Abstract
Purpose
Dietary acid load plays a key role in regulating serum uric acid levels. We hypothesized that dietary acid load indices would be positively associated with the odds of hyperuricemia. We aimed to test this hypothesis in a representative sample of Iranian adult population.
Methods
In this cross-sectional study, a total of 6145 participants aged 35–65 years were recruited from MASHAD cohort study. Dietary intakes were assessed using a 24-h dietary recall. Diet-based acid load was assessed as the potential renal acid load (PRAL), net endogenous acid production (NEAP), and dietary acid load (DAL). Hyperuricemia was defined as serum uric acid greater than the 75th percentile. Multivariable logistic regression models were applied to determine the association between diet-based acid load scores and hyperuricemia.
Results
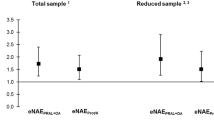
The mean age of participants was 48.89 ± 8.09 years. Overall, 25.7% had hyperuricemia. According to the full-adjusted model, there was a significant association between higher tertile of PRAL, and DAL and hyperuricemia (Q3 PRAL; OR (95% CI): 1.23 (1.05–1.43), Q3 DAL; OR (95% CI): 1.22 (1.05–1.42)). Regarding NEAP, there was no significant association with hyperuricemia. We also found that dietary intake of total sugars, fiber, calcium, and magnesium was associated with the odds of hyperuricemia in our population.
Conclusion
This study showed a significant positive association between two indicators of dietary acid load (PRAL, and DAL) and odds of hyperuricemia among Iranian adults.
Similar content being viewed by others
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to university data ownership policies, but are available from the corresponding author on reasonable request.
References
Borghi C, Piani F (2020) Uric acid and estimate of renal function. Let’s stick together. Int J Cardiol 310:157–158. https://doi.org/10.1016/j.ijcard.2020.01.046. (in eng)
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V (2016) Regulation of uric acid metabolism and excretion. Int J Cardiol 213:8–14. https://doi.org/10.1016/j.ijcard.2015.08.109. (in eng)
Benn CL et al (2018) Physiology of hyperuricemia and urate-lowering treatments. Front Med 5:160. https://doi.org/10.3389/fmed.2018.00160. (in eng)
George C, Minter DA (2022) Hyperuricemia. In: StatPearls. StatPearls Publishing, Treasure Island
Maloberti A et al (2020) Hyperuricemia and risk of cardiovascular outcomes: the experience of the URRAH (Uric Acid Right for Heart Health) Project. High Blood PressCardiovasc Prev 27(2):121–128. https://doi.org/10.1007/s40292-020-00368-z. (in eng)
Kou F, Yang S, Wang S, Liu M (2021) Association between serum uric acid and major chronic diseases among centenarians in China: based on the CHCCS study. BMC Geriatrics 21(1):231. https://doi.org/10.1186/s12877-021-02185-y
Yip K, Cohen RE, Pillinger MH (2020) Asymptomatic hyperuricemia: is it really asymptomatic? Curr Opin Rheumatol 32(1):71–79
Skoczyńska M, Chowaniec M, Szymczak A, Langner-Hetmańczuk A, Maciążek-Chyra B, Wiland P (2020) Pathophysiology of hyperuricemia and its clinical significance—a narrative review. Reumatologia 58(5):312–323. https://doi.org/10.5114/reum.2020.100140. (in eng)
Zhang W et al (2006) "EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 65(10):1312–1324. https://doi.org/10.1136/ard.2006.055269. (in eng)
Danve A, Sehra ST, Neogi T (2021) Role of diet in hyperuricemia and gout. Best Pract Res Clin Rheumatol 35(4):101723. https://doi.org/10.1016/j.berh.2021.101723. (in eng)
Kanbara A, Miura Y, Hyogo H, Chayama K, Seyama I (2012) "Effect of urine pH changed by dietary intervention on uric acid clearance mechanism of pH-dependent excretion of urinary uric acid. Nutr J 11:39. https://doi.org/10.1186/1475-2891-11-39. (in eng)
Esche J, Krupp D, Mensink GB, Remer T (2020) Estimates of renal net acid excretion and their relationships with serum uric acid and hyperuricemia in a representative German population sample. Eur J Clin Nutr 74(Suppl 1):63–68. https://doi.org/10.1038/s41430-020-0688-2. (in eng)
Esche J, Krupp D, Mensink GBM, Remer T (2018) Dietary potential renal acid load is positively associated with serum uric acid and odds of hyperuricemia in the German adult population. J Nutr 148(1):49–55. https://doi.org/10.1093/jn/nxx003. (in eng)
Ghayour-Mobarhan M et al (2015) Mashhad stroke and heart atherosclerotic disorder (MASHAD) study: design, baseline characteristics and 10-year cardiovascular risk estimation. Int J Public Health 60(5):561–572. https://doi.org/10.1007/s00038-015-0679-6. (in eng)
James WPT, Schofield EC (1990) Human energy requirements. A manual for planners and nutritionists. Oxford University Press
Bolton-Smith C, Woodward M, Tunstall-Pedoe H (1992) The Scottish Heart Health Study. Dietary intake by food frequency questionnaire and odds ratios for coronary heart disease risk. II. The antioxidant vitamins and fibre. Eur J Clin Nutr 46(2):85–93
Organization WH (1985) Energy and protein requirements: report of a joint FAO/WHO/UNU expert consultation. In: Energy and protein requirements: report of a joint FAO/WHO/UNU expert consultation, 1985, pp 206–206
Pedone C et al (2010) Quality of diet and potential renal acid load as risk factors for reduced bone density in elderly women. Bone 46(4):1063–1067. https://doi.org/10.1016/j.bone.2009.11.031. (in eng)
Scialla JJ et al (2011) Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 6(7):1526–1532. https://doi.org/10.2215/cjn.00150111. (in eng)
Scialla JJ, Anderson CA (2013) Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 20(2):141–149. https://doi.org/10.1053/j.ackd.2012.11.001. (in eng)
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93(1):62–66. https://doi.org/10.1016/s0022-3476(78)80601-5. (in eng)
Shao SS, Lin CZ, Zhu YF, Chen C, Wu QJ, Chen RR (2022) "Higher dietary acid load is associated with hyperuricemia in Chinese adults: a case-control study. BMC Endocr Disord 22(1):286. https://doi.org/10.1186/s12902-022-01192-3. (in eng)
Shin D, Lee KW (2021) Dietary acid load is positively associated with the incidence of hyperuricemia in middle-aged and older korean adults: findings from the Korean Genome and Epidemiology Study. Int J Environ Res Public Health 18(19):10260. https://doi.org/10.3390/ijerph181910260
Sorensen LB, Levinson DJ (1975) Origin and extrarenal elimination of uric acid in man. Nephron 14(1):7–20. https://doi.org/10.1159/000180432. (in eng)
Mandal AK, Mount DB (2015) The molecular physiology of uric acid homeostasis. Annu Rev Physiol 77:323–345. https://doi.org/10.1146/annurev-physiol-021113-170343. (in eng)
Mount DB, Kwon CY, Zandi-Nejad K (2006) Renal urate transport. Rheum Dis Clin N Am 32(2):313, vi-vi331. https://doi.org/10.1016/j.rdc.2006.02.006. (in eng)
Lowry M, Ross BD (1980) Activation of oxoglutarate dehydrogenase in the kidney in response to acute acidosis. Biochem J 190(3):771–780. https://doi.org/10.1042/bj1900771. (in eng)
Curthoys NP, Moe OW (2014) Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol CJASN 9(9):1627–1638. https://doi.org/10.2215/cjn.10391012. (in eng)
Acknowledgements
The support provided by Mashhad University of Medical Sciences (MUMS) to conduct this study is highly acknowledged.
Funding
This work was supported by the Vice chancellor of Research of Mashhad University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Study concept and design: NS and MG; Data collection: MN and H.B; data analysis and interpretation of data: GK and ZAH and MA; drafting of the manuscript: AR, HB, and NS; supervision and critical revision: GAF, and MG. All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethics Approval and Consent to Participate
All experiments were performed in accordance with the declaration of Helsinki and Mashhad University of Medical Sciences ethical guidelines and regulations. The research protocol was approved by the School of Medicine, Mashhad University of Medical Sciences, Biomedical Research Ethics Committee (IR.MUMS.MEDICAL.REC.1398.228). All participants signed a written informed consent before participating in the study.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seifi, N., Bahari, H., Nosrati, M. et al. Higher dietary acid load is associated with the risk of hyperuricemia. Int Urol Nephrol 56, 1743–1749 (2024). https://doi.org/10.1007/s11255-023-03876-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03876-8