Abstract
Purpose
The aims of this study were to compare cerebral hemodynamics and maximal oxygen uptake (VO2peak) in patients with end-stage renal disease (ESRD) vs. age-matched healthy controls during maximal exercise.
Methods
Twelve patients with ESRD and twelve healthy adults (CTR group) performed exhaustive incremental exercise test. Throughout the exercise test, near-infrared spectroscopy allowed the investigation of changes in oxyhemoglobin (∆O2Hb), deoxyhemoglobin (∆HHb), and total hemoglobin (∆THb) in the prefrontal cortex.
Results
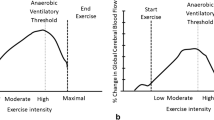
Compared to CTR, VO2peak was significantly lower in ESRD group (P < 0.05). Increase in ∆THb (i.e., cerebral blood volume) was significantly blunted in ESRD (P < 0.05). ESRD patients also had impaired changes in cerebral ∆HHb and ∆O2Hb during high intensity of exercise (P < 0.05). Finally, no significant correlation was observed between VO2peak and changes in cerebral hemodynamics parameters in both groups (All P > 0.05).
Conclusion
Maximal exercise highlights subtle disorders of both hemodynamics and neuronal oxygenation in the prefrontal cortex in patients with ESRD. This may contribute to both impaired cognitive function and reduced exercise tolerance throughout the progression of the disease.



Similar content being viewed by others
Data availability
The data may be shared upon reasonable request to the corresponding author if the request is accepted by the Regional Research Committee for Medical and Health Research Ethics and the local Data Protection Official.
References
Steinbach EJ, Harshman LA (2022) Impact of chronic kidney disease on brain structure and function. Front Neurol 13:797503. https://doi.org/10.3389/fneur.2022.797503
Yu H et al (2023) Morphological brain alterations in dialysis- and non-dialysis-dependent patients with chronic kidney disease. Metab Brain Dis 38(4):1311–1321. https://doi.org/10.1007/s11011-022-01150-x
Arnold R et al (2016) Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis 5:2048004016677687. https://doi.org/10.1177/2048004016677687
Theodorakopoulou MP et al (2023) Muscle oxygenation and microvascular reactivity across different stages of CKD: a near-infrared spectroscopy study. Am J Kidney Dis. https://doi.org/10.1053/j.ajkd.2022.11.013
Yaffe K et al (2010) Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58(2):338–345. https://doi.org/10.1111/j.1532-5415.2009.02670.x
Kalirao P et al (2011) Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis 57(4):612–620. https://doi.org/10.1053/j.ajkd.2010.11.026
Tariq H, Ramakrishnan M, Gupta A (2022) Insights into cognitive brain health in chronic kidney disease. Gerontol Geriatr. https://doi.org/10.26420/gerontolgeriatrres.2022.1074
Stauffer ME, Fan T (2014) Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE 9(1):e84943. https://doi.org/10.1371/journal.pone.0084943
Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23(10):1631–1634. https://doi.org/10.1681/asn.2011111078
Vorstrup S et al (1992) Increased cerebral blood flow in anemic patients on long-term hemodialytic treatment. J Cereb Blood Flow Metab 12(5):745–749. https://doi.org/10.1038/jcbfm.1992.105
Zheng G et al (2016) Anemia rather than hypertension contributes to cerebral hyperperfusion in young adults undergoing hemodialysis: a phase contrast MRI study. Sci Rep 6:22346. https://doi.org/10.1038/srep22346
Cheng BC et al (2019) Decreased cerebral blood flow and improved cognitive function in patients with end-stage renal disease after peritoneal dialysis: an arterial spin-labelling study. Eur Radiol 29(3):1415–1424. https://doi.org/10.1007/s00330-018-5675-9
Wang H et al (2023) Cerebral blood flow regulates iron overload in the cerebral nuclei of hemodialysis patients with anemia. J Cereb Blood Flow Metab 43(5):749–762. https://doi.org/10.1177/0271678x221147363
Kuwabara Y et al (2002) Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int 61(2):564–569. https://doi.org/10.1046/j.1523-1755.2002.00142.x
Ishida K et al (2018) Cerebrovascular CO(2) reactivity during isoflurane-nitrous oxide anesthesia in patients with chronic renal failure. J Anesth 32(1):15–22. https://doi.org/10.1007/s00540-017-2422-3
Slessarev M et al (2021) Hemodialysis patients have impaired cerebrovascular reactivity to CO(2) compared to chronic kidney disease patients and healthy controls: a pilot study. Kidney Int Rep 6(7):1868–1877. https://doi.org/10.1016/j.ekir.2021.04.005
Malyszko J (2010) Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta 411(19–20):1412–1420. https://doi.org/10.1016/j.cca.2010.06.019
Jiménez-Bonilla JF et al (2001) Assessment of cerebral perfusion and cerebrovascular reserve in insulin-dependent diabetic patients without central neurological symptoms by means of 99mTc-HMPAO SPET with acetazolamide. Eur J Nucl Med 28(11):1647–1655. https://doi.org/10.1007/s002590100595
Ogoh S, Ainslie PN (2009) Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 107(5):1370–1380. https://doi.org/10.1152/japplphysiol.00573.2009
Subudhi AW et al (2008) Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circ Physiol 294(1):H164–H171. https://doi.org/10.1152/ajpheart.01104.2007
Perrey S (2008) Non-invasive NIR spectroscopy of human brain function during exercise. Methods 45(4):289–299. https://doi.org/10.1016/j.ymeth.2008.04.005
Poole DC, Wilkerson DP, Jones AM (2008) Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. Eur J Appl Physiol 102(4):403–410. https://doi.org/10.1007/s00421-007-0596-3
Midgley AW et al (2009) Evaluation of true maximal oxygen uptake based on a novel set of standardized criteria. Appl Physiol Nutr Metab 34(2):115–123. https://doi.org/10.1139/h08-146
Tagougui S et al (2015) Regional cerebral hemodynamic response to incremental exercise is blunted in poorly controlled patients with uncomplicated type 1 diabetes. Diabetes Care 38(5):858–867. https://doi.org/10.2337/dc14-1792
Tagougui S et al (2015) Muscle oxygen supply impairment during exercise in poorly controlled type 1 diabetes. Med Sci Sports Exerc 47(2):231–239. https://doi.org/10.1249/mss.0000000000000424
Barstow TJ (2019) Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol 126(5):1360–1376. https://doi.org/10.1152/japplphysiol.00166.2018
Rooks CR et al (2010) Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol 92(2):134–150. https://doi.org/10.1016/j.pneurobio.2010.06.002
Tryc AB et al (2011) Cerebral metabolic alterations and cognitive dysfunction in chronic kidney disease. Nephrol Dial Transplant 26(8):2635–2641. https://doi.org/10.1093/ndt/gfq729
Qiu Y et al (2014) Structural and functional brain alterations in end stage renal disease patients on routine hemodialysis: a voxel-based morphometry and resting state functional connectivity study. PLoS ONE 9(5):e98346. https://doi.org/10.1371/journal.pone.0098346
Moodalbail DG et al (2013) Systematic review of structural and functional neuroimaging findings in children and adults with CKD. Clin J Am Soc Nephrol 8(8):1429–1448. https://doi.org/10.2215/cjn.11601112
Guo H et al (2021) Structural and functional brain changes in hemodialysis patients with end-stage renal disease: DTI analysis results and ALFF analysis results. Int J Nephrol Renovasc Dis 14:77–86. https://doi.org/10.2147/ijnrd.s295025
Sabri O et al (1999) Neuropsychological impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke 30(3):556–566. https://doi.org/10.1161/01.str.30.3.556
Sabri O et al (1998) Correlation of neuropsychological, morphological and functional (regional cerebral blood flow and glucose utilization) findings in cerebral microangiopathy. J Nucl Med 39(1):147–154
Nielsen HB, Boesen M, Secher NH (2001) Near-infrared spectroscopy determined brain and muscle oxygenation during exercise with normal and resistive breathing. Acta Physiol Scand 171(1):63–70. https://doi.org/10.1046/j.1365-201X.2001.00782.x
Sprick JD et al (2020) Cerebral blood flow regulation in end-stage kidney disease. Am J Physiol Renal Physiol 319(5):F782-f791. https://doi.org/10.1152/ajprenal.00438.2020
Macdonald JH et al (2012) Exertional fatigue in patients with CKD. Am J Kidney Dis 60(6):930–939. https://doi.org/10.1053/j.ajkd.2012.06.021
Kanai H et al (2001) Depressed cerebral oxygen metabolism in patients with chronic renal failure: a positron emission tomography study. Am J Kidney Dis 38(4 Suppl 1):S129–S133. https://doi.org/10.1053/ajkd.2001.27421
Ekkekakis P (2009) Illuminating the black box: investigating prefrontal cortical hemodynamics during exercise with near-infrared spectroscopy. J Sport Exerc Psychol 31(4):505–553. https://doi.org/10.1123/jsep.31.4.505
Acknowledgements
We are thankful to all the participants who dedicated their time and effort to complete this study. We would like to equally acknowledge the contributions of the nurses at Taher Sfar hospital.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study conception and design: all authors. Material preparation and data collection and analysis: Amal Machfer, Mohamed Amine Bouzid, Nadia Fekih. Writing—original draft: Amal Machfer. Writing—review and editing: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study received approval from the south institutional human research ethics committee (CPP SUD N° 0459/2022)) and followed the ethical principles of the Declaration of Helsinki (2013).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Machfer, A., Bouzid, M.A., Fekih, N. et al. Blunted cerebral hemodynamic responses to incremental exercise in patients with end-stage renal disease. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-03991-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-03991-0