Abstract
Background
Functional hypogonadism is a condition in which some, but not all, older men have low testosterone levels. Rather than chronological age per se, the causality of hypogonadism includes obesity and impaired general health (e.g., metabolic syndrome). An association between testosterone deficiency and lower urinary tract symptoms (LUTS) has been reported, yet due to prostate safety concerns, men with severe LUTS (IPSS score > 19) have invariably been excluded from entering testosterone trials. Irrespective, exogenous testosterone has not been demonstrated to cause de novo or worsen mild to moderate LUTS.
Objective
This study investigated whether long-term testosterone therapy (TTh) could have a protective effect on improving the symptoms of LUTS in hypogonadal men. However, the exact mechanism by which testosterone exerts is beneficial effect remains uncertain.
Patients and methods
In this study 321 hypogonadal patients with an average age of 58.9 ± 9.52 years received testosterone undecanoate in 12-week intervals for 12 years. One hundred and forty-seven of these males had the testosterone treatment interrupted for a mean of 16.9 months before it was resumed. Total testosterone, International Prostate Symptom Scale (IPSS), post-voiding residual bladder volume and aging male symptoms (AMS) were measured over the study period.
Results
Prior to TTh interruption, it was observed that testosterone stimulation improved the men’s IPSS, AMS and post-voiding residual bladder volume, while their prostate volume significantly increased. During the TTh interruption, there was a significant worsening in these parameters, although the increase in prostate volume continued. When TTh was resumed, these effects were reversed, implying that hypogonadism may require lifelong treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional hypogonadism is characterized by low serum levels of testosterone and associated symptoms in males, demonstrating a higher prevalence as men age [1]. Low testosterone may lead to, and be exacerbated by, concomitant diseases such as metabolic syndrome (MetS), type 2 diabetes (T2D) and obesity, which tend to develop through age [2]. Low testosterone levels affect approximately 20% of men over the age of 60 years, 30% over 70 years and 50% over 80 years [3] and the associations between functional hypogonadism and its clinical features (including absence or regression of secondary sex characteristics, insulin resistance or T2D, hypertension, dyslipidemia, anemia, muscle wasting, reduced bone mass or bone mineral density, oligospermia, decreased libido, decreased sexual function and abdominal adiposity) and comorbidities leads to a lower quality of life (QoL) and often increased mortality [4]. Considering this, some studies have shown that testosterone therapy (TTh) has great therapeutic potential due to the favorable effects of testosterone on the comorbidities associated with androgenic deficiency [2]. However, there have been limited studies into the long-term effects of TTh and whether lifelong treatment is required.
Erectile dysfunction (ED) and lower urinary tract symptoms (LUTS) are significant contributors to age-related QoL in men and are both associated with several features of MetS, including obesity in epidemiological studies [5,6,7]. LUTS are often regarded as a hallmark of benign prostatic hyperplasia (BPH) with an increased incidence as men age [8]. Some studies have identified a correlation between functional hypogonadism and BPH whereby men may have significantly larger prostate volumes (PV) (> 31 mL) [3, 9] compared to men with normal testosterone levels [3]. Furthermore, a study by Schatzl et al. reported that approximately 20% of elderly men with LUTS had hypogonadism [10]. Reducing obesity as part of lifestyle intervention has been shown in some studies to lead to improvements in LUTS [11, 12] but not all [13, 14], and weight loss can result in modest increases in serum testosterone levels [15]. While the mechanisms underlying this potential inter-relationship between testosterone, obesity and LUTS are not known currently, evidence from some studies indicates that TTh may be a useful therapy for improving metabolic and urinary symptoms as well as comorbidities of late-onset hypogonadism (LOH) [16,17,18].
One of the concerns regarding TTh in elderly men remains increasing prostate volume and worsening urinary function parameters. Indeed, it is acknowledged that the prostate is an androgen-dependent organ that requires testosterone for growth and development, thus raising concerns about the potential risk of increased PV and therefore worsening of the associated risks (including LUTS and prostate cancer) if men are given TTh [19]. A controlled cross-sectional study conducted by Behre et al. (1994) concluded that TTh in hypogonadal men resulted in a significant increase in PV, comparable to age-matched normal men whereas hypogonadal men without TTh had lower PV [20]. This suggested that TTh resulted in a modest but significant increase in PV, however, still remained within normal limits. In contrast to this, a Chinese study showed after age adjustment, the rate of PV growth in aging patients with low testosterone was significantly greater than the normal testosterone level group, after 4 years [3]. In addition, a longitudinal study found that hypogonadal men who received TTh had a 12% increase in PV size on average [21]. However, another placebo-controlled study of hypogonadal males on TTh found no significant differences in PV between TTh-treated men and those on placebo [22]. Despite a potential testosterone-induced PV increase, current research suggests that TTh does not increase the risk of developing prostate cancer (PCa) [23,24,25] and can even protect against prostatic carcinoma in castration-resistant prostate cancer patients [26, 27] and men with advanced disease, namely, biochemical recurrence or metastatic PCa [28].
The International Prostate Symptom Score (IPSS) is used to assess the severity of LUTS and its related symptoms (for both irritative and obstructive symptoms) which result in a lower QoL; symptoms can include voiding and obstruction (hesitancy, poor/intermittent stream, straining, feeling of incomplete bladder emptying) as well as storage or irritative symptoms (frequency, incontinence and nocturia) [29]. In a Japanese population study of men with moderate to severe LUTS, TTh resulted in clinically significant improvements in the total IPSS score and the storage symptom score, but the voiding symptom score was not statistically improved after treatment [30]. In a meta-analysis of 14 clinical trials of TTh for hypogonadal men, the change in IPSS was similar among men receiving testosterone versus placebo, suggesting that TTh treatment does not worsen LUTS among men with hypogonadism [31]. The mean follow-up time for the studies included in this meta-analysis was 34.4 months and may indicate that long-term treatment is required for significant improvements in LUTS. Few studies have directly investigated the role of long-term TTh in hypogonadal males on the symptoms of LUTS. The protective role of testosterone and its improvements in LUTS in hypogonadal males were noted in our 2014 study [16]. The weight, waist circumference and BMI of the males also improved, further decreasing the risk for LUTS. Indeed, we have demonstrated that TTh interruption, and consequential reduced total testosterone levels, results in worsening of symptoms including obesity parameters, aging male symptoms (AMS), IPSS, residual voiding volume and bladder wall thickness, erectile function and prostate-specific antigen (PSA), while prostate volume remained unchanged until treatment resumed whereby these effects were reversed [32]. This suggests that hypogonadism may require lifelong TTh. In this retrospective registry study, we aimed to assess the long-term effects of testosterone treatment on the symptoms of LUTS (assessed by IPSS, AMS, post-voiding residual bladder volume, PV) in a cohort of 321 hypogonadal males with an average age of 58.9 ± 9.52 years in a urological setting.
Patients and methods
In a population-based single-center, prospective, cumulative registry study, 321 hypogonadal men with a mean age of 58.9 ± 9.52 years (age range: 19–84 years), a baseline total testosterone level under 12.1 nmol/L on 2 morning blood samples and with clinical symptoms of hypogonadism along with absence of contraindication received 1000 mg injections of long-acting testosterone undecanoate (TU) (Nebido®, Bayer Pharma, Berlin, Germany) for 12 years in 12-week intervals. As assessed by the Aging Males’ Symptoms (AMS) scale, patients had at least moderate symptoms of hypogonadism with a baseline total testosterone (TT) of ≤ 3.50 ng/mL (≤ 12.1 nmol/L). Patients had documented ED for ≥ 6 months, established using the international definition for ED, the International Index of Erectile Function (IIEF). Patients with clinically significant findings on physical examination or presence of known clinically significant diseases that would prejudice the completion of the study or contraindicate testosterone administration were excluded from the study. Patients with severe diabetes mellitus, International Prostate Symptom Score (IPSS) > 18, prostatitis, hyperprolactinaemia (> 20 ng/mL) or cardiovascular events within the last 6 months were also excluded from this study, as were patients with obstruction due to BPH with residual urine higher than 40 mL. The institution received approval from the ethics committee in accordance with German Ärztekammer (German Medical Association) regulations. Patients were enrolled once they had signed an informed written consent and all data was treated with confidentiality (Table 1).
As previously described [2, 32], the cohort received TTh for a mean duration of 65.5 ± 14.1 months before being temporarily interrupted for 147 men for a mean 16.9 ± 3.3 months. The interruption was due to reimbursement issues in 140 men and 7 men were diagnosed with prostate cancer and had a mean withdrawal of 16.9 months. All men resumed their treatment thereafter. The remaining 174 patients were treated without interruption. All injections were administered and documented during the clinical visit; treatment compliance was 100%. Three men dropped out of the study for unknown reasons. Throughout the trial, blood samples were taken from the participants at every other visit as were prostate volume (transrectal ultrasound) and post-voiding residual bladder volume by ultrasonography. Treatment-emergent adverse events were monitored throughout the study. Physical examination, digital rectal examination (DRE) serum prostate-specific antigen (PSA) levels were also measured at each 3-month period. International Prostate Symptom Scores (IPSS) and Aging Males’ Symptoms (AMS) questionnaires were assessed at each treatment visit. An independent commercial laboratory measured the total testosterone in serum samples. Alpha blockers and 5α-reductase inhibitors were used in 54% of the patients. Medications were not altered during the study. No acute urinary retention or prostate surgery was reported during the follow-up time.
Statistical analysis
Patient data were averaged over the course of their study participation and expressed as mean values with standard deviations at each time point. Before the first TU injection, the baseline parameter values were recorded. The continuous variable change from baseline and change from the prior year were compared using analysis of variance (ANOVA). The Statistical Package for Social Sciences v.18 was used for statistical analysis (SPSS Inc., Chicago, USA) and GraphPad Prism version 8.4.3 (GraphPad Software, La Jolla, CA, USA). A value of p < 0.05 was considered significant.
Results
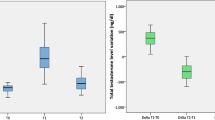
Prior to TTh, total testosterone (TT) levels in the hypogonadal men were measured at a mean 223.22 ± 62 ng/dL and within the first year this significantly increased twofold where it then reached a steady state (years 2 and 4) (Fig. 1). TT levels saw a temporary reduction in the group of patients that had their treatment interrupted (data reported elsewhere [32]) which influenced the mean TT levels for the cohort by reducing levels between 6 and 8 years. Upon retreatment, TT again increased where it was at its greatest level in the final year (year 12) with a difference of 447 ng/dL compared to the baseline (p < 0.0001 vs. baseline).
TTh concurrently reduced the hypogonadal males’ IPSS score (Fig. 2). Initially, it had a mean value of 10.1 ± 5.0 and then had a steady decline until the treatment was interrupted, visualized between years 5 and 8 where it then increased from 6.79 ± 3.63 (year 5) to 8.24 ± 4.15 (year 7). A gradual decline was then visualized again between 8 and 12 years of TTh with the lowest IPSS score being in the final year (5.38 ± 2.17) (p < 0.0001 vs. baseline).
During the TTh, post-voiding residual bladder volume was measured (Fig. 3). It was observed that there was a decrease from 23.8 ± 16.2 mL to 16.7 ± 6.4 mL (p < 0.0001 vs. baseline) with a temporary increase in years 6–8.
Throughout the study, prostate volume was also measured (Fig. 4). Initially, the mean PV was 28.7 ± 8.3 mL and following TTh, there was a significant increase all the way through to year 12 with an average increase of + 10.3 mL to 39.0 ± 6.4 mL (p < 0.0001 vs. baseline) without deviation from the trend during years 6–8, when TTh was interrupted for 147 men.
To measure the health-related quality of life, the Aging Males’ Symptoms Scale was utilized. Figure 5 indicated there was a significant improvement from the baseline with a decrease from 53.7 ± 9.5 to 27.5 ± 4.0 (p < 0.0001 vs. baseline) with a temporary increase in years 6–8.
Discussion
Hypogonadism is commonly associated with concomitant diseases including MetS, obesity, T2D, ED and LUTS. The therapeutic treatment of hypogonadism with testosterone supplementation has shown a protective role in the development and treatment of these diseases, with many studies observing improvements in the parameters of obesity, body composition, glycemic control, ED and QoL [2, 4, 15, 17, 33]. However, the use and safety of long-term TTh is still widely debated due to insufficient randomized controlled clinical trial data and some conflicting studies.
In men, LUTS are often concurrent with BPH, and a number of therapeutic treatments exist which can reduce prostate volume by approximately 25% [34, 35]. In particular, 5-alpha reductase inhibitors (5ARIs) result in an 85% reduction in prostatic dihydrotestosterone and are a common, effective therapy option for men with BPH-related LUTS who have demonstrable prostate enlargement. Treated patients demonstrated decreased prostate volume, improved urinary symptoms, and decreased risk of urinary retention; however, several sexual side effects were present including ED, gynecomastia, decreased libido and decrease in ejaculate volume indicating a lower QoL [36]. The findings of this present study are unique, in that long-term TTh in hypogonadal men improved voiding function, symptoms of LUTS and QoL, independent of prostate volume.
Several studies examining the relationship between prostatic size and LUTS in hypogonadal men receiving TTh indicated no significant increase in prostate size or LUTS with testosterone replacement [22, 31]. Furthermore, there is evidence that TTh can improve LUTS, as Saad et al. found significant improvement in IPSS scores in 28 men with sexual dysfunction and metabolic syndrome treated for 12 months with long-acting testosterone undecanoate [37]. This is supported by our previous study showing significant IPSS score reduction in 261 hypogonadal men with MetS after over 5 years TTh [38, 39]. As with the present study, Karazindiyanoğlu and Cayan showed that 25 hypogonadal men with sexual dysfunction were able to improve LUTS (indicated by IPSS, AMS, IIEF-5), as well as bladder function, following 1 year of TTh compared to placebo-treated controls [40]. Of note, PV significantly increased from 25.24 ± 6.74 mL to 28.8 ± 7.01 mL, although no significant changes in PSA and free PSA levels were shown [40]. Even while PV increased significantly during the present study, the lack of a placebo-controlled group makes it difficult to draw judgments about whether the increase in PV was above that of the normal aging population.
Contraindications for the use of TTh is the increased risk and causality of prostate carcinogenesis/androgen-dependent tumors due to potential PV increase, although increasing evidence suggests that testosterone does not increase PCa and may even be beneficial [41,42,43]. In a recent study, testosterone undecanoate improved urinary function and anthropometric parameters in 412 hypogonadal men and, alongside this, also discovered that TTh had no effect on the prostate parameters with incident prostate cancer reduced in the testosterone-treated group compared to the control group [44]. A randomized, double-blind, placebo-controlled study of 44 hypogonadal men failed to demonstrate a higher risk of prostate cancer in men on TTh, as there was no treatment-related change in prostate histology, tissue biomarkers, gene expression, or cancer incidence or severity (but minor PV increase) when they received 150 mg of testosterone enanthate or matching placebo for a duration of 6 months [45]. Despite this, the relationship between testosterone and PCa remains complex, and recommendations suggest not to use TTh where there is suspected or confirmed prostate malignancies [46, 47]. These proposed risks require further investigation in long-term controlled trials, and in the clinic they need to be weighed against the potential improved health-related QoL. Contrawise, with growing evidence that lower, not higher, testosterone levels trigger the development of PCa and BPH through androgen receptor over-expression, a paradigm shift in altering the present approach to diagnosing and treating men with hypogonadism is advocated to improve men’s health [48]. Regardless, all patients receiving TTh require close monitoring for safety.
LUTS and BPH negatively impact men’s health-related QoL [49]. The treatment of hypogonadal males with TTh has been previously shown to improve QoL, ED and LUTS [16, 32, 44] and is further observed in this present study. The improvement in QoL of hypogonadal men receiving TTh may be due in part to the relief of LUTS symptoms (e.g., voiding dysfunction) but it is also likely due to improvements in the associated effects on sexual function (ED, ejaculatory dysfunction, libido) [50], as seen in a meta-analysis where TTh provided significant improvement in all aspects of sexual function in hypogonadal men [51].
The mechanistic relationship between testosterone and LUTS is complex and remains poorly understood, although a pathophysiological link between components of MetS and LUTS has been proposed. Studies have revealed a possible link between insulin resistance (diabetes mellitus) and LUTS in BPH patients [52] and according to the findings of a meta-analysis, people with diabetes have more severe LUTS than individuals without diabetes, as determined by IPSS [53]. Furthermore, there is evidence strongly linking obesity and the incidence of LUTS/BPH, and the pathology of both obesity and MetS is underpinned by chronic inflammation which additionally plays a crucial role in BPH [54]. Therefore, the inter-relationship between diabetes, obesity, MetS and inflammation contribute to LUTS progression. Low testosterone in men is associated with these comorbidities and several studies have demonstrated the beneficial effects of TTh on insulin sensitivity, parameters of obesity and inflammation [33, 55,56,57,58]. Specifically, TTh reduced BMI, WC, HbA1c, and CRP in parallel to an improvement in LUTS in the same cohort of hypogonadal men as the present study over an 8-year follow-up [44]. Nitric oxide (NO) is a potent regulator of prostatic innervation and pelvic smooth muscle tone and is considered to play a role in LUTS. NO levels result in more severe LUTS [59]. Accordingly, phosphodiesterase-5 (PDE-5) inhibitors are effective in the treatment of BPH/LUTS as well as erectile function [60,61,62]. Evidence suggests that testosterone may improve LUTS, as well as erectile function and associated QoL at least partly via a mechanistic action on NOS and via NO signaling.
The current research has some limitations. Due to this being an observational study with no placebo-controlled group, the direct effects of treatment versus no treatment could not be evaluated, limiting the interpretation of the data. Additionally, TTh was interrupted in 147 males due to reimbursement issues and/or the diagnosis of prostate cancer. While this may skew the data presented, particularly for years 6–8, we have previously discovered that when TTh is resumed, testosterone levels return to pre-interruption levels [32]. As a result, the interruption is unlikely to have altered the circulation levels of hormones measured during the final 12-year follow-up. Indeed, all parameters measured in this study, except prostate volume, regressed in those patients in whom TTh was temporarily interrupted but started improving again after resuming TTh, suggesting a high degree of reversibility. The worsening after testosterone cessation was also observed in short-term studies [63, 64]. The study by Francomano et al. observed that in severely obese men, metabolic, fat but not lean mass, and blood pressure parameters were maintained for a relatively short duration, whereas cardiac and hormonal parameters returned to baseline post-TTh [33]. It is understood that the maintenance of some of the measures may occur over a short term, but may be gradually lost over longer durations. Notably, many of the positive effects of TTh, especially in relation to body composition and weight, can take between 6 and 12 months to manifest [17]. Therefore, the long-term duration of the data collected here is a strength of the present study.
In conclusion, this study suggests that long-term TTh can improve voiding function and alleviate symptoms of LUTS seemingly independently of prostate volume. These therapeutic benefits develop in parallel to improvements to QoL, as indicated by AMS. TTh may need to be continued indefinitely to retain the favorable effects, as we have previously observed in this cohort a worsening of parameters when TTh is discontinued and the sustained benefits over 12 years in the present study. While TTh may not increase prostate risk despite increases in PV, there is a need for large, placebo-controlled long-term outcome studies to validate current suggestions with more conclusive evidence.
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
Abbreviations
- LUTS:
-
Lower urinary tract symptoms
- IPSS:
-
International Prostate Symptom Score
- TTh:
-
Testosterone therapy
- AMS:
-
Aging male symptoms
- MetS:
-
Metabolic syndrome
- T2D:
-
Type 2 diabetes
- QoL:
-
Quality of life
- ED:
-
Erectile dysfunction
- PV:
-
Prostate volumes
- LOH:
-
Late-onset hypogonadism
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- TU:
-
Testosterone undecanoate
- IIEF:
-
International Index of Erectile Function
- DRE:
-
Digital rectal examination
- TT:
-
Total testosterone
- BPH:
-
Benign prostatic hyperplasia
- BMI:
-
Body mass index
- WC:
-
White blood cell count
- HbA1c:
-
Hemoglobin A1C
- CRP:
-
C-reactive protein
- NOS:
-
Nitrous oxide
- NO:
-
Nitric oxide
References
Michaud JE, Billups KL, Partin AW (2015) Testosterone and prostate cancer: and evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol 7(6):378–387. https://doi.org/10.1177/1756287215597633
Yassin A, Nettleship JE, Talib RA et al (2016) Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male 19(1):64–69. https://doi.org/10.3109/13685538.2015.1126573
Xia B, Zhao S, Chen Z et al (2021) Relationship between serum total testosterone and prostate volume in aging men. Sci Rep. https://doi.org/10.1038/s41598-021-93728-1
Hackett GI (2016) Testosterone replacement therapy and mortality in older men. Drug Saf 39:117–130 (PubMed PMID: 26482385)
Rohrmann S, Smit E, Giovannucci E et al (2004) Associations of obesity with lower urinary tract symptoms and noncancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 159:390–397
Seim A, Hoyo C, Ostbye T et al (2005) The prevalence and correlates of urinary tract symptoms in Norwegian men: the HUNT study. BJU Int 96:88–92
Laven BA, Orsini N, Andersson SO et al (2008) Birth weight, abdominal obesity and the risk of lower urinary tract symptoms in a population based study of Swedish men. J Urol 179:1891–1895
Donnell RF (2011) Benign prostate hyperplasia: a review of the year’s progress from bench to clinic. Curr Opin Urol 21:22–26
Pejčić T, Tosti T, Tešić Z et al (2017) Testosterone and dihydrotestosterone levels in the transition zone correlate with prostate volume. Clin Urol 77(10):1082–1092. https://doi.org/10.1002/pros.23365. (PubMed PMID: 28594074)
Schatzl G, Brössner C, Schmid S, The Prostate Study Group of the Austrian Society of Urology et al (2000) Endocrine status in elderly men with lower urinary tract symptoms: correlation of age, hormonal status, and lower urinary tract function. Urology 55:397–402. https://doi.org/10.1016/s0090-4295(99)00473-2. (PubMed PMID: 10699620)
Khoo J, Ling PS, Chen RY et al (2014) Comparing the effects of meal replacements with an isocaloric reduced-fat diet on nutrient intake and lower urinary tract symptoms in obese men. J Hum Nutr Diet 27(3):219–226
Khoo J, Piantadosi C, Duncan R et al (2011) Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 8(10):2868–2875
Yee CH, So WY, Yip SK et al (2015) Effect of weight reduction on the severity of lower urinary tract symptoms in obese male patients with benign prostatic hyperplasia: a randomized controlled trial. Korean J Urol 56(3):240–246. https://doi.org/10.4111/kju.2015.56.3.240
Ng CF, Yee CH, So WY et al (2017) Effect of weight reduction on severity of lower urinary tract symptoms in obese men with benign prostatic hyperplasia. Hong Kong Med J 23(Suppl 2(3)):35–37 (PMID: 29938670)
Corona G, Rastrelli G, Monami M et al (2013) Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol 168(6):829–843. https://doi.org/10.1530/EJE-12-0955. (PMID: 23482592)
Yassin DJ, Douaihy YE, Yassin AA et al (2014) Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol 32:1049–1054. https://doi.org/10.1007/s00345-013-1187-z. (PubMed PMID: 24135918)
Kelly DM, Jones TH (2015) Testosterone and obesity. Obes Rev 16:581–606. https://doi.org/10.1111/obr.12282. (PubMed PMID: 25982085)
Al-Zoubi R, Yassin A, Alwani M et al (2021) A systematic review on the latest developments in testosterone therapy: innovations, advances, and paradigm shifts. Arab J Urol 19(3):370–375. https://doi.org/10.1080/2090598X.2021.1959260
Zitzmann M, Depenbusch M, Gromoll J et al (2003) Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab 88(5):2049–2054. https://doi.org/10.1210/jc.2002-021947. (PubMed PMID: 12727953)
Behre HM (1994) Prostate volume in treated and untreated hypogonadal men in comparison to age-matched controls. Clin Endocrinol 40:341–349 (PubMed PMID: 7514512)
Holmang S, Marin P, Lindstedt G et al (1993) Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal middle-aged men. Prostate 23:99–106 (PubMed PMID: 7690956)
Tenover JL (1998) Androgen deficiency in aging men. Aging male 1:16–21
Calof OM, Singh AB, Lee ML et al (2005) Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60:1451–1457
Feneley MR, Carruthers M (2012) Is testosterone treatment good for the prostate? Study of safety during long-term treatment. J Sex Med 9:2138–2149
Eisenberg ML, Li S, Betts P et al (2015) Testosterone therapy and cancer risk. BJU Int 115:317–321
Schweizer MT, Antonarakis ES, Wang H et al (2015) Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci Transl Med 7(269):269ra2. https://doi.org/10.1126/scitranslmed.3010563. (PMID: 25568070; PMCID: PMC4507510)
Zhang X, Zhong Y, Saad F et al (2020) Testosterone therapy may reduce prostate cancer risk due to testosterone deficiency at a young age via stabilizing serum testosterone levels. Aging Male 23(2):112–118. https://doi.org/10.1080/13685538.2019.1578739.Mar12. (PMID: 30857458)
Morgentaler A, Abello A, Bubley G (2021) Testosterone therapy in men with biochemical recurrence and metastatic prostate cancer: initial observations. Androgens Clin Res Therap 2:121–128. https://doi.org/10.1089/andro.2021.0001
Lepor H (2005) Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol 7(7):3–11 (PubMed PMID: 16986059)
Okada K, Miyake H, Ishida T et al (2018) improved lower urinary tract symptoms associated with testosterone replacement therapy in Japanese men with late-onset hypogonadism. Am J Mens Health 12(5):1403–1408. https://doi.org/10.1177/1557988316652843. (PMCID: PMC6142172)
Kohn TP, Mata DA, Ramasamy R et al (2016) Effects of testosterone replacement therapy on lower urinary tract symptoms: a systematic review and meta-analysis. Eur Urol 69(6):1083–1090. https://doi.org/10.1016/j.eururo.2016.01.043
Yassin A, Almehmadi Y, Saad F et al (2016) Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf) 84(1):107–114. https://doi.org/10.1111/cen.12936. (PubMed PMID: 26331709)
Francomano D, Lenzi A, Aversa A (2014) Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. https://doi.org/10.1155/2014/527470. (PubMed PMID: 24688542)
Lepor H (2007) Alpha Blockers for the treatment of benign prostatic hyperplasia. Rev Urol 9(4):181–190 (PMID: 18231614)
Kaplan SA, Roehrborn CG, McConnell JD et al (2008) Long-term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in men enrolled in the MTOPS trial. J Urol 180:1030–1032
Laborde E, McVary K (2009) Medical management of lower urinary tract symptoms. Rev Urol 11:19–25 (PMID: 20126608)
Saad F, Gooren L, Haider A et al (2007) An exploratory study of the effects of 12 month administration of the novel long-acting testosterone undecanoate on measures of sexual function and the metabolic syndrome. Arch Androl 53(6):353–357. https://doi.org/10.1080/01485010701730880
Yassin DJ, Doros G, Hammerer PG et al (2014) Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med 11(6):1567–1576. https://doi.org/10.1111/jsm.12523. (PMID: 24712761)
Haider KS, Haider A, Doros G et al (2018) Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol 199(1):257–265. https://doi.org/10.1016/j.juro.2017.07.039. (PMID: 28728990)
Karazindiyanoğlu S, Cayan S (2008) The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male 11:146–149. https://doi.org/10.1080/13685530802290438
Khera M, Crawford D, Morales A et al (2014) A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol 65(1):115–123. https://doi.org/10.1016/j.eururo.2013.08.015. (PubMed PMID: 24011426)
Morgentaler A (2013) Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol 189:26–33. https://doi.org/10.1016/j.juro.2012.11.028. (PubMed PMID: 23234627)
Eisenberg M (2015) Testosterone replacement therapy and prostate cancer incidence. World J Mens Health 33(3):125–129. https://doi.org/10.5534/wjmh.2015.33.3.125. (PMID: 26770932)
Saad F, Doros G, Haider KS et al (2018) Hypogonadal men with moderate-to-severe lower urinary tract symptoms have a more severe cardiometabolic risk profile and benefit more from testosterone therapy than men with mild lower urinary tract symptoms. Investig Clin Urol 59(6):399–409. https://doi.org/10.4111/icu.2018.59.6.399. (PubMed PMID: 30402573)
Marks L, Mazer N, Mostaghel E et al (2006) Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA 296(19):2351–2361. https://doi.org/10.1001/jama.296.19.2351
Lenenfeld B, Saad F, Hoesl CE (2005) ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male 8(2):59–74. https://doi.org/10.1080/13685530500163416
Tremblay RR, Morales A (1998) Canadian practice recommendations for screening, monitoring and treating men affected by andropause or partial androgen deficiency. Aging Male 1(3):213–218. https://doi.org/10.3109/13685539809146110
Diokno AC (2022) The role of testosterone in men’s health: is it time for a new approach? Int Urol Nephrol 54(11):2767–2774. https://doi.org/10.1007/s11255-022-03292-4. (Epub 2022 Aug 1. PMID: 35909146)
Speakman M, Kirby R, Doyle S et al (2014) Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH)- Focus on the UK. BJU Int 115(4):598–519. https://doi.org/10.1111/bju.12745
Rosen RC, Giuliano F, Carson CC (2005) Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH). Eur Urol 47(6):824–837. https://doi.org/10.1016/j.eururo.2004.12.013
Isidori AM, Gianetta E, Gianfrilli D et al (2005) Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol 63:381–394
Wang Z, Olumi AF (2011) Diabetes, growth hormone-insulin-like growth factor pathways and association to benign prostatic hyperplasia. Differentiation 82(4):261–271. https://doi.org/10.1016/j.diff.2011.04.004
Xin C, Fan H, Xie J et al (2022) Impact of diabetes mellitus on lower urinary tract symptoms in benign prostatic hyperplasia patients: a meta analysis. Endocrinol. https://doi.org/10.3389/fendo.2021.741748
Kohnen PW, Drach GW (1979) Patterns of inflammation in prostatic hyperplasia: a histologic and bacteriologic study. J Urol 121:755–760
Dhindsa S, Ghanim H, Batra M et al (2016) Insulin resistance and inflammation in hypogonadotropic 28 hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 39(1):82–91. https://doi.org/10.2337/dc15-1518
Janjgava S, Zerekidze T, Uchava L et al (2014) Influence of testosterone replacement therapy on metabolic disorders in male patients with type 2 diabetes mellitus and androgen deficiency. Eur J Med Res. https://doi.org/10.1186/s40001-014-0056-6
Jones TH, Arver S, Behre H et al (2011) Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 34(4):828–837. https://doi.org/10.2337/dc10-1233
Haider KS, Haider A, Saad F et al (2020) Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes Obes Metab 22(11):2055–2068. https://doi.org/10.1111/dom.14122. (PMID: 32558149; PMCID: PMC7689919)
Zhao C, Kim SH, Lee SW et al (2011) Activity of phosphodiesterase type 5 inhibitors in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. BJU Int 107:1943–1947
Gacci M, Corona G, Salvi M et al (2012) A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with alpha-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol 61:994–1003
McVary KT, Roehrborn CG, Kaminetsky JC et al (2007) Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 177:1401–1407
Stief CG, Porst H, Neuser D et al (2008) A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol 53:1236–44
Schroeder ET, Zheng L, Ong MD et al (2004) Effects of androgen therapy on adipose tissue and metabolism in older men. J Clin Endocrinol Metab 89:4863–4872. https://doi.org/10.1210/jc.2004-0784. (PubMed PMID: 15472177)
Francomano D, Bruzziches R, Barbaro G et al (2014) Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest 37:401–411. https://doi.org/10.1007/s40618-014-0066-9. (PubMed PMID: 24639122)
Acknowledgements
Editorial support for this manuscript was provided by Astra-Health, www.Astra-Health.com.
Funding
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AY received partial compensation for data entry, honoraria and occasionally travel grants from Bayer AG and is a Member of the Advisory Board for Testosterone, Besin Health Care, Pharma. DMK has received honoraria and occasionally travel grants from Bayer AG.
Ethical approval
This study was conducted in accordance with German Ärztekammer (German Medical Association) regulations. Due to the nature of this study, explicit ethical approval was not required. However, the researchers ensured that the study was conducted with integrity, transparency, and respect for all relevant regulations and ethical considerations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yassin, A., Alwani, M., Al-Zoubi, R.M. et al. Voiding function improves under long-term testosterone treatment (TTh) in hypogonadal men, independent of prostate size. Int Urol Nephrol 55, 1649–1658 (2023). https://doi.org/10.1007/s11255-023-03602-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03602-4