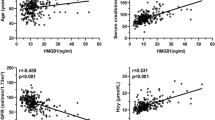
Abstract
Percutaneous coronary intervention (PCI) is a crucial diagnostic and therapeutic approach for coronary heart disease. Contrast agents’ exposure during PCI is associated with a risk of contrast-induced acute kidney injury (CI-AKI). CI-AKI is characterized by a sudden decline in renal function occurring as a result of exposure to intravascular contrast agents, which is associated with an increased risk of poor prognosis. The pathophysiological mechanisms underlying CI-AKI involve renal medullary hypoxia, direct cytotoxic effects, endoplasmic reticulum stress, inflammation, oxidative stress, and apoptosis. To date, there is no effective therapy for CI-AKI. High-mobility group box 1 (HMGB1), as a damage-associated molecular pattern molecule, is released extracellularly by damaged cells or activated immune cells and binds to related receptors, including toll-like receptors and receptor for advanced glycation end product. In renal injury, HMGB1 is expressed in renal tubular epithelial cells, macrophages, endothelial cells, and glomerular cells, involved in the pathogenesis of various kidney diseases by activating its receptors. Therefore, this review provides a theoretical basis for HMGB1 as a therapeutic intervention target for CI-AKI.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N et al (2010) Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int 78(8):803–809. https://doi.org/10.1038/ki.2010.258
James MT, Samuel SM, Manning MA, Tonelli M, Ghali WA, Faris P et al (2013) Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv 6(1):37–43. https://doi.org/10.1161/CIRCINTERVENTIONS.112.974493
Heyman SN, Rosenberger C, Rosen S, Khamaisi M (2013) Why is diabetes mellitus a risk factor for contrast-induced nephropathy? Biomed Res Int 2013:123589. https://doi.org/10.1155/2013/123589
Silvain J, Nguyen LS, Spagnoli V, Kerneis M, Guedeney P, Vignolles N et al (2018) Contrast-induced acute kidney injury and mortality in ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart 104(9):767–772. https://doi.org/10.1136/heartjnl-2017-311975
Sun L, Zhu W, Chen X, Jiang J, Ji Y, Liu N et al (2020) Machine learning to predict contrast-induced acute kidney injury in patients with acute myocardial infarction. Front Med (Lausanne) 7:592007. https://doi.org/10.3389/fmed.2020.592007
Kim JH, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH et al (2014) Predictors of outcomes of contrast-induced acute kidney injury after percutaneous coronary intervention in patients with chronic kidney disease. Am J Cardiol 114(12):1830–1835. https://doi.org/10.1016/j.amjcard.2014.09.022
Flaherty MP, Pant S, Patel SV, Kilgore T, Dassanayaka S, Loughran JH et al (2017) Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ Res 120(4):692–700. https://doi.org/10.1161/CIRCRESAHA.116.309738
Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F (2012) Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation 125(25):3099–3107. https://doi.org/10.1161/CIRCULATIONAHA.111.085290
Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B et al (2015) Impact of contrast-induced acute kidney injury after percutaneous coronary intervention on short- and long-term outcomes: pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv 8(8):e002475. https://doi.org/10.1161/CIRCINTERVENTIONS.114.002475
Chen Q, Guan X, Zuo X, Wang J, Yin W (2016) The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm Sin B 6(3):183–188. https://doi.org/10.1016/j.apsb.2016.02.004
Andersson U, Tracey KJ (2011) HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29:139–162. https://doi.org/10.1146/annurev-immunol-030409-101323
Yang H, Wang H, Chavan SS, Andersson U (2015) High mobility group box protein 1 (HMGB1): the prototypical endogenous danger molecule. Mol Med 21(Suppl 1):S6–S12. https://doi.org/10.2119/molmed.2015.00087
Cho E, Ko GJ (2022) The pathophysiology and the management of radiocontrast-induced nephropathy. Diagnostics (Basel) 12(1):180. https://doi.org/10.3390/diagnostics12010180
McCullough PA (2008) Contrast-induced acute kidney injury. J Am Coll Cardiol 51(15):1419–1428. https://doi.org/10.1016/j.jacc.2007.12.035
Ad-hoc working group of ERBP, Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L et al (2012) A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 27(12):4263–4272. https://doi.org/10.1093/ndt/gfs375
Seeliger E, Sendeski M, Rihal CS, Persson PB (2012) Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 33(16):2007–2015. https://doi.org/10.1093/eurheartj/ehr494
Sendeski MM (2011) Pathophysiology of renal tissue damage by iodinated contrast media. Clin Exp Pharmacol Physiol 38(5):292–299. https://doi.org/10.1111/j.1440-1681.2011.05503.x
Sendeski MM, Persson AB, Liu ZZ, Busch JF, Weikert S, Persson PB et al (2012) Iodinated contrast media cause endothelial damage leading to vasoconstriction of human and rat vasa recta. Am J Physiol Renal Physiol 303(12):F1592–F1598. https://doi.org/10.1152/ajprenal.00471.2012
Yang Y, Yang D, Yang D, Jia R, Ding G (2014) Role of reactive oxygen species-mediated endoplasmic reticulum stress in contrast-induced renal tubular cell apoptosis. Nephron Exp Nephrol 128(1–2):30–36. https://doi.org/10.1159/000366063
Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A et al (2008) Contrast agents and renal cell apoptosis. Eur Heart J 29(20):2569–2576. https://doi.org/10.1093/eurheartj/ehn197
Zhu X, Li S, Lin Q, Shao X, Wu J, Zhang W et al (2021) αKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol Res 167:105531. https://doi.org/10.1016/j.phrs.2021.105531
Dai B, Su Q, Liu X, Mi X, Dou L, Zhou D et al (2023) 2, 2-dimethylthiazolidine hydrochloride protects against experimental contrast-induced acute kidney injury via inhibition of tubular ferroptosis. Biochem Biophys Res Commun 679:15–22. https://doi.org/10.1016/j.bbrc.2023.08.052
Liu X, Li Q, Sun L, Chen L, Li Y, Huang B et al (2021) miR-30e-5p regulates autophagy and apoptosis by targeting Beclin1 involved in contrast-induced acute kidney injury. Curr Med Chem 28(38):7974–7984. https://doi.org/10.2174/0929867328666210526125023
Seeliger E, Flemming B, Wronski T, Ladwig M, Arakelyan K, Godes M et al (2007) Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol 18(11):2912–2920. https://doi.org/10.1681/ASN.2006111216
Kim K, Jeong B, Lee YM, Son HE, Ryu JY, Park S et al (2022) Three-dimensional kidney-on-a-chip assessment of contrast-induced kidney injury: osmolality and viscosity. Micromachines (Basel) 13(5):688. https://doi.org/10.3390/mi13050688
Li Q, Pan S (2022) Contrast-associated acute kidney injury: advances and challenges. Int J Gen Med 15:1537–1546. https://doi.org/10.2147/IJGM.S341072
Heyman SN, Rosen S, Khamaisi M, Idée JM, Rosenberger C (2010) Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol 45(4):188–195. https://doi.org/10.1097/RLI.0b013e3181d2eed8
Cheng AS, Li X (2023) The potential biotherapeutic targets of contrast-induced acute kidney injury. Int J Mol Sci 24(9):8254. https://doi.org/10.3390/ijms24098254
Heyman SN, Rosen S, Rosenberger C (2008) Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol 3(1):288–296. https://doi.org/10.2215/CJN.02600607
Yang Z, Qiao Y, Wang D, Yan G, Tang C (2023) Association between inflammatory biomarkers and contrast-induced acute kidney injury in ACS patients undergoing percutaneous coronary intervention: a cross-sectional study. Angiology 19:33197231185445. https://doi.org/10.1177/00033197231185445
Machado RA, Constantino Lde S, Tomasi CD, Rojas HA, Vuolo FS, Vitto MF et al (2012) Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transplant 27(8):3136–3140. https://doi.org/10.1093/ndt/gfr807
Bianchi ME, Falciola L, Ferrari S, Lilley DM (1992) The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. EMBO J 11(3):1055–1063. https://doi.org/10.1002/j.1460-2075.1992.tb05144.x
Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X et al (2003) Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med 9(1–2):37–45
Cai J, Wen J, Bauer E, Zhong H, Yuan H, Chen AF (2015) The role of HMGB1 in cardiovascular biology: danger signals. Antioxid Redox Signal 23(17):1351–1369. https://doi.org/10.1089/ars.2015.6408
Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE et al (2004) Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 101(1):296–301. https://doi.org/10.1073/pnas.2434651100
Stros M (1998) DNA bending by the chromosomal protein HMG1 and its high mobility group box domains. Effect of flanking sequences. J Biol Chem 273(17):10355–10361
Wang Q, Zeng M, Wang W, Tang J (2007) The HMGB1 acidic tail regulates HMGB1 DNA binding specificity by a unique mechanism. Biochem Biophys Res Commun 360(1):14–19. https://doi.org/10.1016/j.bbrc.2007.05.130
Liu T, Li Q, Jin Q, Yang L, Mao H, Qu P et al (2023) Targeting HMGB1: a potential therapeutic strategy for chronic kidney disease. Int J Biol Sci 19(15):5020–5035. https://doi.org/10.7150/ijbs.87964
Carta S, Castellani P, Delfino L, Tassi S, Venè R, Rubartelli A (2009) DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol 86(3):549–555. https://doi.org/10.1189/jlb.1008598
Yang H, Antoine DJ, Andersson U, Tracey KJ (2013) The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93(6):865–873. https://doi.org/10.1189/jlb.1212662
Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A et al (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 2(20):5551–5560. https://doi.org/10.1093/emboj/cdg516
Ito I, Fukazawa J, Yoshida M (2007) Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem 282(22):16336–16344. https://doi.org/10.1074/jbc.M608467200
Youn JH, Shin JS (2006) Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol 177(11):7889–7897. https://doi.org/10.4049/jimmunol.177.11.7889
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418(6894):191–195. https://doi.org/10.1038/nature00858
Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA (2008) Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 29(1):21–32. https://doi.org/10.1016/j.immuni.2008.05.013
Matsuura R, Komaru Y, Miyamoto Y, Yoshida T, Yoshimoto K, Yamashita T et al (2023) HMGB1 is a prognostic factor for mortality in acute kidney injury requiring renal replacement therapy. Blood Purif 52(7–8):660–667. https://doi.org/10.1159/000530774
Lau A, Wang S, Liu W, Haig A, Zhang ZX, Jevnikar AM (2014) Glycyrrhizic acid ameliorates HMGB1-mediated cell death and inflammation after renal ischemia reperfusion injury. Am J Nephrol 40(1):84–95. https://doi.org/10.1159/000364908
Zhao Z, Hu Z, Zeng R, Yao Y (2020) HMGB1 in kidney diseases. Life Sci 259:118203. https://doi.org/10.1016/j.lfs.2020.118203
Zhang C, Dong H, Chen F, Wang Y, Ma J, Wang G (2019) The HMGB1-RAGE/TLR-TNF-α signaling pathway may contribute to kidney injury induced by hypoxia. Exp Ther Med 17(1):17–26. https://doi.org/10.3892/etm.2018.6932
Miura K, Sahara H, Sekijima M, Kawai A, Waki S, Nishimura H et al (2014) Protective effect of neutralization of the extracellular high-mobility group box 1 on renal ischemia-reperfusion injury in miniature swine. Transplantation 98(9):937–943. https://doi.org/10.1097/TP.0000000000000358
Wang Y, Zhang H, Chen Q, Jiao F, Shi C, Pei M et al (2020) TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif 53(6):e12829. https://doi.org/10.1111/cpr.12829
Guan XF, Chen QJ, Zuo XC, Guo R, Peng XD, Wang JL et al (2017) Contrast media-induced renal inflammation is mediated through HMGB1 and its receptors in human tubular cells. DNA Cell Biol 36(1):67–76. https://doi.org/10.1089/dna.2016.3463
Neyra JA, Chawla LS (2021) Acute kidney disease to chronic kidney disease. Crit Care Clin 37(2):453–474. https://doi.org/10.1016/j.ccc.2020.11.013
Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK (2015) Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26(8):1765–1776. https://doi.org/10.1681/ASN.2015010006
Zhao ZB, Marschner JA, Iwakura T, Li C, Motrapu M, Kuang M et al (2023) Tubular epithelial cell HMGB1 promotes AKI-CKD transition by sensitizing cycling tubular cells to oxidative stress: a rationale for targeting HMGB1 during AKI recovery. J Am Soc Nephrol 34(3):394–411. https://doi.org/10.1681/ASN.0000000000000024
Tanaka S, Tanaka T, Nangaku M (2014) Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 307(11):F1187–F1195. https://doi.org/10.1152/ajprenal.00425.2014
Mo C, Ma X, Jian W, Huang Q, Zheng W, Yang Z et al (2022) High mobility group box 1 and homocysteine as preprocedural predictors for contrast-induced acute kidney injury after percutaneous coronary artery intervention. Int Urol Nephrol 54(7):1663–1671. https://doi.org/10.1007/s11255-021-03050-y
Oh H, Choi A, Seo N, Lim JS, You JS, Chung YE (2021) Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on post-contrast acute kidney injury. Sci Rep 11(1):15625. https://doi.org/10.1038/s41598-021-94928-5
Zhao H, Liu Z, Shen H, Jin S, Zhang S (2016) Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur J Pharmacol 781:92–99. https://doi.org/10.1016/j.ejphar.2016.04.006
Sohn EJ, Kang DG, Lee HS (2003) Protective effects of glycyrrhizin on gentamicin-induced acute renal failure in rats. Pharmacol Toxicol 93(3):116–122. https://doi.org/10.1034/j.1600-0773.2003.930302.x
Thakur V, Nargis S, Gonzalez M, Pradhan S, Terreros D, Chattopadhyay M (2017) Role of glycyrrhizin in the reduction of inflammation in diabetic kidney disease. Nephron 137(2):137–147. https://doi.org/10.1159/000477820
Yue RZ, Li YJ, Su BH, Li CJ, Zeng R (2023) Atorvastatin reduces contrast media-induced pyroptosis of renal tubular epithelial cells by inhibiting the TLR4/MyD88/NF-κB signaling pathway. BMC Nephrol 24(1):25. https://doi.org/10.1186/s12882-023-03066-9
Awad AM, Elshaer SL, Gangaraju R, Abdelaziz RR, Nader MA (2023) Ameliorative effect of montelukast against STZ induced diabetic nephropathy: targeting HMGB1, TLR4, NF-κB, NLRP3 inflammasome, and autophagy pathways. Inflammopharmacology. https://doi.org/10.1007/s10787-023-01301-1
Shen J, Wang L, Jiang N, Mou S, Zhang M, Gu L et al (2016) NLRP3 inflammasome mediates contrast media-induced acute kidney injury by regulating cell apoptosis. Sci Rep 6:34682. https://doi.org/10.1038/srep34682
Luo M, Liu Z, Hu Z, He Q (2022) Quercetin improves contrast-induced acute kidney injury through the HIF-1α/lncRNA NEAT1/HMGB1 pathway. Pharm Biol 60(1):889–898. https://doi.org/10.1080/13880209.2022.2058558
Shelke V, Kale A, Dagar N, Habshi T, Gaikwad AB (2023) Concomitant inhibition of TLR-4 and SGLT2 by phloretin and empagliflozin prevents diabetes-associated ischemic acute kidney injury. Food Funct 14(11):5391–5403. https://doi.org/10.1039/d3fo01379k
Katare PB, Nizami HL, Paramesha B, Dinda AK, Banerjee SK (2020) Activation of toll like receptor 4 (TLR4) promotes cardiomyocyte apoptosis through SIRT2 dependent p53 deacetylation. Sci Rep 10(1):19232. https://doi.org/10.1038/s41598-020-75301-4
Zhou S, Lu S, Guo S, Zhao L, Han Z, Li Z (2021) Protective effect of ginsenoside Rb1 nanoparticles against contrast-induced nephropathy by inhibiting high mobility group box 1 Gene/toll-Like receptor 4/NF-κB signaling pathway. J Biomed Nanotechnol 17(10):2085–2098. https://doi.org/10.1166/jbn.2021.3163
Huang Q, Yang Z, Zhou JP, Luo Y (2017) HMGB1 induces endothelial progenitor cells apoptosis via RAGE-dependent PERK/eIF2α pathway. Mol Cell Biochem 431(1–2):67–74. https://doi.org/10.1007/s11010-017-2976-2
He F, Gu L, Cai N, Ni J, Liu Y, Zhang Q et al (2022) The HMGB1-RAGE axis induces apoptosis in acute respiratory distress syndrome through PERK/eIF2α/ATF4-mediated endoplasmic reticulum stress. Inflamm Res 71(10–11):1245–1260. https://doi.org/10.1007/s00011-022-01613-y
Kim S, Joe Y, Surh YJ, Chung HT (2018) Differential regulation of toll-like receptor-mediated cytokine production by unfolded protein response. Oxid Med Cell Longev 2018:9827312. https://doi.org/10.1155/2018/9827312
Ferrè S, Deng Y, Huen SC, Lu CY, Scherer PE, Igarashi P et al (2019) Renal tubular cell spliced X-box binding protein 1 (Xbp1s) has a unique role in sepsis-induced acute kidney injury and inflammation. Kidney Int 96(6):1359–1373. https://doi.org/10.1016/j.kint.2019.06.023
Liu J, Huang K, Cai GY, Chen XM, Yang JR, Lin LR et al (2014) Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell Signal 26(1):110–121. https://doi.org/10.1016/j.cellsig.2013.10.002
Lai HJ, Zhan YQ, Qiu YX, Ling YH, Zhang XY, Chang ZN et al (2021) HMGB1 signaling-regulated endoplasmic reticulum stress mediates intestinal ischemia/reperfusion-induced acute renal damage. Surgery 170(1):239–248. https://doi.org/10.1016/j.surg.2021.01.042
Zhang J, Chen Q, Dai Z, Pan H (2023) miR-22 alleviates sepsis-induced acute kidney injury via targeting the HMGB1/TLR4/NF-κB signaling pathway. Int Urol Nephrol 55(2):409–421. https://doi.org/10.1007/s11255-022-03321-2
Michel HE, Menze ET (2019) Tetramethylpyrazine guards against cisplatin-induced nephrotoxicity in rats through inhibiting HMGB1/TLR4/NF-κB and activating Nrf2 and PPAR-γ signaling pathways. Eur J Pharmacol 857:172422. https://doi.org/10.1016/j.ejphar.2019.172422
Zhang GZ, Zhang K, Yang SQ, Zhang Z, Chen S, Hou BJ et al (2020) VASPIN reduces inflammation and endoplasmic reticulum stress of renal tubular epithelial cells by inhibiting HMGB1 and relieves renal ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci 24(17):8968–8977. https://doi.org/10.26355/eurrev_202009_22839.
Shelke V, Kale A, Anders HJ, Gaikwad AB (2023) Toll-like receptors 2 and 4 stress signaling and sodium-glucose cotransporter-2 in kidney disease. Mol Cell Biochem 478(9):1987–1998. https://doi.org/10.1007/s11010-022-04652-5
Zhang M, Guo Y, Fu H, Hu S, Pan J, Wang Y et al (2015) Chop deficiency prevents UUO-induced renal fibrosis by attenuating fibrotic signals originated from Hmgb1/TLR4/NFκB/IL-1β signaling. Cell Death Dis 6(8):e1847. https://doi.org/10.1038/cddis.2015.206
Zeng KW, Zhang T, Fu H, Liu GX, Wang XM (2012) Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur J Pharmacol 692(1–3):29–37. https://doi.org/10.1016/j.ejphar.2012.05.030
Sun Y, Peng PA, Ma Y, Liu XL, Yu Y, Jia S et al (2017) Valsartan protects against contrast-induced acute kidney injury in rats by inhibiting endoplasmic reticulum stress-induced apoptosis. Curr Vasc Pharmacol 15(2):174–183. https://doi.org/10.2174/1570161114666161025100656
Peng PA, Wang L, Ma Q, Xin Y, Zhang O, Han HY et al (2015) Valsartan protects HK-2 cells from contrast media-induced apoptosis by inhibiting endoplasmic reticulum stress. Cell Biol Int 39(12):1408–1417. https://doi.org/10.1002/cbin.10521
Acknowledgements
We thank Dr. Xiao Ma from the First Affiliated Hospital of Guangxi Medical University for his kind support in reviewing the manuscript for integrity check and giving precious opinions.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82260060 to Dr. Qingwei Ji).
Author information
Authors and Affiliations
Contributions
Manuscript designing and outlining: Changhua Mo, Gui Chun, and Qingwei Ji; manuscript writing: Changhua Mo and Qili Huang; figure and table creating: Changhua Mo, Lixia Li, Yusheng Long, Guihua Li, and Lingyue Qiu; and clinical consulting and revision: Ying Shi, Zhengde Lu, Ning Wu, Qingkuan Li, and Huayuan Zeng. All the authors read the submitted version and approved it.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest with the contents of this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mo, C., Huang, Q., Li, L. et al. High-mobility group box 1 and its related receptors: potential therapeutic targets for contrast-induced acute kidney injury. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-03981-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-03981-2