Abstract
Purpose
To develop and internally validate nomograms to help choose the optimal biopsy strategy among no biopsy, targeted biopsy (TB) only, or TB plus systematic biopsy (SB).
Patients and Methods
This retrospective study included a total of 385 patients who underwent magnetic resonance imaging (MRI)-guided TB and/or SB at our institute after undergoing multiparametric MRI (mpMRI) between 2015 and 2018. We developed models to predict clinically significant prostate cancer (csPCa) based on suspicious lesions from a TB result and based on the whole prostate gland from the results of TB plus SB or SB only. Nomograms were generated using logistic regression and evaluated using receiver-operating characteristic (ROC) curve analysis, calibration curves and decision analysis. The results were validated using ROC curve and calibration on 177 patients from 2018 to 2019 at the same institute.
Results
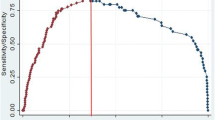
In the multivariate analyses, prostate-specific antigen level, prostate volume, and the Prostate Imaging Reporting and Data System score were predictors of csPCa in both nomograms. Age was also included in the model for suspicious lesions, while obesity was included in the model for the whole gland. The area under the curve (AUC) in the ROC analyses of the prediction models was 0.755 for suspicious lesions and 0.887 for the whole gland. Both models performed well in the calibration and decision analyses. In the validation cohort, the ROC curve described the AUCs of 0.723 and 0.917 for the nomogram of suspicious lesions and nomogram of the whole gland, respectively. Also, the calibration curve detected low error rates for both models.
Conclusion
Nomograms with excellent discriminative ability were developed and validated. These nomograms can be used to select the optimal biopsy strategy for individual patients in the future.







Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–137.
Thompson JE, Moses D, Shnier R, et al. Multiparametric magnetic resonance imaging guided diagnostic biopsy detects significant prostate cancer and could reduce unnecessary biopsies and over detection: a prospective study. J Urol. 2014;192(1):67–74.
C.Weinreb. J, O.Barentsz. J, L.Choyke. P, et al. PI-RADS prostate imaging—reporting and data system: 2015. Version 2. Eur Urol. 2016;69(1):16–40.
Abd-Alazeez M, Kirkham A, Ahmed HU, et al. Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies as the reference standard. Prostate Cancer Prostatic Dis. 2014;17(1):40–6.
Porpiglia F, Manfredi M, Mele F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer. Eur Urol. 2017;72(2):282–8.
Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65(4):809–15.
Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–22.
Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–77.
Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer. 2016;122(6):884–92.
Calio BP, Sidana A, Sugano D, et al. Risk of upgrading from prostate biopsy to radical prostatectomy pathology—does saturation biopsy of index lesion during multiparametric magnetic resonance imaging-transrectal ultrasound fusion biopsy help? J Urol. 2018;199(4):976–82.
Hofbauer SL, Maxeiner A, Kittner B, et al. Validation of prostate imaging reporting and data system version 2 for the detection of prostate cancer. J Urol. 2018;200(4):767–73.
Radtke JP, Kuru TH, Boxler S, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol. 2015;193(1):87–94.
Baco E, Rud E, Eri LM, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol. 2016;69(1):149–56.
Sathianathen NJ, Warlick CA, Weight CJ, et al. A clinical prediction tool to determine the need for concurrent systematic sampling at the time of magnetic resonance imaging-guided biopsy. BJU Int. 2018;123:612–7.
van Leeuwen PJ, Hayen A, Thompson JE, et al. A multiparametric magnetic resonance imaging-based risk model to determine the risk of significant prostate cancer prior to biopsy. BJU Int. 2017;120(6):774–81.
Truong M, Wang B, Gordetsky JB, et al. Multi-institutional nomogram predicting benign prostate pathology on magnetic resonance/ultrasound fusion biopsy in men with a prior negative 12-core systematic biopsy. Cancer. 2018;124(2):278–85.
Yudong Cao, Min Cao, Yuke Chen, et al. The combination of prostate imaging reporting and data system version 2 (PI-RADS v2) and periprostatic fat thickness on multi-parametric MRI to predict the presence of prostate cancer. Oncotarget. 2017;8:44040–9.
Lee SM, Liyanage SH, Wulaningsih W, et al. Toward an MRI-based nomogram for the prediction of transperineal prostate biopsy outcome: a physician and patient decision tool. Urol Oncol. 2017;35(11):664e611–8.
Guo LH, Wu R, Xu HX, et al. Comparison between ultrasound guided transperineal and transrectal prostate biopsy: a prospective, randomized, and controlled trial. Sci Rep. 2015;5:16089.
Singh PB, Anele C, Dalton E, et al. Prostate cancer tumour features on template prostate-mapping biopsies: implications for focal therapy. Eur Urol. 2014;66(1):12–9.
Osses D, van Asten J, Kieft GD, Tijsterman J. Prostate cancer detection rates of magnetic resonance imaging-guided prostate biopsy related to prostate imaging reporting and data system score. World J Urol. 2017;35:207–12.
Hamoen EHJ, de Rooij M, Alfred Witjes J, Barentsz J, M Rovers M. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol. 2014;67:1112–21.
Venderink W, van Luijtelaar A, Bomers JG, et al. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol. 2017;73:353–60.
Pepe P, Garufi A, Priolo GD, Galia A, Fraggetta F, Pennisi M. Is it time to perform only magnetic resonance imaging targeted cores? our experience with 1,032 men who underwent prostate biopsy. J Urol. 2018;200(4):774–8.
Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology. 2013;81(6):1142–6.
Scattoni V, Roscigno M, Raber M, et al. Initial extended transrectal prostate biopsy—are more prostate cancers detected with 18 cores than with 12 cores? J Urol. 2008;179(4):1327–31 (discussion 1331).
Abd TT, Goodman M, Hall J, et al. Comparison of 12-core versus 8-core prostate biopsy: multivariate analysis of large series of US veterans. Urology. 2011;77(3):541–7.
Puech P, Rouvière O, Renard-Penna R, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy—prospective multicenter study. Radiology. 2013;268(2):461–9.
Wegelin O, Exterkate L, van der Leest M, et al. The FUTURE trial: a multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur Urol. 2018;75:582–90.
Radtke JP, Wiesenfarth M, Kesch C, et al. Combined clinical parameters and multiparametric magnetic resonance imaging for advanced risk modeling of prostate cancer-patient-tailored risk stratification can reduce unnecessary biopsies. Eur Urol. 2017;72(6):888–6.
Acknowledgement
This work was supported by the Program for Chinese National Natural Science Fund (81602220) and Chinese Shanghai Municipal Planning Commission of science and Research Fund (201540182).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
None of the authors have any conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, BM., Shi, ZK., Li, HS. et al. A Novel Prediction Tool Based on Multiparametric Magnetic Resonance Imaging to Determine the Biopsy Strategy for Clinically Significant Prostate Cancer in Patients with PSA Levels Less than 50 ng/ml. Ann Surg Oncol 27, 1284–1295 (2020). https://doi.org/10.1245/s10434-019-08111-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08111-2