Abstract
Background
Abnormal hematologic parameters before patients undergoing prostate biopsy play a pivotal role in guiding the surgical management of prostate cancer (PCa) incidence. This study aims to establish the first nomogram for predicting PCa risk for better surgical management.
Methods
We retrospectively reviewed and analyzed the data including basic information, preoperative hematologic parameters, and imaging examination of 540 consecutive patients who underwent transrectal ultrasound (TRUS)-guided prostate biopsy for elevated prostate-specific antigen (PSA) in our medical center between 2017 and 2021. Logistic regression analysis was used to determine the risk factors for PCa occurrence, and the nomogram was constructed to predict PCa occurrence. Finally, the data including 121 consecutive patients in 2022 were prospectively collected to further verify the results.
Results
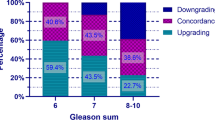
In retrospective analyses, univariate and multivariate logistic analyses identified that three variables including age, diabetes, and De Ritis ratio (aspartate transaminase/alanine transaminase, AST/ALT) were determined to be significantly associated with PCa occurrence. A nomogram was constructed based on these variables for predicting the risk of PCa, and a satisfied predictive accuracy of the model was determined with a C-index of 0.765, supported by a prospective validation group with a C-index of 0.736. The Decision curve analysis showed promising clinical application. In addition, our results also showed that the De Ritis ratio was significantly correlated with the clinical stage of PCa patients, including T, N, and M stages, but insignificantly related to the Gleason score.
Conclusions
The increased De Ritis ratio was significantly associated with the risk and clinical stage of PCa and this nomogram with good discrimination could effectively improve individualized surgical management for patient underdoing prostate biopsy.


Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript.
Abbreviations
- PCa:
-
Prostate cancer
- BPH:
-
Benign prostatic hyperplasia
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PLR:
-
Platelet-to-lymphocyte ratio
- PSA:
-
Prostate-specific antigen
- De Ritis ratio:
-
Aspartate transaminase/alanine transaminase
- SD:
-
Standard Deviation
- OR:
-
Odds ratio
- DCA:
-
Decision curve analysis
- AUC:
-
Areas under the receiver-operating characteristic curve
References
Sung H, Ferlay J, Siegel RL (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Pernar CH, Ebot EM, Wilson KM, Mucci LA (2018) The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med 8(12):a030361
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V et al (2014) Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 384(9959):2027–2035
Daniyal M, Siddiqui ZA, Akram M, Asif HM, Sultana S, Khan A (2014) Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac J Cancer Prev 15(22):9575–9578
Hayes JH, Barry MJ (2014) Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 311(11):1143–1149
Kearns JT, Faino AV, Newcomb LF, Brooks JD, Carroll PR, Dash A et al (2018) Role of surveillance biopsy with no cas a prognostic marker for reclassification: results from the Canary Prostate Active Surveillance Study. Eur Urol 73(5):706–712
Welch HG, Albertsen PC (2009) Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst 101(19):1325–1329
Miotto A Jr, Srougi M, Brito GA, Leite KM, Nesrallah AJ, Ortiz V (2004) Value of various PSA parameters for diagnosing prostate cancer in men with normal digital rectal examination. Int Braz J Urol 30(2):109–113
Wang S, Ji Y, Chen Y, Du P, Cao Y, Yang X et al (2021) The Values of systemic immune-inflammation index and neutrophil-lymphocyte ratio in the localized prostate cancer and Benign prostate hyperplasia: a retrospective clinical study. Front Oncol 11:812319
Wang H, Fang K, Zhang J, Jiang Y, Wang G, Zhang H et al (2017) The significance of De Ritis (aspartate transaminase/alanine transaminase) ratio in predicting pathological outcomes and prognosis in localized prostate cancer patients. Int Urol Nephrol 49(8):1391–1398
Sansa A, Venegas MDP, Valero C (2021) The aspartate aminotransaminase/alanine aminotransaminase (De Ritis) ratio predicts sensitivity to radiotherapy in head and neck carcinoma patients. Head Neck 43(7):2091–2100
Gorgel SN, Kose O, Koc EM, Ates E, Akin Y, Yilmaz Y (2017) The prognostic significance of preoperatively assessed AST/ALT (De Ritis) ratio on survival in patients underwent radical cystectomy. Int Urol Nephrol 49(9):1577–1583
Zhou J, He Z, Ma S, Liu R (2020) AST/ALT ratio as a significant predictor of the incidence risk of prostate cancer. Cancer Med 9(15):5672–5677
Röhnisch HE, Kyrø C, Olsen A, Thysell E, Hallmans G, Moazzami AA (2020) Identification of metabolites associated with prostate cancer risk: a nested case-control study with long follow-up in the Northern Sweden Health and Disease Study. BMC Med 18(1):187
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B et al (2014) Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab 19(3):393–406
Huang X, Liu G, Guo J, Su Z (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14(11):1483–1496
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S et al (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A 104(49):19345–19350
Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ (2009) Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res 69(20):7986–7993
Elf SE, Chen J (2014) Targeting glucose metabolism in patients with cancer. Cancer 120(6):774–780
Sookoian S, Pirola CJ (2015) Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol 21(3):711–725
Acknowledgements
Not applicable.
Funding
The authors declare that no funding was used for this study from any sources.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interest.
Ethical approval
This study was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and received approval from the ethics committee of the Second Affiliated Hospital of Chongqing Medical University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, YD., Ren, Zj., Gou, YQ. et al. Development and validation of a model for predicting the risk of prostate cancer. Int Urol Nephrol 56, 973–980 (2024). https://doi.org/10.1007/s11255-023-03837-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03837-1