Abstract
Background
This study aims to explore the association between dietary acid load and hyperuricemia in Chinese adults.
Methods
A case-control study was conducted. Adult participants with hyperuricemia were recruited as the cases and those without hyperuricemia were as the controls. Food consumption was evaluated by food frequency questionnaire (FFQ). Dietary acid load was assessed by potential renal acid load (PRAL) and net endogenous acid production (NEAP). Dietary acid load was divided into four levels: the first quartile (Q1), the second quartile (Q2), the third quartile (Q3) and the fourth quartile (Q4). Logistic regression model was applied for exploring the association between dietary acid load (PRAL and NEAP) and hyperuricemia. Odds ratio (OR) and its correspondence confidence interval (CI) were computed.
Results
A total of 290 participants were eligible in this study, in which there were 143 individuals in case group and 147 in control group. A higher level of PRAL was found to be associated with odds of hyperuricemia. ORs of hyperuricemia for Q2, Q3 and Q4 of PRAL were 2.74 (95%CI: 1.94 ~ 3.88, p-value: 0.004), 2.90 (95%CI: 2.05 ~ 4.10, p-value: 0.002) and 3.14 (95%CI: 2.22 ~ 4.45, p-value: 0.001), respectively. There was a positive association between elevated NEAP and hyperuricemia. OR of hyperuricemia for Q2 was not material significance (OR:1.54, 95%CI: 0.93 ~ 2.53, p-value: 0.210), however, ORs of hyperuricemia for Q3 (OR: 2.40, 95%CI: 1.70 ~ 3.38, p-value: 0.011) and Q4 (OR: 3.27, 95%CI: 2.31 ~ 4.62, p-value: 0.001) were statistically significant.
Conclusion
Higher level of dietary acid load was found to be associated with hyperuricemia in Chinese adults, indicative of advocation of a well-balanced diet in this population.
Similar content being viewed by others
Introduction
Hyperuricemia is characterized by abnormally high serum uric acid, and it may lead to deposition of sodium urate crystal, especially in arthrosis, if without any treatments [1]. The long-term accumulation of urate crystal might cause harm to the joint structure and bring huge aching feeling to the patients, which affect their life quality [2, 3]. Previously studies also reported a higher risk of chronic diseases in patients with hyperuricemia, such as cardiovascular disease, diabetes, kidney disease etc. [4,5,6] In addition, increasing evidence showed that a higher prevalence of hyperuricemia was associated with a higher all-cause mortality [7,8,9,10,11]. The impacts of hyperuricemia on general population in some regions have been underestimated and ignored [12].
Prevalence of hyperuricemia varies in different ethnic groups, countries and territories, and it shows a positive correlation between economic growth and the prevalence of hyperuricemia [12]. A prevalence rate of 21.4% in the American adults was reported according to the Health and Nutrition Examination Survey in a period between 2007 and 2008 [13]. When it comes to China, 13.3% of the total population were found to have abnormally high serum uric acid from 2000 to 2014 [14]. More recently, two Chinese nationally representative cross-sectional surveys demonstrated a higher rate in men (24.4%) than women (3.6%) in 2018 ~ 2019 [15], which indicates that men are more susceptible to hyperuricemia than women.
A variety of factors contribute to the long-run increase of hyperuricemia in Chinese adults, and diet pattern modification in the last decades might be the most important one [16, 17]. The consumption of animal source food has been increased sharply in last a few years, especially livestock and poultry meat, animal fishery products etc., which contain rich purine [16, 17]. As uric acid is the final product of purine metabolism, an increased risk of hyperuricemia in people with higher animal source food consumption was documented [18]. However, some studies suggest that a higher consumption of fruit and vegetable was associated with a lower risk of hyperuricemia [19, 20]. In order to comprehensively assess effect of consuming different food group, dietary acid load was brought up [21]. Acid precursors and base precursors from various kinds of consuming food are evaluated [21]. Among the compute methods of dietary acid load, potential renal acid load (PRAL) and net endogenous acid production (NEAP) were widely applied [21]. Chronic acid-base imbalance, especially high dietary acid load, has been found to be associated with several kinds of diseases, such as hypertension, diabetes, cancer etc. [21,22,23,24,25], while its impact on hyperuricemia is not fully understood. This study based on case-control design aims to explore the association between dietary acid load and hyperuricemia in Chinese adults.
Methods
Study design and participants
This study was based on case-control design. Participants were recruited from Wenzhou hospital of traditional Chinese medicine affiliated to Zhejiang Chinese medicine university in a period from January 2017 to December 2021. Participants diagnosed with hyperuricemia were included in case group and should also meet the following criteria: (1) with an age > 18 years; (2) with basic communication capability and memory; (3) without cancer, neuropsychological diseases, diabetes and gastrointestinal diseases, as these diseases have large impact on food pattern; (4) the food pattern was not largely changed in last 12 months. Almost the same number of participants without hyperuricemia were included in the control group. Male proportion and age distribution in the control group were similar to the case group, while other eligible criteria for the control group were also in accord with the case group. This study was performed in line with the principles of the Declaration of Helsinki, and approved by the Ethical Committee of Wenzhou hospital of traditional Chinese medicine affiliated to Zhejiang Chinese medicine university. Informed consent was obtained from all individual participants included in the study.
Anthropological data and health-related information were derived from electronic patient record. Anthropological data included age, gender, weight (kg), height (cm) and body mass index (BMI). Health-related information included diastolic blood pressure (mmHg), systolic blood pressure (mmHg), heart rate (beats/minutes), serum uric acid (μmol/L), smoking, drinking and chronic diseases. Smoking statues were divided into current smoking, ever smoking and never smoking. Ever smoking was defined as those who quit smoking for more than 12 months. Alcohol drinking statues were classified as current drinking, ever drinking and never drinking. Ever drinking was defined as those who quit drinking for more than 12 months. The list of chronic diseases was as follows: hypertension, coronary heart disease, osteoporosis, chronic obstructive pulmonary disease, chronic kidney disease and hyperlipidemia. These chronic diseases required a clear clinical diagnosis, and self-reporting was not considered in this study as the lack of uniform standards of diseases assessment from different participants.
Definition of hyperuricemia
We used a Chinese general criterion to diagnose hyperuricemia in this study. Hyperuricemia for men was defined as fasting serum uric acid > 420 μmol/L in two separate days, and for women was > 360 μmol/L. [26] In addition, individuals diagnosed as gout or underwent hyperuricemia treatment were also classified as hyperuricemia.
Food consumption survey by food frequency questionnaire
A validated food frequency questionnaire (FFQ) was applied for food consumption survey. Briefly, FFQ in this study was adapted from China Health and Nutrition Survey which is an ongoing open cohort and designed to examine the effects of the health, nutrition status and other factors on the study population [27]. Given the differences in local diet pattern, 12 representative food groups in Zhejiang province were set in the FFQ, inclusive of grains, potato, vegetable, fruit, soy, fish, red meat, poultry, dairy, egg, beverages and edible oil. A small-scale pre-survey was also conducted to verify FFQ validity. Food consumption survey was performed though face-to-face interview by well-trained investigators. The participants were asked to answer diet-related information in a period of last 12 months. Eating amount (g/time or ml/time) and eating frequency (times/day, times/week, times/month or times/year) were recorded. Daily consumption of the 12 food groups were calculated, respectively.
Food consumption and dietary acid load
In order to compute daily nutrient intake, the related data from the Chinese food composition table (2012 version) were used. Energy intake and three macro-nutrients including carbohydrate, fat and protein were calculated. In order to evaluate dietary acid load (PRAL and NEAP), additional nutrients including sodium, magnesium, phosphorus, magnesium and calcium were also computed. According to previously studies [21], PRAL and NEAP were calculated by the following formulas, respectively:
-
(1)
PRAL (mEq/d) =0.4888 × protein intake (g/d) + 0.0366 × phosphorus (mg/d) - 0.0205 × potassium (mg/d) - 0.0125 × calcium (mg/d) - 0.0263 × magnesium (mg/d);
-
(2)
NEAP (mEq/d) = (54.5 × protein intake (g/d) ÷ potassium intake (mEq/d))-10.2
Statistical analysis
Variables on discrete distribution were as number and proportion, while if variables on continuous distribution were in accord with normal distribution than they were exhibited as mean and standard deviation (SD), otherwise were exhibited as median and range interquartile (IQR). Variable difference was evaluated by χ2 test, student’s test or Wilcoxon rank sum test. Logistic regression model was applied to assess the association between dietary acid load (PRAL and NEAP) and the odds of hyperuricemia. Dietary acid load was divided into four level: the first quartile (Q1), the second quartile (Q2), the third quartile (Q3), and the fourth quartile (Q4), in which each group has an approximately same number of participants. Q1 was considered as a reference group and odd ratios (ORs) and the correspondent confidence intervals (CIs) of Q2, Q3 and Q4 were computed. Additionally, possible risk factors, such as age and sex, as well as multiply variables (age, sex, BMI, diastolic blood pressure, systolic blood pressure, smoking, drinking and chronic disease) were put in the Logistic regression model to obtain adjusted effects. Statistical analysis was conducted in R language (version 3.5.1, R Core Development Team). Forest plots were produced based on R language software package named FORESTPLOT. In this study, a p-value < 0.05 was perceived as statistical significance.
Results
Characteristic of participants
A total of 290 participants were eligible in this study, in which there were 143 cases (diagnosed with hyperuricemia) and 147 controls (without hyperuricemia). Participant selection was exhibited in flowing chart (Fig. 1). In the case group, male participant was accounting for 53.85 and 55.78% in control group. An average age in the case group was 43.29 ± 12.93 years and 42.91 ± 10.44 years in the control group. Except for serum uric acid, other baseline variables including age, male proportion, weight, height, BMI, diastolic blood pressure, systolic blood pressure, heart rate, smoking, drinking and chronic disease, were lack of material significance between participants in case group and control group (p-value < 0.05). Characteristic of participants was summarized in Table 1.
Food consumption, nutrients intake and dietary acid load
As respect to food groups, vegetable consumption and fruit consumption in the case group were material lower than in the control group (p-value < 0.05). Fish consumption in case group were material higher than in control group (p-value < 0.05). In terms of other food groups including grains, potato, soy, red meat, poultry, dairy, egg, beverages and edible oil, there were lack of significant differences between participants in case group and control group, shown in Table 2.
As for nutrients intake including carbohydrate, fat, protein, sodium, magnesium, phosphorus, magnesium and calcium, there were lack of statistically significant difference between participants in case group and control group, shown in Table 2.
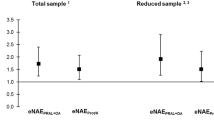
PRAL in the case group was significantly higher than the control group. In addition, NEAP in the case group was also higher than in the control group, shown in Table 2 and Fig. 2.
The association between PRAL and hyperuricemia
Higher PRAL was found to be associated with hyperuricemia. Crude ORs of hyperuricemia for Q2, Q3 and Q4 of PRAL were 2.74 (95%CI: 1.94 ~ 3.88, p-value: 0.004), 2.90 (95%CI: 2.05 ~ 4.10, p-value: 0.002) and 3.14 (95%CI: 2.22 ~ 4.45, p-value: 0.001), respectively and p-value for trend was < 0.001. The positively significant associations were remained after adjusting for age and sex, or for multiple variables, shown in Table 3 and Fig. 3.
Association between NEAP and hyperuricemia
There was a positive association between elevated NEAP and hyperuricemia. Crude OR of hyperuricemia for Q2 was not material significance (OR:1.54, 95%CI: 0.93 ~ 2.53, p-value: 0.210), however, ORs of hyperuricemia for Q3 (OR: 2.40, 95%CI: 1.70 ~ 3.38, p-value: 0.011) and Q4 (OR: 3.27, 95%CI: 2.31 ~ 4.62, p-value: 0.001) were statistically significant. P-value for trend was < 0.001 indicative of a dose-response relationship between NEAP and hyperuricemia. After adjusting for age and sex, or for multiple variables, the positive associations did not fluctuate largely, shown in Table 4 and Fig. 4.
Discussion
To our best understanding, this is the first study to explore the association between dietary acid load and hyperuricemia in mainland China. Results from the current study showed a positive association between higher dietary acid load and hyperuricemia. We applied two assessment indicators of dietary acid load including PRAL and NEAP, and the results were consistent. Our results were relative robustness as they were lack of large fluctuations after adjusting a broad of possible risk factors as we could. Since the prevalence of hyperuricemia in Chinese adults is increasing continuously, advocation of well-balanced diet is urgently needed to prevent and control the disease. In addition, we also identified vegetable and fruit consumption were lower in participants with hyperuricemia, but fish consumption higher. Patients with hyperuricemia should eat more vegetable and fruit but less rich-purines foods, such fish, on the premise of dietary diversity.
According to previous reports, high dietary acid load is characterized by both a high consumption of acid precursors and a low intake of base precursors [21]. Acid precursors are mainly from phosphorus and proteins, which have rich sulfur-containing amino acids, while base precursors are largely derived from potassium, magnesium, calcium etc. [23] The aforementioned reveals that high dietary acid load may attribute to a diet pattern with rich in animal-derived food but low in plant-derived food. Taking the diet pattern with high acid load in a long term might change the acid–base balance to acidosis [28]. Even though the potential mechanisms involved in dietary acid load and the risk of hyperuricemia is indeterminate, a previous study indicated that an alkaline urine pH would help excrete uric acid excretion, but not an acidic urine pH [29].
Other studies also exhibited a positive relationship between higher dietary acid load and risk of hyperuricemia. Dayeon et al. [29], found a 21% higher risk for hyperuricemia in middle-aged and older Korean adults with the highest quartile of PRAL, and a 17% higher risk in NEAP according to the Korean Genome and Epidemiology Study [29]. Another cross-sectional study conducted in German and with 6894 adults also identified a significant association between higher dietary acid load and risk of hyperuricemia [30, 31]. The German adults with lower PRAL were found to have a lower odd for hyperuricemia (OR: 0.60; 95% CI: 0.43, 0.83). Our study was in accord with these findings [30, 31]. Even though these studies were observational design, the effect of the higher dietary acid load on chronic diseases cannot be ignored.
There are some limitations in this study which should be notably taken into consideration. Firstly, this study was based on case-control design, which cannot avoid biases, such as recalling bias. Food consumption frequency and amount of some kinds of food might exist deviations with reality or error reporting, in particular in the senior citizens who had impaired memory. FFQ including 12 food groups was applied for food consumption survey, while there must be large bias of the estimation of nutrients intake as the nutrients content of specific foods within the same group may vary greatly, causing inaccurate estimates of nutrients. Secondly, a case-control study could only provide clues to the cause of hyperuricemia and cannot draw a causal conclusion. Thirdly, only adult participants were included in this study, indicative of limited extrapolation of the results. Hyperuricemia is commonly acknowledged as a chronic disease. Even though the age of onset of hyperuricemia trends to be younger, the majority of cases are still among adults, especially those middle-aged and elderly group. Fourthly, the dose-response relationship between dietary acid load and hyperuricemia was less understood. Although we gave p-value for trend according to Logistic regression analysis in different group divided by level of dietary acid load, analysis of dose-response relationship was not available as the limited number of participants in this study.
Two aspects should be noted in the future study. The first one is that prospective studies are urgently needed to verify the association between dietary acid load and the risk of hyperuricemia and its possible mechanisms should be also uncovered. The second one is that dose-response relationship between dietary acid load and hyperuricemia should be further studied.
Conclusion
This study based on case-control design identified a significantly positive association between higher dietary acid load and the odds of hyperuricemia in Chinese adults. Both of PRAL and NEAP had consistent results, indicative of advocation of a well-balanced diet in Chinese population. Given the limitation of case-control study, further prospective studies with large population are urgently needed.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Li Q, Li X, Wang J, et al. Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements [J]. BMJ Open. 2019;9(8):e26677.
Kaneko K, Aoyagi Y, Fukuuchi T, et al. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia [J]. Biol Pharm Bull. 2014;37(5):709–21.
Shen X, Wang C, Liang N, et al. Serum metabolomics identifies dysregulated pathways and potential metabolic biomarkers for hyperuricemia and gout [J]. Arthritis Rheumatol. 2021;73(9):1738–48.
Lai X, Gao B, Zhou G, et al. The association between baseline, changes in uric acid, and renal failure in the elderly Chinese individuals: a prospective study with a 3-year follow-up [J]. Int J Endocrinol. 2022;2022:4136373.
Liu L, Zhang X, Peng L, et al. Hyperuricemia is related to the risk of cardiovascular diseases in ethnic Chinese elderly women [J]. Glob Heart. 2022;17(1):12.
Li Y, Zhu B, Xie Y, et al. Effect modification of hyperuricemia, cardiovascular risk, and age on chronic kidney disease in China: a cross-sectional study based on the China health and nutrition survey cohort [J]. Front Cardiovasc Med. 2022;9:853917.
Zhang L, An K, Mou X, et al. Effect of urate-lowering therapy on the progression of kidney function in patients with asymptomatic hyperuricemia: a systematic review and Meta-analysis [J]. Front Pharmacol. 2021;12:795082.
Wang M, Wang C, Zhao M, et al. Uric acid variability and all-cause mortality: a prospective cohort study in northern China [J]. J Nutr Health Aging. 2021;25(10):1235–41.
Masulli M, D'Elia L, Angeli F, et al. Serum uric acid levels threshold for mortality in diabetic individuals: the URic acid right for heArt health (URRAH) project [J]. Nutr Metab Cardiovasc Dis. 2022.
Mortada I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: an emerging association [J]. Curr Hypertens Rep. 2017;19(9):69.
Donkeng M, Kuaté D, Koudjou PN, et al. Association between hyperuricemia and glycated hemoglobin in type 2 diabetes at the district Hospital of Dschang [J]. Pan Afr Med J. 2021;40:177.
Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors [J]. Nat Rev Rheumatol. 2020;16(7):380–90.
Chen-Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: the National Health and nutrition examination survey, 2007-2016[J]. Arthritis Rheumatol. 2019;71(6):991–9.
Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and Meta-analysis [J]. Biomed Res Int. 2015;2015:762820.
Zhang M, Zhu X, Wu J, et al. Prevalence of hyperuricemia among Chinese adults: findings from two nationally representative cross-sectional surveys in 2015-16 and 2018-19[J]. Front Immunol. 2021;12:791983.
Li M, Shi Z. Ultra-processed food consumption associated with overweight/obesity among Chinese adults-results from China health and nutrition survey 1997-2011[J]. Nutrients. 2021;13(8).
Xue H, Wu Y, Wang X, et al. Time trends in fast food consumption and its association with obesity among children in China [J]. PLoS One. 2016;11(3):e151141.
Aihemaitijiang S, Zhang Y, Zhang L, et al. The association between purine-rich food intake and hyperuricemia: a cross-sectional study in Chinese adult residents [J]. Nutrients. 2020;12(12).
Li X, Song P, Li J, et al. Relationship between hyperuricemia and dietary risk factors in Chinese adults: a cross-sectional study [J]. Rheumatol Int. 2015;35(12):2079–89.
Gao Y, Cui LF, Sun YY, et al. Adherence to the dietary approaches to stop hypertension diet and hyperuricemia: a cross-sectional study [J]. Arthritis Care Res (Hoboken). 2021;73(4):603–11.
Mozaffari H, Siassi F, Guilani B, et al. Association of dietary acid-base load and psychological disorders among Iranian women: a cross-sectional study [J]. Complement Ther Med. 2020;53:102503.
Ronco AL, Martínez-López W, Calderón JM, et al. Dietary acid load and lung cancer risk: a case-control study in men [J]. Cancer Treat Res Commun. 2021;28:100382.
Mirmiran P, Houshialsadat Z, Bahadoran Z, et al. Dietary acid load and risk of cardiovascular disease: a prospective population-based study [J]. BMC Cardiovasc Disord. 2021;21(1):432.
Mohammadifard N, Karimi G, Khosravi A, et al. High dietary acid load score is not associated with the risk of metabolic syndrome in Iranian adults [J]. Int J Vitam Nutr Res. 2021;91(1-2):152–63.
Parohan M, Sadeghi A, Nasiri M, et al. Dietary acid load and risk of hypertension: a systematic review and dose-response meta-analysis of observational studies [J]. Nutr Metab Cardiovasc Dis. 2019;29(7):665–75.
Bardin T, Richette P. Definition of hyperuricemia and gouty conditions [J]. Curr Opin Rheumatol. 2014;26(2):186–91.
Huang L, Wang H, Wang Z, et al. Regional disparities in the association between cereal consumption and metabolic syndrome: results from the China health and nutrition survey [J]. Nutrients. 2019;11(4).
Abbasalizad FM, Vajdi M, Najafi M. Dietary acid load significantly predicts 10-years survival in patients underwent coronary artery bypass grafting (CABG) surgery [J]. PLoS One. 2019;14(10):e223830.
Shin D, Lee KW. Dietary acid load is positively associated with the incidence of hyperuricemia in middle-aged and older Korean adults: findings from the Korean genome and epidemiology study [J]. Int J Environ Res Public Health. 2021;18(19).
Esche J, Krupp D, Mensink G, et al. Dietary potential renal acid load is positively associated with serum uric acid and odds of hyperuricemia in the German adult population [J]. J Nutr. 2018;148(1):49–55.
Esche J, Krupp D, Mensink GB, et al. Estimates of renal net acid excretion and their relationships with serum uric acid and hyperuricemia in a representative German population sample [J]. Eur J Clin Nutr. 2020;74(Suppl 1):63–8.
Acknowledgements
We would like to thank Dr. Wei-xia Huang, chief physician of Wenzhou hospital of traditional Chinese medicine affiliated to Zhejiang Chinese medicine university, for her careful guidance for topic selection and experiment design.
Funding
NA.
Author information
Authors and Affiliations
Contributions
R.R.C. and S.S.S. contributed to study concept and design. S.S.S. contributed to literature review and manuscript writing. C.Z.L., Y.F.Z., C.C. and Q.J.W. contributed to data collection and analysis. C.Z.L. and Y.F.Z. contributed to data collection. All authors approved the final version of the article for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee approval was obtained from Wenzhou hospital of traditional Chinese medicine affiliated to Zhejiang Chinese medicine university. This study was performed in line with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not Applicable.
Competing interests
NA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shao, Ss., Lin, Cz., Zhu, Yf. et al. Higher dietary acid load is associated with hyperuricemia in Chinese adults: a case-control study. BMC Endocr Disord 22, 286 (2022). https://doi.org/10.1186/s12902-022-01192-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01192-3