Abstract
Background
Membranous nephropathy (MN) and IgA nephropathy (IgAN) are the most common primary glomerulopathies worldwide. The systemic metabolic changes in the progression of MN and IgAN are not fully understood.
Methods
A total of 87 and 70 patients with MN and IgAN, respectively, and 30 healthy controls were enrolled in this study. Untargeted metabolomics was performed to explore the differential metabolites and metabolic pathways in the early stage of MN and IgAN. To judge the diagnostic ability of biomarkers, receiver operating characteristic curve analysis (ROC) were performed.
Results
Principal component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA) suggested that patients with MN and IgAN showed an obvious separation trend from the healthy controls. In addition, 155 and 148 metabolites were identified to be significantly altered in the MN and IgAN groups, respectively. Of these, 70 metabolites were markedly altered in both disease groups; six metabolites, including L-tryptophan, L-kynurenine, gamma-aminobutyric acid (GABA), indoleacetaldehyde, 5-hydroxyindoleacetylglycine, and N-alpha-acetyllysine, showed the opposite tendency. The most affected metabolic pathways included the amino acid metabolic pathways, citrate cycle, pantothenate and CoA biosynthesis, and hormone signaling pathways.
Conclusions
Substantial metabolic disorders occurred during the progression of MN and IgAN. L-tryptophan, L-kynurenine, GABA, indoleacetaldehyde, 5-hydroxyindoleacetylglycine, and N-alpha-acetyllysine may show potential as biomarkers for the identification of MN and IgAN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is one of the leading causes of death worldwide. In China, the incidence of CKD has reached 10.8%, and the number is still rapidly growing [1]. Primary glomerular diseases, including membranous nephropathy (MN) and IgA nephropathy (IgAN), are among the leading causes of CKD [2]. Early stages of MN and IgAN typically exhibit an asymptomatic onset with nephrotic-range proteinuria, hypoalbuminemia, edema, and hyperlipidemia. However, these symptoms are neither specific nor sensitive enough for early diagnosis of primary glomerulopathy and still require histopathological evaluation by kidney biopsy [3]. To better determine disease diagnosis and guide the course of treatment, clarifying disease-related metabolic changes and screening potential biomarkers are crucial.
The rapid development of high-throughput technologies, including high-resolution mass spectrometry, sequencing technologies, and microarray, has significantly advanced our understanding of the discovery of biomarkers for primary glomerulopathy. Metabolomics is a newly emerging field of omics research that aims to study global metabolic metabolites in biological systems [4]. In general, the expression of upstream genes and proteins can be amplified by downstream metabolite signals, which makes it easier to detect differences by analyzing metabolite profiling [5]. Therefore, we used a UPLC-Q/Orbitrap-HRMS-based untargeted metabolomics to systematically explore the differential metabolites and metabolic pathways in the early stage of MN and IgAN compared with the healthy population. Furthermore, receiver operating characteristic (ROC) curve analysis was used to judge the diagnostic ability of potential biomarkers.
Methods
Study population
From March 2018 to September 2020, 87 and 70 patients with biopsy-proven primary idiopathic MN and biopsy-proven IgAN, respectively, at the Second Affiliated Hospital of Soochow University were enrolled in this study. Renal specimens were evaluated using direct immunofluorescence, light microscopy, and electron microscopy. Patients with secondary forms of glomerulopathy, including hepatitis B viral infection, lupus nephritis, and tumor, were excluded. Only angiotensin receptor blockers (ARBs) were given to a subset of patients before serum sample collection for reducing urine protein excretion. None of these patients received immunosuppressive medications or other therapeutic agents before the diagnosis was established. The corresponding immunosuppressive agents, including methylprednisolone, cyclophosphamide, tripterygium, tacrolimus, cyclosporin, and leflunomide, were administered immediately after the diagnosis was established. Moreover, 30 healthy adult volunteers (HC group) were enrolled in this metabolomics study. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University.
Sample collection, preparation, and UPLC-Q/Orbitrap-HRMS analysis
To prepare the serum, 5 mL of fasting blood samples collected from the patients and healthy adult volunteers were centrifuged at 2,500 g for 10 min at 4 °C. All serum samples were immediately stored in a freezer at − 80 °C for further processing.
Next, 400 µL of methanol was added to 100 µL of serum sample in an EP tube. The mixture was vortexed for 1 min and centrifuged at 15,000 g for 10 min at 4 °C. The supernatant was subsequently transferred into another EP tube and evaporated to dry using a vacuum centrifugal concentrator. The residue was resuspended in 150 μL of 80% methanol and filtered through a 0.22-µm nylon syringe filter.
The separation of the target compounds was performed on a Waters ACQUITY UPLC HSS T3 (2.1 × 150 mm, 1.8 µm) liquid chromatography column at 40 °C with an ACQUITY UPLC CSH C18 VanGuard Pre-column (2.1 × 5 mm, 1.7 µm) using a Dionex Ultimate 3000 UPLC system. In positive ion mode, the mobile phase contained 0.1% aqueous formic acid and 0.1% formic acid in acetonitrile. In negative ion mode, the mobile phase contained a 5-mM ammonium formate aqueous buffer and acetonitrile. The mobile phase flow rate was 0.25 mL/min, and the injection volume was 5 µL in both positive and negative ion modes. The Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, USA) equipped with an ESI interface was applied for mass spectrometry analysis. The detailed gradient elution conditions and the optimal mass spectrometry parameters were the same as previously described [6]. All mass spectrometry spectra were acquired and analyzed using the Xcalibur 4.0 software (Thermo Fisher Scientific).
Data processing and metabolite identification
The data processing and metabolite identification progress was performed as described in our previous study [6]. After the raw data files were converted into an mzXML format using the ProteoWizard software (v3.0.8789), the freely available XCMS software was used to perform peak identification, filtration, alignment, and integration. The three-dimensional data matrix, including retention time, mass-to-charge ratio, and intensity, was converted into a table for further process analysis. To compare the data of different magnitudes, the peak area of the data was batch normalized before multivariate statistical analysis. Subsequently, to perform principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA), the data were uploaded into SIMCA-P 14.0. Autoscaling was used in all the models to acquire more scientific, reliable, and intuitive results. PCA analysis is an unsupervised multivariate statistical method that can reflect the original state of metabolomic data. OPLS-DA is a multivariate statistical analysis method with supervised pattern recognition, which can effectively propose the effects unrelated to the study, to screen the differential metabolites. To select the potential metabolites in the study, the variable importance in the project values (VIP) of the OPLS-DA model and p values were calculated as a threshold value (VIP > 1 combined with p < 0.05). These potential metabolites were subsequently subjected to pathway analysis performed through METLIN (http://metlin.scripps.edu/), MoNA (https://mona.fiehnlab.ucdavis.edu/), and BioDeepDB (https://query.biodeep.cn/). To elucidate metabolic differences between the MN and IgAN groups, the identified metabolites were compared. Moreover, to judge the diagnostic ability of biomarkers, ROC curve analyses were performed using MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/).
Results
Clinicopathological characteristics of patients
In this study, 87 and 70 patients with MN and IgAN, respectively, and 30 healthy adult volunteers were enrolled to determine metabolic changes in the progression of MN and IgAN. The baseline characteristics of the healthy volunteers and the patients before renal biopsies are presented in Table 1. The pathological features of patients are shown in Supplementary Material.
Multivariate statistical analysis
To screen for the specific metabolites in the development and progression of idiopathic MN, PCA and OPLS-DA were used for grasping the overall situation and identifying inter-group differences in the data, respectively. As shown in Figs. 1 and 2, the MN and IgAN groups showed an obvious separation trend from the healthy controls. The following were the R2Y (represents the seed setting rate) and Q2 (represents the prediction ability of the model) values of the OPLS-DA model in this study: MN/HC-ESI(+), R2Y = 0.980, Q2 = 0.956; MN/HC-ESI(−), R2Y = 0.987, Q2 = 0.944; IgAN/HC-ESI( +), R2Y = 0.993, Q2 = 0.961; IgAN/HC-ESI(−), R2Y = 0.987, Q2 = 0.920. These results showed that the models had a strong explanatory ability and could be reliably used to screen for differential metabolites.
Multivariate data analysis of the membranous nephropathy (MN) and healthy control (HC) groups. A Principal component analysis (PCA) score map for the positive ion mode, B PCA score map for the negative ion mode, C Orthogonal partial least squares discriminant analysis (OPLS-DA) score map for the positive ion mode, and D OPLS-DA score map for the negative ion mode
Metabolite screening in untargeted metabolomics analysis
Using VIP > 1.0 and p < 0.05 as the thresholds in our study, 155 and 148 metabolites were identified to be significantly altered in the MN and IgAN groups, respectively. In detail, 71 metabolites were significantly increased and 84 metabolites were significantly decreased in the MN group, whereas 85 metabolites were significantly increased and 63 metabolites were significantly decreased in the IgAN group. Furthermore, the results indicated that 70 metabolites were markedly altered in both disease groups. Detailed information on these metabolites is shown in Table 2.
Metabolic pathway analysis
To further search for the metabolic pathways that potential biomarkers may participate in, MetaboAnalyst 5.0 was used to analyze the potential metabolite data obtained above. As shown in Fig. 3, a total of 11 pathways were identified to be statistically significant (p < 0.05), which may be potentially associated with MN, including (1) phenylalanine metabolism; (2) tyrosine metabolism; (3) phenylalanine, tyrosine, and tryptophan biosynthesis; (4) valine, leucine, and isoleucine biosynthesis; (5) prolactin signaling pathway; (6) citrate cycle; (7) arginine and proline metabolism; (8) tryptophan metabolism; (9) pantothenate and CoA biosynthesis; (10) ovarian steroidogenesis; and (11) D-arginine and D-ornithine metabolism. Meanwhile, six pathways were identified to be statistically significant in the IgAN group, including (1) phenylalanine metabolism; (2) tyrosine metabolism; (3) valine, leucine, and isoleucine biosynthesis; (4) prolactin signaling pathway; (5) arginine and proline metabolism; and (6) arginine biosynthesis.
Identification of potential biomarkers for MN and IgAN
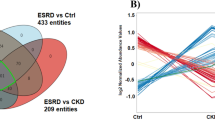
Six metabolites exerted the opposite tendency in the MN and IgAN groups, including L-tryptophan, L-kynurenine, GABA, indoleacetaldehyde, 5-hydroxyindoleacetylglycine, and N-alpha-acetyllysine (Fig. 4). The values of the area under the ROC curve were 0.867, 0.941, 0.896, 0.884, 0.943, and 0.891, respectively, suggesting the high predictive performance of these metabolites for the identification of MN and IgAN (Fig. 5).
Discussion
Metabolomics has become an important diagnostic tool that can directly reflect the state of organisms [7]. Compared with conventional identification methods, such as biopsy, metabolomics has the advantage that the contents of various metabolites can be analyzed and distinguished from disease populations [8]. To investigate the metabolic changes during the progression of MN and IgAN, untargeted metabolomics research was first performed. The results showed that 155 and 148 metabolites were identified to be significantly altered in the MN and IgAN groups, respectively. Through further metabolic pathway analysis, we observed that 11 and 6 metabolic pathways were significantly affected in the patients with MN and IgAN, including amino acid metabolic pathways, citrate cycle, pantothenate and CoA biosynthesis, and hormone signaling pathway. Here, we attempted to provide a detailed discussion on these affected metabolic pathways and compare the metabolic differences between the MN and IgAN groups.
Substantial metabolic disorders occurred during the progression of primary glomerulopathy; according to the metabolomics findings, amino acid metabolic pathways were the most affected. In humans, the kidneys play a significant role in the metabolism and reabsorption of amino acids. In our study, in the MN group, the relative contents of L-glutamic acid, L-valine, and L-leucine significantly increased, whereas those of L-cysteine, L-tryptophan, L-phenylalanine, and L-tyrosine significantly decreased. In the IgAN group, the relative contents of L-glutamic acid, L-valine, L-leucine, L-isoleucine, L-tryptophan, and L-proline significantly increased, whereas those of L-arginine, L-asparagine, and L-cysteine significantly decreased. The abnormal metabolism of amino acids may be caused by the increase in protein-decomposing metabolism or the change of energy demand in the inflammatory state. Therefore, the patients should acquire a low-protein diet containing sufficient essential amino acids, which can improve the imbalance of amino acid metabolism and subsequently increase protein synthesis.
The tricarboxylic acid (TCA) cycle is a common metabolic pathway in organisms, which is performed in the mitochondria of eukaryotic cells. It is not only the final metabolic pathway of the three nutrients (e.g., sugars, lipids, and amino acids) but also the link between them [9]. In this study, the relative contents of citric, fumaric, and succinic acids in the MN and IgAN groups decreased, suggesting that the TCA cycle is decelerated in patients with MN and IgAN. This result was consistent with the results of a previous study that patients with MN with more impaired kidney filtration function exhibited lower citric acid levels in serum [10]. In addition, the abnormal levels of citric, fumaric, and succinic acids suggest that mitochondrial function is damaged, which indirectly reflects the obvious oxidative stress in patients with MN. This may be the result of renal ischemia and hypoxia-induced cell necrosis, which is consistent with the previous results reported in vitro [11, 12].
Furthermore, our results showed that pantothenate and CoA biosynthesis was significantly affected during the progression of MN. Pantothenate is an essential vitamin as it is the pivotal precursor of CoA, which plays an essential role in the TCA cycle and energy metabolism [13]. The pantothenate and CoA biosynthesis pathway is actively involved in the inflammatory response and host defense by generating cysteamine. Cysteamine can directly inhibit the activity of γ-glutamylcysteine synthase, the rate-limiting enzyme involved in the metabolism from glutamate to γ-glutamy-L-cysteine (the precursor of glutathione) [14]. We observed a significant decrease in the γ-glutamy-L-cysteine level with a significant increase in the glutamate level, suggesting that glutathione biosynthesis was blocked during the progression of MN. Our study indicates that pantothenate and glutathione metabolism may play a significant role in MN development.
Moreover, the kidney is an essential site for the synthesis and degradation of several hormones [15, 16]. We observed that multiple hormone signaling pathways, including the prolactin signaling pathway, glucagon signaling pathway, and ovarian steroidogenesis, may participate in the pathogenesis of endocrine abnormalities in patients with MN and IgAN. The results indicate that various endocrine disorders may have occurred in the early stage of primary glomerulopathy.
In addition to these significantly affected metabolic pathways, the changes of specific metabolites must be paid attention to. We observed that a multitude of gut-derived protein-bound uremic toxins, including hippuric acid, kynurenine, indole-3-acetate, quinolinic acid, and spermidine, were all significantly downregulated in the early stage of MN. Similar results were also obtained in the IgAN group, wherein multiple uremic toxins, including hippuric acid, guanidinoacetate, and N-acetyl-L-phenylalanine, presented a significant decline. Previous studies reported that the accumulation of gut-derived protein-bound uremic toxins in patients with CKD was a critical risk factor for cardiovascular damage [17]. Therefore, we can infer that the disturbed metabolic processes in MN may also dynamically change as the disease develops. The damage caused by the elevated uremic toxin levels in these patients is not alarming, at least in the early stages of disease development. Moreover, our results showed that the levels of pyridoxic and homovanillic acids, which are two novel endogenous serum biomarkers of organic anion transporter (OAT)1 and OAT3, were all significantly upregulated in the MN and IgAN groups [18]. The abovementioned evidence indicated that the decline in the tubular secretion function may be earlier than the decline in the glomerular filtration function. Therefore, the use of drugs that are mainly eliminated by active secretion may deserve more attention in the early stage of primary glomerulopathy.
This study had several limitations. First, differences in the baseline characteristics of the patients in the two groups during sample collection were noted, making disease progression a confounding factor for differential diagnosis between the two groups. We considered propensity score matching for comparison; however, we did not perform it because of the small number of participants after matching. This study may truly differentiate patients with IgAN and primary MN from healthy controls; however, potential biomarkers are yet to be concluded. These biomarkers did not differentiate the aforementioned diseases from other kidney diseases, including CKD and secondary glomerular disease. Therefore, using these metabolites as alone markers might not be specific indicators for the clinical diagnosis. Still, further study will be needed. Furthermore, we were unable to control for diet among all patients, which might have an influence on the energy metabolism pathway and related metabolites.
The most significantly affected pathways were observed to be highly overlapped during the progression of MN and IgAN. To screen for potential biomarkers to distinguish between the MN and IgAN groups, metabolites, including tryptophan, kynurenine, GABA, indoleacetaldehyde, 5-hydroxyindoleacetylglycine, and N-alpha-acetyllysine, were observed to exert opposite tendency in the MN and IgAN groups compared with the healthy controls. The ROC curve analysis confirmed the potential diagnostic value of all six metabolites. Notably, four (e.g., tryptophan, kynurenine, indoleacetaldehyde, and 5-hydroxyindoleacetylglycine) of the six metabolites were associated with the tryptophan metabolism pathway. Further analysis suggested that the kynurenine pathway, the major (95%) catabolic route for tryptophan [19], was significantly downregulated in the MN group. Multiple intermediate or end-product metabolites of kynurenine, including 3-hydroxyanthranilic, quinolinic, and xanthurenic acids, significantly decreased in the MN group. In the IgAN group, apart from the increased tryptophan and kynurenine levels, no notable changes were observed in the levels of the metabolites of kynurenine. Existing studies had indicated that the disorder of tryptophan metabolism in IgAN patients might be associated with the disturbed intestinal microbiome. De Angelis’s study revealed that tryptophan levels increased with progressed disease stage of IgAN [20], which was corroborated with the findings in our study. Wu’s study also revealed that the conversion of tryptophan to kynurenine was enhanced in IgAN patients [21]. They proposed that the accumulation of kynurenine in the circulation system was related to the reduced renal function and the abnormal kynurenine metabolism caused by immune dysregulation and chronic inflammation in the host [21, 22]. It remained unclear how the progression of MN affected tryptophan metabolism. Our results indicated that tryptophan and its metabolites were potential biomarkers for discriminating between IgAN and MN. First, the patients in the IgAN group had worse renal function as compared to the patients in the MN group, which might lead to the reduced glomerular filtration of tryptophan and its metabolites. Meanwhile, there were significant differences in the gut microbial community composition and abundance between IgAN patients and MN patients, which might contribute to the difference in disorder of intestinal tryptophan–indole metabolic pathway [23, 24]. Moreover, considering the importance of the kynurenine pathway for its role in anti-inflammation in kidney disease [25], we inferred that the metabolic differences in this pathway might also be related to the difference in the pathological basis between the two glomerulopathies. However, there is currently scant evidence to support this conclusion. To further investigate the prognostic performance of these potential biomarkers, targeted metabolomics analysis will be performed in our future research.
Data availability
The data sets used during the current study are available from the corresponding author on reasonable request.
References
Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J et al (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379:815–822
Pippias M, Kramer A, Noordzij M, Afentakis N, de la Torre RA, Ambühl PM et al (2017) The European renal association—European dialysis and transplant association registry annual report 2014: a summary. Clin Kidney J 10:154–169
Krata N, Foroncewicz B, Zagożdżon R, Moszczuk B, Zielenkiewicz M, Pączek L et al (2021) Peroxiredoxins as markers of oxidative stress in IgA nephropathy, membranous nephropathy and lupus nephritis. Arch Immunol Ther Exp (Warsz) 70:3
Fiehn O (2002) Metabolomics–the link between genotypes and phenotypes. Plant Mol Biol 48:155–171
Rinschen MM, Ivanisevic J, Giera M, Siuzdak G (2019) Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol 20:353–367
Qu Y, Su C, Zhao Q, Shi A, Zhao F, Tang L et al (2022) Gut microbiota-mediated elevated production of secondary bile acids in chronic unpredictable mild stress. Front Pharmacol 13:837543
Oldiges M, Lütz S, Pflug S, Schroer K, Stein N, Wiendahl C (2007) Metabolomics: current state and evolving methodologies and tools. Appl Microbiol Biotechnol 76:495–511
Crutchfield CA, Thomas SN, Sokoll LJ, Chan DW (2016) Advances in mass spectrometry-based clinical biomarker discovery. Clin Proteomics 13:1
Akram M (2014) Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys 68:475–478
Gao X, Chen W, Li R, Wang M, Chen C, Zeng R et al (2012) Systematic variations associated with renal disease uncovered by parallel metabolomics of urine and serum. BMC Syst Biol 6(Suppl 1):S14
Thurman JM (2007) Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 123:7–13
Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE et al (2003) Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol Renal Physiol 284:F532–F537
Patassini S, Begley P, Xu J, Church SJ, Kureishy N, Reid SJ et al (2019) Cerebral vitamin B5 (D-Pantothenic Acid) deficiency as a potential cause of metabolic perturbation and neurodegeneration in Huntington’s disease. Metabolites 9(6):113
Ma T, Liu T, Xie P, Jiang S, Yi W, Dai P et al (2020) UPLC-MS-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and CoA biosynthesis pathway in diabetic kidney disease. Life Sci 258:118160
Valdivielso JM, Jacobs-Cachá C, Soler MJ (2019) Sex hormones and their influence on chronic kidney disease. Curr Opin Nephrol Hypertens 28:1–9
Kuczera P, Adamczak M, Wiecek A (2015) Endocrine abnormalities in patients with chronic kidney disease. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 36:109–118
Graboski AL, Redinbo MR (2020) Gut-derived protein-bound uremic toxins. Toxins (Basel) 12:590
Shen H, Nelson DM, Oliveira RV, Zhang Y, Mcnaney CA, Gu X et al (2018) Discovery and validation of pyridoxic acid and homovanillic acid as novel endogenous plasma biomarkers of organic anion transporter (OAT) 1 and OAT3 in cynomolgus monkeys. Drug Metab Dispos 46:178–188
Maddison DC, Giorgini F (2015) The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol 40:134–141
De Angelis M, Montemurno E, Piccolo M et al (2014) Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One 9(6):e99006
Wu H, Tang D, Yun M et al (2022) Metabolic dysfunctions of intestinal fatty acids and tryptophan reveal immuno-inflammatory response activation in IgA nephropathy. Front Med (Lausanne) 9:811526
Cervenka I, Agudelo LZ, Ruas JL (2017) Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357(6349):eaaf9794
Ardalan M, Ahmadian E, Hosseiniyan Khatibi SM et al (2022) Microbiota and glomerulonephritis: an immunological point of view. Am J Med Sci 364(6):695–705
Park JI, Kim TY, Oh B et al (2020) Comparative analysis of the tonsillar microbiota in IgA nephropathy and other glomerular diseases. Sci Rep 10(1):16206
Wee HN, Liu JJ, Ching J, Kovalik JP, Lim SC (2021) The kynurenine pathway in acute kidney injury and chronic kidney disease. Am J Nephrol 52(10–11):771–787
Acknowledgements
Sincere thanks should be given to PANOMIX Biomedical Tech Co., LTD, Suzhou.
Funding
This work was supported by Suzhou Pharmaceutical Association (SYSD2020184).
Author information
Authors and Affiliations
Contributions
YQ and YW wrote the paper. AS, ZH, CQ, CS and JP designed the research. YZ conducted the diagnosis and the follow-up of the patients. YQ and YW carried out the sample handling and performed statistical analysis. AS gave the final approval of the version to be published.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This study was reviewed and approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University. Informed consent was obtained from all participants. All procedures involving human participants complied with the ethical standards and were performed in accordance with the Declaration of Helsinki. All patients signed an informed consent form before renal biopsy.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qu, Y., Wang, Y., Hu, Z. et al. Role of metabolomic profile as a potential marker to discriminate membranous nephropathy from IgA nephropathy. Int Urol Nephrol 56, 635–651 (2024). https://doi.org/10.1007/s11255-023-03691-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03691-1