminuria, and/or decreased kidney function (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2), persisting for at least 3 months [1]. It represents a prevalent non-communicable health condition characterized by the gradual loss (or stability in the most favorable situations) of renal function over time. Epidemiological studies have consistently demonstrated a striking prevalence of CKD, with estimates ranging from 9 to 10% within the general population, worldwide. Prevalence and incidence are progressively growing, being almost duplicated over the past decades [2].
The presence of CKD, irrespective of other individual demographic and clinical characteristics such as age, sex, presence of diabetes, hypertension and cardiovascular (CV) disease, exposes patients to an increased risk for future negative health events [3,4,5]. Considered together, these data revealed that CKD represents an urgent ‘public health’ problem and prompts the improvement investigation of all aspects of disease, namely etiology, risk factors, and optimal management strategies. Understanding the epidemiological landscape of CKD, indeed, is pivotal for informing healthcare policies, allocating resources, and initiating interventions aimed at reducing its burden and improving patient outcomes on a global scale.
Chronic kidney disease is characterized by a variety of complications (i.e. hypertension, mineral bone disorders, hyperkaliemia, albuminuria, anemia) that, when combined, have the potential to significantly alter the individual patients’ prognosis. All these variables can present in CKD patients especially when levels of kidney function (eGFR) significantly decrease but their prognostic weight is not the same in all affected patients. The most important variables investigated in terms of prognosis of CKD are eGFR and albuminuria (the abnormal presence of albumin in urine), the so-called ‘kidney measures’ that account for most of the prediction power in the currently available models [6]. However, prediction models with acceptable performance, kidney measures only do not fully predict the future risk of CKD progressing to kidney failure (KF), fatal and non-fatal cardiovascular (CV) events and mortality. This is due to the fact that kidney function related risk factors in CKD patients do not uniformly contribute to the abovementioned future events. Moreover, not all CKD patients respond with the same magnitude to the nephroprotective treatment and thus a significant portion of patients remain at high CV and renal risk under even the best possible care [8,9,10,10]. One reason for this low reliability in prediction is the complexity and heterogenicity of mechanisms underlying kidney damage with several factors playing variable roles. Strategies to face such complexity are represented by the construction of prediction models that allow to “adjust” for a multitude of variables, also called “confounders”. Another strategy is to furtherly simplify the information by creating risk scores in which point of risks can be assigned to each variable (e.g. 1, 2, or 3 points to increasing categories of albuminuria < 30, 30–300, > 300 mg/g depending on the predicted risk). The emergence of artificial intelligence (AI) in the past few years may represent an opportunity to improve risk prediction in CKD. Artificial intelligence already showed great potential in several fields of medicine [11, 12].
The aim of the present article is to review the state of art of renal and CV risk prediction in CKD and to highlight how AI can provide simple and complex answers to clinical and research questions.
Prognosis in chronic kidney disease patients
The association between CKD and a future worse prognosis is an interesting matter of discussion [13]. In fact, the mechanisms underlying the abovementioned associations involve a series of pathophysiological processes. Proteinuria often signifies renal damage and inflammation, while a low eGFR reflects reduced renal capacity to filter and eliminate metabolic waste products. It was also demonstrated that proteinuria is a systemic marker of CV and endothelial damage, according to the ‘Steno hypothesis’, other than a marker of kidney disease itself. Both these factors, proteinuria and eGFR, can contribute to the progression of kidney disease and increased cardiovascular risk through shared processes such as endothelial dysfunction, activation of the renin–angiotensin–aldosterone system, and systemic inflammation. Understanding and managing these pathological mechanisms are crucial for the prevention and optimal treatment of CKD and its cardiovascular and renal complications.
Including the kidney measures, namely eGFR and proteinuria confers several advantages in predicting mortality risk, ESKD, and CV events compared to traditional risk factors and monitoring these factors over time may help to relent CKD progression and to reduce CV risk. This approach enables more personalized risk management [15,16,16].
The association between proteinuria, eGFR, and the risk of mortality, ESKD, and cardiovascular events has been proved in different population settings. Several studies have highlighted that both a reduction in eGFR and an increase in proteinuria are strongly correlated with an elevated risk of mortality and the development of ESKD. Specifically, an eGFR below 15 ml/min per 1.73 m2, below the threshold of 45 ml/min per 1.73 m2, has been associated with a significantly increased risk of mortality (hazard ratio (HR): 1.47, p = 0.000) and ESKD (HR: 6.24, p < 0.001), while an eightfold higher albumin or protein–creatinine ratio has been correlated with a greater risk of mortality (HR: 1.40, p = 0.101) and ESKD (HR: 3.04, p < 0.001). These associations have been found both in the general population and in high-risk populations, such as diabetic patients or those with pre-existing cardiovascular diseases [17].
Furthermore, it has been demonstrated that both reduced eGFR and the presence of proteinuria are independent (non-traditional) CV risk factors. They have about a similar prognostic weight of that provided by the sum of ‘traditional’ risk factors, such as hypertension, sex, smoking habit, serum cholesterol levels.
Other important risk factors influence prognosis in CKD patients. Among these, as complications of CKD itself, we remember: anemia, hyperkaliemia, metabolic acidosis, hypertension and hyperphosphatemia.
Anemia, defined as hemoglobin (Hb) < 13.0 g/dl for men and < 12.0 g/dl for women, is a common complication of CKD. It has multifactorial causes like EPO deficiency, uremic-induced inhibitors of erythropoiesis, reduced erythrocyte survival, disordered iron homeostasis and chronic inflammation [18]. The negative effects on health and development outcomes result from the impacts of decreased oxygen delivery to tissues, so multiple organ systems may be affected [19]. The prevalence increases with CKD stage. In the NHANES analysis, it was 17.4%, 50.3%, and 53.4% in stages 3, 4, and 5 CKD, respectively. Based on observational data, anemia is associated with an increased risk of cardiovascular events, all-cause mortality and CKD progression [20]. In fact, it was shown, for every 1-g/dL decrease Hb concentration, there is 11% greater chance of time to dialysis therapy and doubling of serum creatinine concentration. The most emblematic relationship between anemia and heart disease is the development of left ventricular hypertrophy (LVH). LVH is strongly and independently associated with increased mortality risk [21].
Hyperkalemia, defined as a serum potassium > 5.0 mmol/L, is one of the most frequent electrolyte abnormalities in CKD as result of reduced renal potassium excretion, renin–angiotensin–aldosterone system (RAAS) inhibitors and mineralocorticoid receptor antagonists (MRA). When the eGFR decreases from 90 to < 20 mL/min/1.73 m2 or stage 5, the prevalence of hyperkalemia increases from 2 to 42% and to 56.7%, respectively [22]. This can lead to changes in muscle function including the heart muscle, and cause arrhythmia. Persistent HK results in poorer clinical outcomes, which are evident from the early stages 1-3a of CKD. Hyperkalemia has consistently been associated with an increased risk of adverse events (mainly all-cause and cardiovascular mortality) in patients with CKD [23].
Metabolic acidosis in CKD is diagnosed in patients with plasma or venous blood bicarbonate concentration < 22 mmol/L. It occurs in about 20% of patients with CKD and increases with the stage of CKD. The causes of metabolic acidosis in these patients are insufficient production of bicarbonate in distal tubule cells in the process of ammonia genesis, disturbed secretion of protons (as titratable acidity) in the proximal and distal tubules, and impaired reabsorption of bicarbonate in the kidney tubule. With serum bicarbonate concentration < 22 mmol/L, the progression of CKD is about 3 times more pronounced than in patients with serum bicarbonate concentration > 26 mmol/L. Furthermore, even if less studied, metabolic acidosis shows to be linked to cardiovascular disease [24].
Hypertension, defined as a systolic BP > 140 mm Hg or a diastolic BP > 90 mm Hg, is a common comorbidity in patients with chronic kidney disease (CKD). The prevalence ranges from 60 to 90% depending on the stage of CKD and its cause. The mechanisms of hypertension in CKD include volume overload, sympathetic overactivity, salt retention, endothelial dysfunction, and alterations in hormonal systems that regulate blood pressure (BP). Chronic kidney disease (CKD) is both a common cause of hypertension and CKD is also a complication of uncontrolled hypertension. It is associated with an increased risk of cardiovascular morbidity and mortality [25, 26].
Worsening of kidney function as a consequence of an elevated BP is evident by a direct relationship between relative risk of developing end-stage kidney disease (ESKD) and BP severity [27].
Hyperphosphatemia occurs rather late in the process of CKD progression, usually from stage 4. Excessive retention of phosphate can cause a wide range of conditions, such as vascular calcifications, impaired bone mineralization, and dysregulated cell signaling and cell death [28]. Several studies found an association between phosphate, even in the normal range, and all-cause mortality, cardiovascular events, or cardiovascular mortality, which showed a 35 percent increase in mortality per mg increase in phosphorus above normal values (95% CI 1.16–1.57). It affect, also, CKD progression and time to ESKD [29]. All these risk factors impose tailored approaches to prevent and treat all CKD complications [30].
To date, prognostic models on kidney disease were built using the risk of ESKD over time. The principal applied score of 2 and 5 year risk of ESKD is the Kidney Failure Risk Equation (KFRE) [6]. The KFRE included age, gender, race, albuminuria and eGFR and demonstrated a good statistical power (c-statistic of about 0.9). Other risk factors of future events such as arterial hypertension, anemia, serum phosphate, calcium and potassium, smoking habit, body mass index and the presence of diabetes have been included in model prediction, but they did not provide sufficient evidence to be considered for large population screening and assessment [31, 32]. The topic is even more intriguing if we consider that a striking number of CKD patients remain, despite the optimal treatment, having high proteinuria and low eGFR and thus at high CV an ESKD risk. Hence, optimizing risk stratification with modern tools can definitely help to make progress in the future management of CKD patients.
Prognostic models in CKD
The development of prognostic models for CKD patients has seen a good implementation in the past decade, with the development of increasingly sophisticated and accurate tools to predict the course of the disease and associated clinical outcomes. In particular, the main prognostic models in CKD are oriented toward the identification of patients with high-risk for progression to ESKD, CV fatal and non-fatal events and mortality. Usually, the available models integrate a series of different variables that have a plausible biological association with the aforementioned outcomes and that can play a prognostic effect even after “adjusting” for each other in the model. Variables included in the models, that are so called multivariable models, can be clinical, laboratory and demographic. These may include age, sex, race, blood pressure, eGFR levels, proteinuria, presence of diabetes mellitus, hemoglobin, serum phosphorus levels and other risk factors [15, 33, 34].
To date, prognostic models for CKD are mainly based on the so-called “frequentist approach”. Such an approach is based on the concept that when studying the population, sampling from the population introduces uncertainty. Statistical inference provides us with tools to quantity this uncertainty by answering the question “what would happen if I repeated the study many times?”. In fact, for each estimator of frequentist approach we can compute a confidence interval (usually at 95%) that informs us about the variability of our average value if we repeat the test. And this is applicable to the mean, the odds ratio, the hazard ratio and any other measure derived from the frequentist approach [35].
The study design which is more adaptive to evaluate prognosis in epidemiologic application to CKD is the cohort study, designed to evaluate the role of a certain factor on future events (in terms of temporal sequence) not present at the first evaluation of patients.
Cohort studies are fundamental study designs that follow a group of individuals over time to assess the association between risk factors and the development of diseases or events. They involve the prospective monitoring of groups exposed and not exposed to risk factors, allowing for comparison of the risk of developing the event of interest and identifying causal associations. These studies lead to the identification of risk factors associated with CKD progression, such as hypertension, diabetes, albuminuria, and are also used to assess the effectiveness of a certain treatment in slowing down the progression of the disease.
Kaplan––Meier estimator and Cox proportional hazard models are the principal tools implemented in the context of cohort studies to assess prognosis [23, 36]. The Kaplan–Meier method can be used to estimate the cumulative survival of patients with CKD over time, considering events such as disease progression, dialysis, or kidney transplantation. This method allows for the assessment of the survival probability of CKD patients and for comparing survival curves among subgroups of patients with different risk levels. One significant limitation of the Kaplan–Meier analysis is its allowance for only “crude” risk, namely univariate analysis, which does not account for variables (also known as “confounders”) that may influence the association of exposure with the event. Overcoming this limitation is made possible by the Cox proportional hazards regression. Cox regression is a semi-parametric statistical method used to analyze survival data and assess the effect of independent variables on the risk of an event over time. One of the main measures resulting from the Cox model is the hazard ratio (HR), which represents the instantaneous ratio between the risks of two groups of individuals with different characteristics regarding a variable of interest [37]. The Hazard Ratio provides information on the magnitude and direction of the effect of a variable on the probability of an event occurring over time. Essentially, the Hazard Ratio calculated from the Cox model allows for evaluating the association between a predictive variable and the outcome of interest, providing an estimate of the relative risk (RR) of developing the event in the exposed group compared to the reference group. In the context of CKD, the Cox model enables the identification of the most significant risk factors influencing the progression of kidney disease, assessing the effectiveness of management strategies, and developing personalized interventions to improve patients' clinical outcomes [38]. One variant of the Cox model is the Fine and Gray model [38, 39]. The Fine and Gray model shares some features with the Cox model but is able to compute a relative risk of an event during time, accounting for the competing risk of a second event (e.g. mortality and ESKD in CKD patients) that can occur in a competitive fashion. This type of risk is provided as sub-hazard ration and is gaining momentum in assessing prognosis of CKD patients, especially those with high CV risk at baseline. The respective of Kaplan–Meier curves in the competitive risk scenario is represented by the cumulative incidence, also known as Aalen-Johansen estimator [40, 41]. The cumulative incidence, unlike Kaplan–Meier estimator that ignores the competing event, adjusts the slope of the curve and thus the probability of events after calculating the risk (for each category of patients) of the competing event of interest. One example of risk prediction, in patients with CKD, using the competing risk approach is represented by the novel KD predict [42]. This score computes the 1 (up to 5)-year risks of death and kidney failure by accounting that both are competing events. For example, according to KFRE a 50 year old female with CKD, eGFR of 32 mL/min/1.73m2 and urine albumin-to-creatinine ratio (ACR) of 150 mg/g had a 5-year ESKD risk probability of 12.48% whereas the same probability for the same patient was 11% only with ‘KD predict’ tool. To date, cohort studies and the classical inference estimators are the principal tools used to study the principal outcomes of CKD patients. The main limitations of classical tools are that they provide the risk with a limited number of variables (the investigator should know the variable of interest) and that risk is manifested at a certain moment in time. Moreover, these models provide an average increased risk for each variable, namely a 30% increased risk to CV events in presence of severe proteinuria as compared with moderate or normal proteinuria. But, computing the true risk considering multiple parameters, and possibly the most significant, is often complex with traditional methods. Novel tools, including AI, may intervene in discovering unknown risk factors and in refining the combination of old and novel risk factors to improve with greater accuracy the event prediction.
Artificial intelligence in chronic kidney diseases prediction and management
It is nontrivial to furnish a definition of Artificial Intelligence (AI) due to the inherent complexity in defining the concept of intelligence itself. Alan Turing's landmark paper in 1950 established a test, known as imitation game, for assessing machine intelligence, proposing that for a machine to be considered intelligent, it must respond in ways indistinguishable from human behavior. Consequently, Artificial Intelligence is acknowledged as the capacity of a machine or a software to perform tasks necessitating human intelligence. Despite the large number of techniques included, one approach known as Machine Learning (ML) has gained undisputed fame. Machine learning can be seen as a method to enable a machine or a program to acquire that ability through a training process. According to Tom M. Mitchell [43], a computer program is said to learn from experience E with respect to some class of tasks T and performance measure P, if its performance at tasks in T, as measured by P, improves with experience E.
Machine learning techniques can be broadly categorized into supervised learning and unsupervised learning.
The former, involves training a model on labeled data, where the outcome (target variable) is known. This category includes both classification and regression techniques, based on the need to predict categorical outcome (e.g., yes/no, disease/no disease) or continuous outcome, respectively.
The second one involves training a model on data without labeled outcomes. The goal is to uncover hidden patterns or structures in the data (e.g., clustering or dimensional reduction techniques).
Examples of Machine Learning techniques are linear regression, logistic regression, Lasso, Ridge, support vector machines (SVM), k-nearest neighbors, decision trees, ensembles (e.g., random forest, XGBoost), k-means clustering, principal component analysis (PCA) and others.
Lasso (Least Absolute Shrinkage and Selection Operator) regression is a type of linear regression that uses L1 regularization. This technique not only minimizes the residual sum of squares between the observed and predicted values but also adds a penalty proportional to the absolute value of the coefficients. This penalty can shrink some coefficients to zero, effectively performing variable selection.
Ridge regression is a type of linear regression that uses L2 regularization. This technique adds a penalty proportional to the square of the magnitude of the coefficients to the residual sum of squares. The penalty term discourages large coefficients, thereby addressing issues of multicollinearity and improving the model’s generalization.
Decision trees are versatile supervised learning techniques used for both classification and regression tasks. They model decisions and their possible consequences as a tree-like structure. A decision tree consists of nodes (i.e., decision points) and branches (i.e., outcomes). The root node represents the entire dataset, which is recursively split based on feature values. At each node, the data is split based on a feature that results in the best separation according to a certain criterion (e.g., Gini impurity, information gain for classification; mean squared error for regression).The terminal nodes (leaves) represent the final decision or prediction [44].
XGBoost is an ensemble of weak models (e.g., decision trees) based on the gradient boosting framework. It constructs additive models by sequentially fitting a weak learner (usually a decision tree) to the residuals of the model from the previous iteration. The boosting process starts with an initial prediction, then at each iteration fit a new tree to the residual errors (i.e., the difference between the actual and predicted values). The new tree is added to the ensemble to reduce the residuals. The final model is the combination of all the individual trees. XGBoost includes regularization terms to control the complexity of the model, preventing overfitting like L1 (Lasso) and L2 (Ridge) regularization [45].
The aforementioned machine learning techniques offer significant advantages over traditional statistical models like the Cox model by not requiring strong assumptions such as proportional hazards or linearity of covariate effects. Their flexibility in handling high-dimensional data and complex relationships allows them to outperform classic models, particularly in scenarios where the data structure and relationships are intricate and do not conform to the assumptions of traditional statistical methods.
Deep Learning (DL) is a subset of machine learning that employs artificial neural networks with multiple layers (hence the term "deep") of interconnected nodes called neurons, to learn intricate patterns and representations from vast amounts of data. It is inspired by the structure and function of the human brain's biological neural networks. The latter, composed of billions of interconnected neurons, exhibit complex information processing, learning, and adaptation abilities. Deep learning attempts to mimic this behavior using artificial neural networks where each neuron receives inputs, applies a transformation using weights and biases, and produces an output that serves as input to the neurons in the next layer. In fact, neural networks are generally organized into layers. The input layer receives the input data, then there is a stack of multiple hidden layers that perform transformations on the data, finally an output layer which produce the final output. During the training process, the “Backpropagation” is performed for each training pace. It calculates the gradients of the loss function with respect to each weight and bias, propagating errors backward through the network and updating the weights and biases to reduce the loss. The loss function measures the difference between the network’s predictions and the actual values. Common loss functions include Mean Squared Error (MSE) for regression and Cross-Entropy for classification.
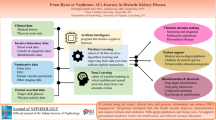
The integration of artificial intelligence into medical research and practice is showing a rapidly growing trend powered by the increasing availability of healthcare digital data. Common applications include performing early diagnosis, improving risk prediction and management, improving communication between patient and specialist, drug discovering, mental health issues identification [46] and others (Fig. 1).
Furthermore, in the last decade, several AI-based devices have been approved by the Food and Drug Administration (FDA)Footnote 1 and a lot of them have been successfully implemented.
Due to the lack of education in digital medicine, several countries, including Italy, have introduced a pioneering university degree program. One notable institution implementing this initiative is UniCal with its MEDTDFootnote 2 (Medicine and Digital Technologies) course. This program integrates medical and computer engineering curricula, aiming to equip doctors with the skills to understand and apply the latest techniques to traditional medicine.
In the context of CKD, where an accurate and an early prediction of renal and cardiovascular (CV) risk is paramount for effective management, AI-based approaches hold significant potential.
One of the primary advantages of employing AI in CKD research lies in its ability to analyze vast amounts of patient data and identify complex patterns that may not be readily apparent to human observers. Traditional prediction models for CKD prognosis rely heavily on kidney measures such as estimated glomerular filtration rate (eGFR) and proteinuria, which provide valuable insights but may not capture the full spectrum of risk factors contributing to disease progression and adverse outcomes. AI algorithms can complement these measures by integrating additional information, such as genetic markers, biomarkers, comorbidities, socio-economic factors, as well as bioimages (e.g., pictures, CT and ultrasound) to generate more comprehensive risk profiles for individual patients. For instance, according to [47] deep-learning models can be used to identify chronic kidney disease and type 2 diabetes solely from retinal fundus photographs in combination with clinical metadata (age, sex, height, weight, body-mass index and blood pressure), while in [48] deep-learning models trained instead on external photographs of the eyes can be used to detect diabetic retinopathy (DR), diabetic macular oedema and poor blood glucose control.
Furthermore, AI-based approaches offer the flexibility to adapt and refine predictive models over time as new data becomes available, enabling continuous improvement in accuracy and performance. Deep learning algorithms, in particular, excel at extracting hierarchical representations from complex datasets, allowing for more nuanced and personalized risk assessments in CKD patients [49]. By leveraging techniques such as convolutional neural networks and recurrent neural networks, deep learning models can discern subtle relationships between disparate variables and uncover novel predictive insights. A summary of the main paper concepts is reported in Table 1.
Artificial intelligence: challenges to address and pitfalls
However, despite the potential advantages of AI-based approaches, several pitfalls and challenges must be addressed to ensure their effective integration into clinical practice. One notable concern is the "black box" nature of some AI algorithms which may obscure the rationale behind their predictions and limit interpretability for clinicians. In particular, due the complexity of the structure of deep learning models, it is usually impossible to know why they return an output given a specific input because it is not possible to follow the full path from the input to the output. Nonetheless, in recent years, researchers are focusing on the Explainable AI (XAI) approach to mitigate this issue. Explainable AI aims to enhance the transparency of AI models by providing interpretable explanations for their decisions, thus enabling clinicians to understand and trust the outputs generated by these algorithms. Through techniques such as feature importance analysis, attention mechanism, and model-agnostic approaches, Explainable AI seeks to uncover the underlying factors driving AI predictions [50]. Feature importance analysis calculates a score for all the features included in a model, so that the larger is the score the larger is the predictive effect of a certain feature. The Attention mechanism is a technique used to improve the model performance by assigning to features a weight to each feature. These weights determine the level of importance of the feature in the model. Interestingly, model agnostic approach is a methods that aims at interpreting the predictive response of a black box model rather than the response of the original data [51]. By gaining insights into the decision-making process of AI systems, clinicians can better assess the reliability of AI-generated recommendations and incorporate them into their clinical decision-making process more confidently. Additionally, addressing the black box problem is crucial for ensuring accountability and compliance with regulatory standards in healthcare, as opaque algorithms may raise concerns regarding patient safety, privacy, and ethical implications. Therefore, while the inherent opacity of some AI algorithms poses a significant challenge, the emergence of Explainable AI methodologies offers promising solutions to promote the responsible and effective integration of AI in clinical settings.
Ensuring transparency and explainability in AI models is crucial for building trust and facilitating their adoption in real-world healthcare settings. Artificial intelligence may be involved in several steps of clinical decision making in nephrology, from diagnosis to treatment decision (based on the early prediction of response to treatment) and to an improvement of routine clinical procedures (e.g. outpatients evaluation and kidney transplant list assessment).
Conclusions
In summary, the application of AI in CKD research holds promise for enhancing risk prediction and personalized management strategies. By harnessing the power of machine learning and deep learning techniques, researchers and clinicians can unlock new insights into the complex dynamics of CKD progression and improve patient outcomes. However, it is essential to address the challenges posed by AI adoption and ensure responsible and ethical use of these technologies in clinical decision-making.
Data availability
No datasets were generated or analysed during the current study.
References
Levey AS, Coresh J (2012) Chronic kidney disease. The lancet 379(9811):165–180
Ong KLi, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, Dalton BE et al (2023) Global, regional, and national burden of diabetes from 1990 to 2021 with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet (London, England) 402(10397):203–234. https://doi.org/10.1016/S0140-6736(23)01301-6
Hu L, Napoletano A, Provenzano M, Garofalo C, Bini C, Comai G, La Manna G (2022) Mineral bone disorders in kidney disease patients: the ever-current topic. Int J Mol Sci 23(20):12223. https://doi.org/10.3390/ijms232012223
Andreucci M, Provenzano M, Faga T, Michael A, Patella G, Mastroroberto P, Serraino GF, Bracale UM, Ielapi N, Serra R (2021) Aortic aneurysms, chronic kidney disease and metalloproteinases. Biomolecules 11(2):194. https://doi.org/10.3390/biom11020194
Simeoni M, Borrelli S, Garofalo C, Fuiano G, Esposito C, Comi A, Provenzano M (2021) Atherosclerotic-nephropathy: an updated narrative review. J Nephrol 34(1):125–136. https://doi.org/10.1007/s40620-020-00733-0
Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS (2011) A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305(15):1553–1559. https://doi.org/10.1001/jama.2011.451
Provenzano M, De Nicola L, Pena MJ, Capitoli G, Garofalo C, Borrelli S, Andreucci M (2020) Precision nephrology is a non-negligible state of mind in clinical research: remember the past to face the future. Nephron 144(10):463–478
Provenzano M, Coppolino G, De Nicola L, Serra R, Garofalo C, Andreucci M, Bolignano D (2019) Unraveling cardiovascular risk in renal patients: a new take on old tale. Front Cell Developmental Biol 7:314
Minutolo R, Andreucci M, Balletta MM, Russo D (2000) Effect of posture on sodium excretion and diuretic efficacy in nephrotic patients. Am J Kidney Dis 36(4):719–727
Petrykiv SI, de Zeeuw D, Persson F, Rossing P, Gansevoort RT, Laverman GD, Heerspink HJ (2017) Variability in response to albuminuria-lowering drugs: true or random? Br J Clin Pharmacol 83(6):1197–1204
Sawhney R, Malik A, Sharma S, Narayan V (2023) A comparative assessment of artificial intelligence models used for early prediction and evaluation of chronic kidney disease. Decision Anal J 6:100169
Niel O, Bastard P (2019) Artificial intelligence in nephrology: core concepts, clinical applications, and perspectives. Am J Kidney Dis 74(6):803–810
Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Ärnlöv J (2015) Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. The Lancet Diabet Endocrinol 3(7):514–525
Gansevoort RT, Matsushita K, van der Velde M et al (2011) Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80(1):93–104. https://doi.org/10.1038/ki.2010.531
Matsushita K, van der Velde M, Astor BC et al (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375(9731):2073–2081. https://doi.org/10.1016/S0140-6736(10)60674-5
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, Eknoyan G (2005) Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 67(6):2089–2100
Astor BC, Matsushita K, Gansevoort RT, Van Der Velde M, Woodward M, Levey AS (2011) Chronic kidney disease prognosis consortium. lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79(12):1331–1340
Chaparro CM, Suchdev PS (2019) Anemia epidemiology, pathophysiology and etiology in low- and middle-income countries. Ann N Y Acad Sci 1450(1):15–31. https://doi.org/10.1111/nyas.14092
Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23(10):1631–1634. https://doi.org/10.1681/ASN.2011111078
Hanna RM, Streja E, Kalantar-Zadeh K (2021) Burden of anemia in chronic kidney disease: beyond erythropoietin. Adv Ther 38(1):52–75. https://doi.org/10.1007/s12325-020-01524-6
Pereira AA, Sarnak MJ (2003) Anemia as a risk factor for cardiovascular disease. Kidney Int Suppl 87:S32–S39. https://doi.org/10.1046/j.1523-1755.64.s87.6.x
Valdivielso J, Balafa O, Ekart R, Ferro C, Mallamaci F et al (2021) Hyperkalemia in chronic kidney disease in the new era of kidney protection therapies. Drugs 81(13):1467–1489
Provenzano M, De Francesco M, Iannazzo S, Garofalo C, Andreucci M, Genualdo R, Borrelli S, Minutolo R, Conte G, De Nicola L (2020) Cost-analysis of persistent hyperkalaemia in non-dialysis chronic kidney disease patients under nephrology care in Italy. Int J Clin Pract 74(5):e13475
Căpuşă C, Ştefan G, Stancu S, Lipan M, Tsur LD, Mircescu G (2017) Metabolic acidosis of chronic kidney disease and subclinical cardiovascular disease markers: friend or foe? Medicine (Baltimore) 96(47):e8802. https://doi.org/10.1097/MD.0000000000008802
Ku E, Lee BJ, Wei J, Weir MR (2019) Hypertension in CKD: Core Curriculum 2019. Am J Kidney Dis 74(1):120–131. https://doi.org/10.1053/j.ajkd.2018.12.044
Hamrahian SM, Falkner B (2017) Hypertension in chronic kidney disease. Adv Exp Med Biol 956:307–325. https://doi.org/10.1007/5584_2016_84
Judd E, Calhoun DA (2015) Management of hypertension in CKD: beyond the guidelines. Adv Chronic Kidney Dis 22(2):116–122. https://doi.org/10.1053/j.ackd.2014.12.001
Vervloet MG, van Ballegooijen AJ (2018) Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int 93(5):1060–1072. https://doi.org/10.1016/j.kint.2017.11.036
Rastogi A, Bhatt N, Rossetti S, Beto J (2021) Management of hyperphosphatemia in end-stage renal disease: a new paradigm. J Ren Nutr 31(1):21–34. https://doi.org/10.1053/j.jrn.2020.02.003
Lerario S, Monti L, Ambrosetti I, Luglio A, Pietra A, Aiello V, Montanari F, Bellasi A, Zaza G, Galante A, Salera D, Capelli I, La Manna G, Provenzano M (2024) Fabry disease: a rare disorder calling for personalized medicine. Int Urol Nephrol. https://doi.org/10.1007/s11255-024-04042-4
De Nicola L, Provenzano M, Chiodini P, D’Arrigo G, Tripepi G, Del Vecchio L, Conte G, Locatelli F, Zoccali C, Minutolo R (2015) Prognostic role of LDL cholesterol in non-dialysis chronic kidney disease: multicenter prospective study in Italy. Nutr Metab Cardiovasc Dis 25(8):756–762. https://doi.org/10.1016/j.numecd.2015.04.001
Provenzano M, Rivoli L, Garofalo C, Faga T, Pelagi E, Perticone M, Serra R, Michael A, Comi N, Andreucci M (2020) Renal resistive index in chronic kidney disease patients: possible determinants and risk profile. PLoS ONE 15(4):e0230020. https://doi.org/10.1371/journal.pone.0230020
De Nicola L, Serra R, Provenzano M, Minutolo R, Michael A, Ielapi N, Federico S, Carrano R, Bellizzi V, Garofalo C, Iodice C, Borrelli S, Grandaliano G, Stallone G, Gesualdo L, Chiodini P, Andreucci M (2023) Risk of end-stage kidney disease in kidney transplant recipients versus patients with native chronic kidney disease: multicentre unmatched and propensity-score matched analyses. Nephrol Dial Transplant 38(2):507–516. https://doi.org/10.1093/ndt/gfac131
Provenzano M, Chiodini P, Minutolo R, Zoccali C, Bellizzi V, Conte G, Locatelli F, Tripepi G, Del Vecchio L, Mallamaci F, Di Micco L, Russo D, Heerspink HJL, De Nicola L (2020) Reclassification of chronic kidney disease patients for end-stage renal disease risk by proteinuria indexed to estimated glomerular filtration rate: multicentre prospective study in nephrology clinics. Nephrol Dial Transplant 35(1):138–147. https://doi.org/10.1093/ndt/gfy217
Crugliano G, Provenzano M, Torino C, Garofalo C, Zicarelli M, Coppolino G, Bolignano D, Serra R, Andreucci M (2022) Study designs adopted in epidemiology of chronic diseases. G Ital Cardiol 23(2):100–112. https://doi.org/10.1714/3735.37212
Abd ElHafeez S, D’Arrigo G, Leonardis D, Fusaro M, Tripepi G, Roumeliotis S (2021) Methods to analyze time-to-event data: the cox regression analysis. Oxid Med Cell Longev 30(2021):1302811. https://doi.org/10.1155/2021/1302811
Crugliano G, Provenzano M, Torino C, Garofalo C, Zicarelli M, Coppolino G, Bolignano D, Serra R, Andreucci M (2022) Study designs adopted in epidemiology of chronic diseases. G Ital Cardiol 23(2):100–112. https://doi.org/10.1714/3735.3721
Austin PC, Fine JP (2017) Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 36(27):4391–4400
Provenzano M, Minutolo R, Chiodini P, Bellizzi V, Nappi F, Russo D, Borrelli S, Garofalo C, Iodice C, De Stefano T, Conte G, Heerspink HJL, De Nicola L (2018) Competing-risk analysis of death and end stage kidney disease by hyperkalaemia status in non-dialysis chronic kidney disease patients receiving stable nephrology care. J Clin Med 7(12):499. https://doi.org/10.3390/jcm7120499
Putter H, Spitoni C (2018) Non-parametric estimation of transition probabilities in non-Markov multi-state models: the landmark Aalen-Johansen estimator. Stat Methods Med Res 27(7):2081–2092. https://doi.org/10.1177/0962280216674497
Agarwal R, Joseph A, Anker SD, Filippatos G, Rossing P, Ruilope LM, Pitt B, Kolkhof P, Scott C, Lawatscheck R, Wilson DJ, Bakris GL (2022) Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol 33(1):225–237. https://doi.org/10.1681/ASN.2021070942
Liu P, Sawhney S, Heide-Jørgensen U, Quinn RR, Jensen SK, Mclean A, Christiansen CF, Gerds TA, Ravani P (2024) Predicting the risks of kidney failure and death in adults with moderate to severe chronic kidney disease: multinational, longitudinal, population based, cohort study. BMJ 15(385):e078063. https://doi.org/10.1136/bmj-2023-078063
Mitchell, Tom. (2006). The Discipline of Machine Learning.
Greco R, Papalia T, Lofaro D, Maestripieri S, Mancuso D, Bonofiglio R (2010) Decisional trees in renal transplant follow-up. In Transplantation Proceed 42(4):1134–1136
Chen, Tianqi & Guestrin, Carlos. (2016). XGBoost: A Scalable Tree Boosting System. https://doi.org/10.1145/2939672.2939785.
Greco CM, Simeri A, Tagarelli A, Zumpano E (2023) Transformer-based language models for mental health issues: a survey. Pattern Recognition Lett 167:204–211
Zhang K, Liu X, Xu J, Yuan J, Cai W, Chen T, Wang G (2021) Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nature Biomed Eng 5(6):33–545
Babenko B, Mitani A, Traynis I, Kitade N, Singh P, Maa AY, Liu Y (2022) Detection of signs of disease in external photographs of the eyes via deep learning. Nature Biomed Eng 6(12):1370–1383
Allen A, Iqbal Z, Green-Saxena A, Hurtado M, Hoffman J, Mao Q, Das R (2022) Prediction of diabetic kidney disease with machine learning algorithms, upon the initial diagnosis of type 2 diabetes mellitus. BMJ Open Diabetes Res Care 10(1):e002560. https://doi.org/10.1136/bmjdrc-2021-002560.PMID:35046014;PMCID:PMC8772425
Arrieta AB, Díaz Rodríguez N, Del Ser J, Bennetot A, Tabik S, Barbado A, Herrera F (2020) Explainable artificial intelligence (XAI): concepts taxonomies opportunities and challenges toward responsible AI. Information Fusion 58:82–115
Hagemann V, Rieth M, Suresh A, Kirchner F (2023) Human-AI teams-challenges for a team-centered AI at work. Front Artif Intell 27(6):1252897. https://doi.org/10.3389/frai.2023.1252897.PMID:37829660;PMCID:PMC10565103
Acknowledgements
This work was also funded by: 1.The Next Generation EU—project Tech4You—Technologies for climate change adaptation and quality of life improvement, n. ECS0000009; 2.The National Plan for NRRP Complementary Investments—project n. PNC0000003—AdvaNced Technologies for Human-centrEd Medicine (ANTHEM); 3.POS RADIOAMICA project funded by the Italian Minister of Health (CUP: H53C22000650006); 4.POS CAL.HUB.RIA project funded by the Italian Minister of Health (CUP H53C22000800006); 5.The Next Generation EU—project SERICS (PE00000014) —“SEcurity and RIghts in the CyberSpace”.
Funding
Open access funding provided by Università della Calabria within the CRUI-CARE Agreement. This article was not funded.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.P.; validation: A.S., G.P., R.A., G.P., T.S.T., R.G., P.V., G.G., V.P., M.P. and G.Z.; writing—original draft preparation: A.S., G.P. and M.P.; writing—review and editing: A.S., G.P. and M.P.; visualization: A.S., G.P., R.A., G.P., T.S.T., R.G., P.V., G.G., V.P., M.P. and G.Z.; supervision: M.P., P.V.; G.G., V.P., G.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simeri, A., Pezzi, G., Arena, R. et al. Artificial intelligence in chronic kidney diseases: methodology and potential applications. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-04165-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-04165-8