Abstract
Purpose
To investigate the associations of anion gap (AG) levels before and 1-day after hemodialysis as well as anion gap changes with the mortality in critically ill patients receiving renal replacement therapy (RRT).
Methods
Totally, 637 patients from MIMIC-III were included in this cohort study. The associations between AG (T0), AG (T1), or ∆AG [AG (T0) − AG (T1)], and the risk of 30-day or 1-year mortality were examined by Cox restricted cubic spline regression models. Univariate and multivariate Cox proportional-hazards model was applied to assess the associations between AG (T0), AG (T1), ∆AG with 30-day and 1-year mortality, respectively.
Results
The median follow-up time was 18.60 (8.53, 38.16) days and 263 (41.3%) patients were survived. There was a linear relationship between AG (T0), AG (T1) or ∆AG and the risk of 30-day or 1-year mortality, respectively. The risk of 30-day mortality was higher in AG (T0) > 21 group (HR = 1.723, 95% CI 1.263–2.350), and AG (T1) > 22.3 group (HR = 2.011, 95% CI 1.417–2.853), while lower in AG > 0 group (HR = 0.664, 95% CI 0.486–0.907). The risk of 1-year mortality was increased in AG (T0) > 21 group (HR = 1.666, 95% CI 1.310–2.119), and AG (T1) > 22.3 group (HR = 1.546, 95% CI 1.159–2.064), while decreased in AG > 0 group (HR = 0.765, 95% CI 0.596–0.981). Patients with AG (T0) ≤ 21 had higher 30-day and 1-year survival probability than those with AG (T0) > 21.
Conclusion
AG before and after dialysis as well as the changes of AG were important factors associated with the risk of 30-day and 1-year mortality in critically ill patients receiving RRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal replacement therapy (RRT) is required in patients with critical illness in intensive care units (ICUs) who suffer from a great burden of comorbidities and being unable to tolerate the hemodynamic shifts of conventional hemodialysis [1]. RRT provides organ support in critically ill patients with kidney failure for the management of fluid when the kidneys are unable to meet the metabolic demand of the critically ill disease state [2, 3]. Although the application of RRT increased the survival benefits for many patients in ICUs, the mortality of patient require RRT was as high as 60% in some studies [4,5,6]. To deep investigate more related prognostic biomarkers associated with critically ill patients receiving RRT might help the clinicians to improve the outcomes of these patients.
The serum anion gap (AG) is a mathematical derivation parameter obtained based on the difference in serum cation and anion concentrations [7]. AG reflects the unmeasured concentration of anion, which is considered to be a biomarker assessing the acid–base disorders and helping identify different forms of metabolic acidosis [8]. Along with differential diagnosis of acid–base, this value can be used as a biomarker for predicting prognosis in patients with chronic kidney disease [9]. Previously, the increase of AG in patients with moderate chronic kidney disease was reported to be associated with elevated risk for progression to end-stage renal disease [10]. Cheng et al. revealed that serum AG was an effective predictor for all-cause mortality in critically ill patients with acute kidney injury [7]. A recent study found that there is a “J-shaped” association between AG before dialysis and mortality in elderly patients over 75 years old who receive hemodialysis, indicating too high or too low AG might lead to higher risk of death [11]. The AG is a time-varying factor associated with the renal outcomes [12], but current studies were mainly explored the AG at some time point. Monitoring the AG and its changes at different time points may be instructive for providing appropriate interventions for critically ill patients to improve their outcomes. There was evidence indicated that the acid–base balance before hemodialysis was significantly different from that after hemodialysis in stable hemodialysis patients [13]. The pre and post- hemodialysis serum AG was also different [14]. Whether the changes of AG levels before and after hemodialysis were associated with the mortality in critically ill patients receiving RRT needs exploration.
In this study, the purpose was to investigate the associations of AG levels before and 1-day after hemodialysis as well as anion gap changes at the two time points with the mortality in critically ill patients receiving RRT using the data from the Medical Information Mart for Intensive Care-III (MIMIC-III) database. Subgroup analysis was performed based on the age, the estimated glomerular filtration rate (eGFR), the Elixhauser Comorbidity Index (ECI).
Methods
Study design and population
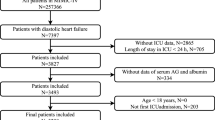
Totally, 687 patients undergoing RRT with the measurement of AG calculation related biomarkers from MIMIC-III between 2001–2012 were involved in this cohort study. MIMIC-III included the health data of over 40,000 patients who were admitted to the intensive care unit (ICU) of Beth Israel Deaconess Medical Center [15]. The information of patients including demographics, vital signs, laboratory findings, imaging reports, organ failure scores, severity of illness scores, comorbidities, diagnosis, treatment, length of stay in hospital, and survival data were collected [16]. Patients ≥ 18 years who received RRT for ≥ 2 days in ICU from the database with measurement of anion gap before and 1-day after RRT were included in our study. The requirement of ethical approval for this was waived by the Institutional Review Board of Affiliated Liutie Central Hospital of Guangxi Medical University, because the data was accessed from MIMIC-III (a publicly available database). All individuals provided informed consent before participating in the study. In our study, patients aged < 18 years, and those with RRT treatment < 2 days were excluded. Finally, 637 participants were included.
Main variables
Age (years), gender, ethnicity (White, Black, Asian, Hispanic/Latino, or others), systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), respiratory rate (time/min), heart rate (time/min), temperature (℃), oxygen saturation (SpO2, %), fraction of inspired oxygen (FiO2, %), white blood cell (WBC, K/UL), neutrophil (%), lymphocytes (K/UL), platelets (PLT, K/UL), hemoglobin (g/dL), red cell distribution width (RDW), total bilirubin (TBIL, μmol/L), albumin (g/dL), blood urea nitrogen (BUN, mg/dL), creatinine (mg/dL), eGFR (mL/min), glucose (mg/dL), lactate (mmol/L), pH, base excess, AG (T0), AG (T1),, urine output, fluid input (mL), length of stay (LOS, day), oxygen therapy, mechanical ventilation, vasopressor, ECI, Glasgow coma scale (GCS), the Simplified Acute Physiology Score II (SAPSII), Sequential Organ Failure Assessment (SOFA).
AG (T0) referred to AG levels before dialysis and AG (T1) indicated AG levels 1-day after dialysis. Values of AG were calculated using the following equation: AG (serum sodium [mmol/L] + serum potassium [mmol/L]) − (serum chloride [mmol/L] + serum bicarbonate [mmol/L]) + 2.5 × (4 − observed albumin [g/dL]) [17]. ∆AG = AG (T0) − AG (T1). The participants were stratified into two groups according to the cut-point of AG (T0), AG (T1), and ∆AG, respectively including AG (T0) ≤ 21 mmol/L and AG (T0) > 21 mmol/L, AG (T1) ≤ 22.3 mmol/L and AG (T1) > 22.3 mmol/L, ∆AG ≤ 0 and ∆AG > 0. AG (T0) ≤ 21 mmol/L, AG (T1) ≤ 22.3, mmol/L ∆AG ≤ 0 were set as the reference groups respectively. The X-tile software was used for cut-point selection.
Outcome variables
Survival information (including survival outcome and time of death) was obtained from the Social Security Death Index records. The follow-up endpoints were all-cause mortality at 30 days and 1 year after ICU admission. The median follow-up after ICU admission to discharge or death was 18.60 (8.53, 38.16) days.
Statistical analysis
Continuous variables were presented as mean and standard deviation (mean ± SD) or median and interquartile range [IQR], and categorical variables were displayed as number (percentages) [n (%)]. Differences in baseline variables between groups stratified by the patients who were survival and dead were compared via Mann–Whitney or t tests for continuous variables, and the Chi-squared test or Fisher’s exact test for dichotomous variables. The associations between AG (T0), AG (T1), or ∆AG, and the risk of 30-day or 1-year mortality were examined by Cox restricted cubic spline regression models. Univariate and multivariate Cox proportional-hazards model was applied to assess the association between AG (T0), AG (T1), ∆AG with 30-day and 1-year mortality, respectively. Survival analysis was performed using standardized Kaplan Meier curves. Subgroup analysis were stratified for age (< 65 years or ≥ 65 years), eGFR (< 15 or ≥ 15), ECI (< 25 or ≥ 25). Multivariate model adjusted for age, respiratory rate, heart rate, SBP, DBP, respiratory failure, SPO2, PLT, RDW, TBIL, eGFR, urine output, oxygen therapy, mechanical ventilation, vasopressor ECI, SAPSII, SOFA. A two-sided P value of < 0.05 was considered statistically significant. All analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Austria, Vienna).
Results
The baseline characteristics of all participants
Overall, 687 patients were involved in this study. We excluded 1 patient aged < 18 years, and 49 patients with RRT treatment < 2 days, and 637 participants were finally included. The screen process of the participants was shown in Fig. 1. Among them, 219 participants died within 30 days, and 335 participants died within 1 year after ICU admission. All participants were divided into the survival group (n = 263) and death group (n = 374) based on the overall survival data from the database. As shown in Table 1, the median age in the death group was older than the survivor group (66.42 years vs 59.04 years). The mean SBP (117.18 mmHg vs 126.97 mmHg) and DBP (62.86 mmHg vs 67.32 mmHg) in the death group were lower than the survival group. The median creatinine in the death group was lower than the survival group (2.40 mg/dL vs 3.10 mg/dL). The median eGFR in the death group was higher than the survival group (29.70 mL/min vs 23.36 mL/min). The mean AG (T0) (22.52 vs 21.15) and AG (T1) (20.43 vs 18.95) in the death group was higher than the survival group. The mean ECI in the death group was higher than the survival group (32.18 vs 23.89).
The associations of AG (T0), AG (T1), and ∆AG with the 30-day mortality or 1-year mortality in patients
The Cox regression model with a restricted cubic spline showed a linear relationship between AG (T0) and the risk of 30-day mortality (Fig. 2) as well as the risk of 1-year mortality (Fig. 3) in the patients. A linear relationship between AG (T1) and the risk of 30-day mortality (Fig. 4) as well as the risk of 1-year mortality (Fig. 5) in the patients were observed in the Cox regression model with a restricted cubic spline. The Cox regression model with a restricted cubic spline showed a linear relationship between ∆AG and the risk of 30-day mortality (Fig. 6) as well as the risk of 1-year mortality in the patients (Fig. 7).
Table 2 shows the results of the Cox proportional-hazards model. After adjustment for the confounding factors including age, respiratory rate, temperature, SpO2, PLT, RDW, TBIL, eGFR, urine output, oxygen therapy, mechanical ventilation, vasopressor, ECI, SAPSII, SOFA, the risk of 30-day mortality was higher in AG (T0) > 21 group (HR = 1.723, 95% CI 1.263–2.350), and AG (T1) > 22.3 group (HR = 2.011, 95% CI: 1.417–2.853), while lower in AG > 0 group (HR = 0.664, 95% CI 0.486–0.907). After adjusting for age, respiratory rate, SBP, DBP, respiratory failure, temperature, SpO2, PLT, RDW, TBIL, eGFR, urine output, oxygen therapy, mechanical ventilation, vasopressor, ECI, SAPSII, SOFA and AG (T0), the risk of 1-year mortality was increased in AG (T0) > 21 group (HR = 1.666, 95% CI 1.310–2.119), and AG (T1) > 22.3 group (HR = 1.546, 95% CI 1.159–2.064), while decreased in AG > 0 group (HR = 0.765, 95% CI 0.596–0.981).
The cumulative survival probability of 30-day mortality and 1-year mortality in patients
The cumulative survival probability of 30-day mortality among participants as stratified by AG (T0) levels was shown in Supplementary Fig. 1, which revealed that patients with AG (T0) ≤ 21 had higher 30-day survival probability than those with AG (T0) > 21. The cumulative survival probability of 1-year mortality among participants with AG (T0) ≤ 21 was higher than those with AG (T0) > 21 (Supplementary Fig. 2). The cumulative survival probability of 30-day mortality (Supplementary Fig. 3) and 1-year mortality (Supplementary Fig. 4) among participants with AG (T1) ≤ 22.3 were higher than those with AG (T1) > 22.3. The cumulative survival probability of 30-day mortality (Supplementary Fig. 5) and 1-year mortality (Supplementary Fig. 6) among participants with ∆AG > 0 levels were higher than those with ∆AG ≤ 0.
Subgroup analysis of the associations of AG (T0), AG (T1), and ∆AG with the 30-day mortality or 1-year mortality in patients
In terms of different age groups, the risk of 30-day mortality was increased in patients aged ≥ 65 years with AG (T0) > 21 (HR = 2.111, 95% CI 1.356–3.286) and patients < 65 years with AG (T1) > 22.3 (HR = 2.558, 95% CI 1.552–4.215) after multivariate adjustment for confounders. In patients with different eGFR, we found that the risk of 30-day mortality was elevated in patients with AG (T0) > 21 in eGFR ≥ 15 group (HR = 2.061, 95% CI 1.485–2.861) and AG (T1) > 22.3 in eGFR < 15 group (HR = 3.757, 95% CI 1.060–13.318). The risk of 30-day mortality was decreased in patients with ∆AG > 0 in eGFR < 15 group (HR = 0.211, 95% CI 0.062–0.719). As for patients with different ECI levels, the increased risk of 30-day mortality was observed in those with AG (T0) > 21 in ECI ≥ 25 group (HR = 1.621, 95% CI 1.142–2.303) as well as AG (T1) > 22.3 in both ECI < 25 group (HR = 4.441, 95% CI 1.617–12.041) and ECI ≥ 25 group (HR = 1.641, 95% CI 1.097–2.454). The increased risk of 30-day mortality was found in patients with ∆AG > 0 in ECI < 25 group (HR = 0.485, 95% CI 0.247–0.951) and ECI ≥ 25 group (HR = 0.678, 95% CI 0.473–0.973) (Table 3).
As shown in Table 4, after multivariate adjustment for confounders, there was a higher risk of 1-year mortality among both age < 65 years group (HR = 1.819, 95% CI 1.261–2.622) and age ≥ 65 years group (HR = 1.579, 95% CI 1.1122–2.222), eGFR ≥ 15 group (HR = 1.851, 95% CI 1.428–2.398), and both ECI < 25 group (HR = 1.466, 95% CI 0.857–2.510) and ECI ≥ 25 group (HR = 1.476, 95% CI 1.115–1.954) in patients with AG (T0) > 21. A higher risk of 1-year mortality was observed in aged < 65 years group (HR = 1.992, 95% CI 1.308–3.032) and ECI < 25 group (HR = 2.547, 95% CI 1.178–5.747) in patients AG (T1) > 22.3. There was a lower risk of 1-year mortality among subjects ECI < 25 group in patients with ∆AG > 0 (HR = 0.582, 95% CI 0.347–0.978).
Sensitivity analysis was performed to explore the associations of AG (T0), AG (T1), and ∆AG with the 30-day mortality or 1-year mortality in patients using AG not adjusting for albumin (Table 5). The analysis showed very similar results to the main analysis shown in Table 2.
Discussion
The current study evaluated the associations of AG (T0), AG (T1), and ∆AG with the 30-day mortality or 1-year mortality in critically ill patients receiving RRT. The results delineated that higher AG (T0) and AG (T1) while lower ∆AG were associated with higher risk of 30-day and 1-year mortality in these patients. The findings of this study might highlight the importance of monitoring AG and its changes at different time points for fluid management before and during RRT in critically ill patients requiring dialysis.
The serum AG is a calculated entity applied for assessing acid–base disorders and differential diagnosis of metabolic acidosis [18]. There was evidence indicated that anions that accumulating in patients on hemodialysis were associated with the lower ionized fraction of magnesium and calcium, which was an effective predictor for ionized magnesium and calcium in patients on hemodialysis [19]. Previously, Chen et al. revealed that the baseline AG ≥ 20 was associated with 1.63-fold increase in the risk of mortality of patients with congestive heart failure and chronic kidney disease [20]. In the study of Asahina et al., the association between AG and renal outcomes was evaluated in 1168 Japanese chronic kidney disease patients, which uncovered that high AG acidosis developed during the later stages of chronic kidney disease and the AG level changed with progression of chronic kidney disease [21]. Another study depicted that elevated serum AG in adults with moderate chronic kidney disease was associated with increased risk for progression to end-stage renal disease [22]. A high AG was reported to be an essential prognostic factor in patients with chronic kidney disease, because veverimer, a nonabsorbable hydrochloride-binding polymer, was validated to improve kidney function and decrease the AG [12]. In our study, we found that both higher AG before dialysis and higher AG 1-day after dialysis were correlated with increased risk of 30-day and 1-year mortality in critically ill patients undergoing RRT. Furthermore, ∆AG < 0, indicating that AG level 1-day after dialysis was lower than AG level before dialysis, was associated with decreased risk of 30-day and 1-year mortality in critically ill patients undergoing RRT. In previous studies, the change of AG was regarded as a novel marker of outcome in critically ill patients [23]. Lipnick et al. demonstrated that the changes between critical care initiation AG and prehospital admission AG was a predictor for mortality in patients with critical illness [24]. The mechanism might due to that the uremic acids such as indoxyl sulfate, p-cresyl sulfate, and trimethylamine N-oxide can cause kidney injury-induced renal fibrosis, which further increase the risk of mortality [25, 26]. This reminded the clinicians to concern on AG levels before and 1-day after dialysis, as well as their differences in critically ill patients undergoing RRT, and timely interventions should be provided to control their AG level.
Increased AG change was identified to be a risk factor for early mortality after starting hemodialysis in the elderly, and patients with high AG presented more advanced chronic kidney disease and more severe hyperphosphatemia [11]. Herein, subgroup analysis showed that increased AG level before dialysis was associated with elevated risk in 30-day mortality and 1-year mortality in those aged ≥ 65 years. High AG and increased AG level can result in irreparable damage in elderly patients including increased muscle protein catabolism with muscle wasting, reduced albumin synthesis with malnutrition, promotion of systemic inflammation, increased bone resorption [27]. Another study delineated that patients with high AG presented mobility impairments at the time of starting hemodialysis and preservation of mobility was one of the most important factors to improve the short-term prognosis after starting dialysis in elderly patients with end-stage renal disease [28]. The eGFR is a biomarker for the evaluation of chronic kidney disease, which can reflect preoperative renal function and is unitized for guiding the postoperative fluid management to prevent postoperative acute kidney injury in surgical populations [29]. In the current study, high AG level before dialysis was associated with high risk of 30-day and 1-year mortality in critically ill patients undergoing RRT who had eGFR ≥ 15; High AG level 1-day after dialysis and ∆AG were correlated with the risk of 30-day mortality in critically ill patients undergoing RRT who had eGFR < 15. For patients with eGFR < 15, clinicians should be pay special attention on AG level after dialysis and the changes of AG level before and after dialysis. ECI measures the clinical conditions that exist before hospital admission, which might be related to the mortality and resource use in the hospital [30]. Patients with high ECI score should be careful with AG levels and changes of AG level before and after dialysis.
The strength of our study was that we analyzed AG level at different time points to clear its changes and association with risk of 30-day and 1-year mortality in critically ill patients undergoing RRT. The finding might help the clinicians better manage these patients and make appropriate treatments for them. Several limitations existed in the current study. Firstly, this was a retrospective study, which might cause bias in the data. Secondly, the reason of patients receiving RRT was not clear, and we conducted the analysis in subgroup based on eGRF to reduce the influence of this limitation. Thirdly, due to the limitations of the MIMIC-III database, the data on the treatments after discharge as well as the detection methods of AG related indexes including sodium, potassium, chloride, bicarbonate and albumin could not be obtained, which might influence the results in our study. In the future, more studies were required to verify the findings of this study.
Conclusion
The present study evaluated the associations of AG (T0), AG (T1), and ∆AG with the 30-day mortality or 1-year mortality in critically ill patients receiving RRT. We found AG before and after dialysis as well as the changes of AG were important prognostic biomarkers in critically ill patients receiving RRT. Dynamic monitoring of the changes helps improve bedside management of clinicians for patients. The results might provide a reference for the clinicians to continuously monitor AG levels and its changes at different time points for fluid management before and during RRT in critically ill patients requiring dialysis.
Data availability
The datasets used and/or analyzed during the current study are available from the MIMIC III database, https://mimic.physionet.org/iii/.
References
Kim KY, Ryu JH, Kang DH, Kim SJ, Choi KB, Lee S (2022) Early fluid management affects short-term mortality in patients with end-stage kidney disease undergoing chronic hemodialysis and requiring continuous renal replacement therapy. BMC Nephrol 23(1):102. https://doi.org/10.1186/s12882-022-02725-7
Naorungroj T, Neto AS, Yanase F, Eastwood G, Wald R, Bagshaw SM, Bellomo R (2021) Time to initiation of renal replacement therapy among critically ill patients with acute kidney injury: a current systematic review and meta-analysis. Crit Care Med 49(8):e781–e792. https://doi.org/10.1097/ccm.0000000000005018
Chappell K, Kimmons LA, Haller JT, Canada RB, He H, Hudson JQ (2021) Levetiracetam pharmacokinetics in critically ill patients undergoing renal replacement therapy. J Crit Care 61:216–220. https://doi.org/10.1016/j.jcrc.2020.10.032
Chaijamorn W, Rungkitwattanakul D, Pattharachayakul S, Singhan W, Charoensareerat T, Srisawat N (2020) Meropenem dosing recommendations for critically ill patients receiving continuous renal replacement therapy. J Crit Care 60:285–289. https://doi.org/10.1016/j.jcrc.2020.09.001
Woodward CW, Lambert J, Ortiz-Soriano V, Li Y, Ruiz-Conejo M, Bissell BD, Kelly A, Adams P, Yessayan L, Morris PE, Neyra JA (2019) Fluid overload associates with major adverse kidney events in critically ill patients with acute kidney injury requiring continuous renal replacement therapy. Crit Care Med 47(9):e753–e760. https://doi.org/10.1097/ccm.0000000000003862
Sharma S, Brugnara C, Betensky RA, Waikar SS (2015) Reductions in red blood cell 2,3-diphosphoglycerate concentration during continuous renal replacement therapy. Clin J Am Soc Nephrol 10(1):74–79. https://doi.org/10.2215/cjn.02160214
Cheng B, Li D, Gong Y, Ying B, Wang B (2020) Serum anion gap predicts all-cause mortality in critically ill patients with acute kidney injury: analysis of the MIMIC-III database. Dis Markers 2020:6501272. https://doi.org/10.1155/2020/6501272
Liu X, Feng Y, Zhu X, Shi Y, Lin M, Song X, Tu J, Yuan E (2020) Serum anion gap at admission predicts all-cause mortality in critically ill patients with cerebral infarction: evidence from the MIMIC-III database. Biomarkers 25(8):725–732. https://doi.org/10.1080/1354750x.2020.1842497
Lee SW, Kim S, Na KY, Cha RH, Kang SW, Park CW, Cha DR, Kim SG, Yoon SA, Han SY, Park JH, Chang JH, Lim CS, Kim YS (2016) Serum anion gap predicts all-cause mortality in patients with advanced chronic kidney disease: a retrospective analysis of a randomized controlled study. PLoS One 11(6):e0156381. https://doi.org/10.1371/journal.pone.0156381
Banerjee T, Crews DC, Wesson DE, McCulloch CE, Johansen KL, Saydah S, Rios Burrows N, Saran R, Gillespie B, Bragg-Gresham J, Powe NR (2019) Elevated serum anion gap in adults with moderate chronic kidney disease increases risk for progression to end-stage renal disease. Am J Physiol Renal Physiol 316(6):F1244–F1253. https://doi.org/10.1152/ajprenal.00496.2018
Arai Y, Tanaka H, Shioji S, Sakamoto E, Kondo I, Suzuki M, Katagiri D, Tada M, Hinoshita F (2020) Anion gap predicts early mortality after starting hemodialysis in the elderly. Clin Exp Nephrol 24(5):458–464. https://doi.org/10.1007/s10157-019-01844-0
Kaimori JY, Sakaguchi Y, Kajimoto S, Asahina Y, Oka T, Hattori K, Doi Y, Isaka Y (2022) Diagnosing metabolic acidosis in chronic kidney disease: importance of blood pH and serum anion gap. Kidney Res Clin Pract 41(3):288–297. https://doi.org/10.23876/j.krcp.21.200
Wieliczko M, Małyszko J (2022) Acid-base balance in hemodialysis patients in everyday practice. Ren Fail 44(1):1090–1097
Hernández-Jaras J, García H, Ferrero JA (2000) Changes in the anion gap in patients undergoing hemodiafiltration. Nefrologia 20(1):66–71
Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA, Mark RG (2016) MIMIC-III, a freely accessible critical care database. Sci Data 3:160035. https://doi.org/10.1038/sdata.2016.35
Wu WT, Li YJ, Feng AZ, Li L, Huang T, Xu AD, Lyu J (2021) Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res 8(1):44. https://doi.org/10.1186/s40779-021-00338-z
Sun T, Cai C, Shen H, Yang J, Guo Q, Zhang J, Zhang B, Ding Y, Zhou Y (2020) Anion gap was associated with inhospital mortality and adverse clinical outcomes of coronary care unit patients. Biomed Res Int 2020:4598462. https://doi.org/10.1155/2020/4598462
Fenves AZ, Emmett M (2021) Approach to patients with high anion gap metabolic acidosis: core curriculum 2021. Am J Kidney Dis 78(4):590–600. https://doi.org/10.1053/j.ajkd.2021.02.341
Sakaguchi Y, Hamano T, Kubota K, Oka T, Yamaguchi S, Matsumoto A, Hashimoto N, Mori D, Obi Y, Matsui I, Isaka Y (2018) Anion gap as a determinant of ionized fraction of divalent cations in hemodialysis patients. Clin J Am Soc Nephrol 13(2):274–281. https://doi.org/10.2215/cjn.07930717
Chen J, Li Y, Liu P, Wu H, Su G (2022) A nomogram to predict the in-hospital mortality of patients with congestive heart failure and chronic kidney disease. ESC Heart Fail. https://doi.org/10.1002/ehf2.14042
Asahina Y, Sakaguchi Y, Kajimoto S, Hattori K, Doi Y, Oka T, Kaimori JY, Isaka Y (2022) Association of time-updated anion gap with risk of kidney failure in advanced CKD: a cohort study. Am J Kidney Dis 79(3):374–382. https://doi.org/10.1053/j.ajkd.2021.05.022
Abramowitz MK, Hostetter TH, Melamed ML (2012) The serum anion gap is altered in early kidney disease and associates with mortality. Kidney Int 82(6):701–709. https://doi.org/10.1038/ki.2012.196
Domínguez-Cherit G, Namendys-Silva SA (2013) Changes in the anion gap: a novel marker of outcome in critically ill patients. Back to the basis. Crit Care Med 41(1):336–337. https://doi.org/10.1097/CCM.0b013e318270e799
Lipnick MS, Braun AB, Cheung JT, Gibbons FK, Christopher KB (2013) The difference between critical care initiation anion gap and prehospital admission anion gap is predictive of mortality in critical illness. Crit Care Med 41(1):49–59. https://doi.org/10.1097/CCM.0b013e31826764cd
Wang X, Yang S, Li S, Zhao L, Hao Y, Qin J, Zhang L, Zhang C, Bian W, Zuo L, Gao X, Zhu B, Lei XG, Gu Z, Cui W, Xu X, Li Z, Zhu B, Li Y, Chen S, Guo H, Zhang H, Sun J, Zhang M, Hui Y, Zhang X, Liu X, Sun B, Wang L, Qiu Q, Zhang Y, Li X, Liu W, Xue R, Wu H, Shao D, Li J, Zhou Y, Li S, Yang R, Pedersen OB, Yu Z, Ehrlich SD, Ren F (2020) Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 69(12):2131–2142. https://doi.org/10.1136/gutjnl-2019-319766
Gupta N, Buffa JA, Roberts AB, Sangwan N, Skye SM, Li L, Ho KJ, Varga J, DiDonato JA, Tang WHW, Hazen SL (2020) Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler Thromb Vasc Biol 40(5):1239–1255. https://doi.org/10.1161/atvbaha.120.314139
Kraut JA, Madias NE (2011) Consequences and therapy of the metabolic acidosis of chronic kidney disease. Pediatr Nephrol (Berlin, Germany) 26(1):19–28. https://doi.org/10.1007/s00467-010-1564-4
Arai Y, Kanda E, Kikuchi H, Yamamura C, Hirasawa S, Aki S, Inaba N, Aoyagi M, Tanaka H, Tamura T, Sasaki S (2014) Decreased mobility after starting dialysis is an independent risk factor for short-term mortality after initiation of dialysis. Nephrology (Carlton) 19(4):227–233. https://doi.org/10.1111/nep.12202
Shimomura A, Obi Y, Fazl Alizadeh R, Li S, Nguyen NT, Stamos MJ, Kalantar-Zadeh K, Ichii H (2017) Association of pre-operative estimated GFR on post-operative pulmonary complications in laparoscopic surgeries. Sci Rep 7(1):6504. https://doi.org/10.1038/s41598-017-06842-4
Moore BJ, White S, Washington R, Coenen N, Elixhauser A (2017) Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care 55(7):698–705. https://doi.org/10.1097/mlr.0000000000000735
Funding
This study was supported by The Basic Ability Enhancement Program for Young and Middle-aged Teachers of Guangxi (2020KY03028 to Y.L.Z.), Liuzhou Scientific Research and Technological Development Program (2019BJ10615 to Y.L.Z.), Guangxi Natural Science Foundation (2018GXNSFAA294055 to C.J.L.).
Author information
Authors and Affiliations
Contributions
YZ and CL designed the study. YZ wrote the manuscript. TZ, GZ, QH and JL collected, analyzed and interpreted the data. YZ critically reviewed, edited and approved the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethics approval and consent to participate
The requirement of ethical approval for this was waived by the Institutional Review Board of Affiliated Liutie Central Hospital of Guangxi Medical University, because the data was accessed from MIMIC-III (a publicly available database). All individuals provided written informed consent before participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11255_2023_3583_MOESM1_ESM.pdf
Supplementary Figure 1 The cumulative survival probability of 30-day mortality among participants as stratified by AG (T0) levels (PDF 76 KB)
11255_2023_3583_MOESM2_ESM.pdf
Supplementary Figure 2 The cumulative survival probability of 1-year mortality among participants as stratified by AG (T0) levels (PDF 74 KB)
11255_2023_3583_MOESM3_ESM.pdf
Supplementary Figure 3 The cumulative survival probability of 30-day mortality among participants as stratified by AG (T1) levels (PDF 75 KB)
11255_2023_3583_MOESM4_ESM.pdf
Supplementary Figure 4 The cumulative survival probability of 1-year mortality among participants as stratified by AG (T1) levels (PDF 73 KB)
11255_2023_3583_MOESM5_ESM.pdf
Supplementary Figure 5 The cumulative survival probability of 30-day mortality among participants as stratified by ∆AG levels (PDF 75 KB)
11255_2023_3583_MOESM6_ESM.pdf
Supplementary Figure 6 The cumulative survival probability of 1-year mortality among participants as stratified by ∆AG levels (PDF 72 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhai, Y., Luo, C., Zhou, T. et al. Associations of continuous anionic gap detection with the mortality in critically ill patients receiving renal replacement therapy. Int Urol Nephrol 55, 2967–2980 (2023). https://doi.org/10.1007/s11255-023-03583-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03583-4