Abstract
Purpose
Advanced age is associated with an impaired humoral immune response to SARS-CoV-2 mRNA vaccination in kidney transplant recipients (KTR). The mechanisms are, however, poorly understood. Frailty syndrome assessment may determine the most vulnerable population.
Methods
This study is a secondary analysis of a prospective study (NCT04832841) regarding seroconversion after BNT162b2 vaccination, including 101 SARS-CoV-2 naïve KTR 70 years and older. The Fried frailty components were evaluated, and antibodies against S1 and S2 subunits of SARS-CoV-2 were examined > 14 days after the second dose of BNT162b2 vaccine.
Results
Seroconversion was observed in 33 KTR. Male gender, eGFR, MMF-free immunosuppression, and a lower frailty score were associated with higher seroconversion rates in univariable regression. Concerning frailty components, physical inactivity had the most negative effect on seroconversion (OR = 0.36, 95% CI 0.14–0.95, p = 0.039). In a multivariable regression adjusted for eGFR, MMF-free immunosuppression, time from transplant and gender, pre-frail (OR = 0.27, 95% CI 0.07–1.00, p = 0.050), and frail status (OR = 0.14, 95% CI 0.03–0.73, p = 0.019) were associated with an increased risk of unresponsiveness to SARS-CoV-2 vaccines.
Conclusion
Frailty was associated with an impaired humoral response to SARS-CoV-2 mRNA vaccination in older SARS-CoV-2 naïve KTR.
Trail registration
This study is registered under the identifier NCT04832841 on ClinicalTrials.gov.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplant recipients (KTR) are considered at high risk for COVID-19 infection-associated morbidity and mortality [1]. Vaccination of this vulnerable population has, therefore, been recommended. However, recent studies have revealed a significantly impaired humoral response to mRNA vaccines in KTR compared to the general population [2]. The use of mycophenolate mofetil, depletive induction therapy, and costimulation blockade, as well as older age were found to be among the main risk factors of limited humoral response to SARS-CoV-2 mRNA vaccines [3,4,5] .
With improvements in KTR survival rates [6], elderly patients constitute a significant part of the KTR population. Elderly patients are at higher risk for infectious complications, which is frequently further aggravated by frailty syndrome [7]. Frailty syndrome, characterized by decreased physiologic reserve and resistance to stressors, further increases COVID-19-associated morbidity and mortality [1, 8].
Some previous studies have considered frailty a risk factor for decrease in flu vaccine effectiveness in the general population [9]. However, their findings are conflicting [10, 11]. Currently available data concerning the effect of frailty on seroconversion rates after SARS-CoV-2 vaccination in the general population are limited, and to the best of our knowledge, no study has yet pointed toward an association between frailty and impaired seroconversion in KTR [12]. We, therefore, aimed to assess the humoral response following vaccination with the mRNA SARS-CoV-2 vaccine (BNT162b2) in frail and non-frail elderly kidney transplant recipients.
Methods
Study design
This is a secondary analysis of a prospective single-center study (NCT04832841) performed in 2021 (April–June) [5]. Our objective is to assess the seroprevalence of anti SARS-CoV-2 S1/S2 IgG assessment following SARS-CoV-2 mRNA vaccination in KTR. Frailty assessment was offered to KTR 70 years and older who were SARS-CoV-2 naïve and had received two doses of mRNA BNT162b2 vaccine. Antibody testing was done at least 14 days after the second dose of BNT162b2 vaccine. Other inclusion and exclusion criteria and a description of antibody testing have already been reported [5, 13]. Finally, 103 KTRs were enrolled in the study (Fig. 1). Two KTR were excluded due to a positive SARS-CoV-2 PCR test following the first vaccine dose and eighty-two KTR declined frailty assessment.
Study flowchart. A total of 203 kidney transplant recipients (KTR) from the original study (NCT04832841) were 70 and more years old. Five KTR were vaccinated while on the waiting list prior to transplantation, thirteen were COVID-19 positive prior to vaccination and eighty-two KTR were enrolled in the original study but were not interested to participate in the frailty assessment. A total of 103 KTR were enrolled in this study and of them 2 experienced COVID-19 PCR positivity after the 1st vaccine dose. Thus, a total of 101 KTR were included in the final analysis
Frailty assessment
Frailty was evaluated according to Fried frailty criteria and on the same day, blood samples for antibody testing were collected. Frailty was determined based on five components: weight loss (self-reported unintentional weight loss of more than 10 lbs in the past year); weakness (grip strength below an established cutoff based on gender and BMI); exhaustion (self-reported); physical inactivity (kcals/week below an established cutoff); slower walking speed (walking time of 15 feet below an established cutoff by gender and height) [8]. Each of the five components was scored 0 or 1 depending on the absence or the presence of the component, respectively. The cumulative Fried frailty score was calculated as the sum of the component scores (range 0–5). Non-frail status was defined as a score of 0, pre-frail status was defined as a score of 1–2, and frail status was defined as a score of 3 and higher [8].
Statistical analysis
Continuous variables are expressed as medians (min, max) and categorical variables are expressed as n and a percentage of the total and compared by Pearson Chi-square test. Univariable and multivariable binary regression analyses were used to predict the responsiveness to SARS-CoV-2 vaccination. The multivariable binary logistic regression model was based on the multivariable model of the primary study and included a variable for frailty as well as variables which were previously found to be independently associated with vaccine immune response. The independent variables associated with vaccine immune response in the primary study were age, gender, time from transplant to vaccination, eGFR, mycophenolate use, and depleting therapy in the last year [5]. However, age was not included in the current final model, as all the KTR were elderly within few years of each other. Furthermore, no elderly KTR received depleting therapy in the last year, and thus this variable could also not be included. Aside from mycophenolate use, no other immunosuppression variable was entered into the model, as none were independently associated with vaccine response in the original study. Sensitivity analyses of multivariable binary logistic regression model was calculated using bootstrap resampling. Additional sensitivity analysis was performed where all statisticaly significant variables in univariable regression were also included in the multivariable model. A p value of less than 0.05 was considered statistically significant. There were no missing data of variables of interest. Statistical analysis was performed using IBM SPSS Statistics, Version 24 (International Business Machines Corp.) and R-studio version 4.0.3. (2020-10-10).
Results
A total of 101 elderly KTR naïve to COVID-19 were included in the analysis. Males and females were represented equally. The median age was 73 years, most patients had undergone the first transplantation, and the median follow-up from transplantation was 6.2 years. Details on demography and immunosuppression are provided in Table 1. In the entire cohort, seroconversion after two doses of mRNA BNT162b2 vaccine was observed in 33% KTRs who were older than 70 years of age.
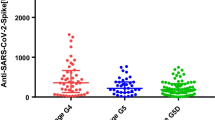
Twenty-seven (27%) patients were classified as frail and forty-eight (48%) patients as pre-frail. General characteristics of patients divided by frailty status are provided in the Supplementary Table 1. Exhaustion (45%) and physical inactivity (36%) were the most frequently observed Fried frailty components. Weakness, slow walking speed, and weight loss were observed in 29%, 24%, and 12%, respectively. Seroconversion after vaccination was observed in 12 out of 27 (44%) non-frail, 15 out of 48 (31%) pre-frail, and 6 out of 26 (23%) frail KTR, respectively. Interestingly, there were 36 (36%) KTR who were physically inactive, and 29 of them (80%) did not mount antibodies after vaccination (p = 0.046) (Table 2).
Seroconversion was further used as the dependent variable for univariable and multivariable binary logistic regression. The variables associated with seroconversion in univariable regression were eGFR (OR = 1.03, 95% CI 1.01–1.06, p = 0.001), MMF-free immunosuppression (IS) regimen (OR = 4.32, 95% CI 1.69–11.07, p = 0.002), CNI-based IS (OR = 0.22, 95% CI 0.07–068, p = 0.009), and mTOR inhibitor IS (OR = 4.03, CI 1.21–13.52, p = 0.024). Male gender (OR = 2.09, 95% CI 0.89–4.9, p = 0.091), depletive induction IS (OR = 0.43, CI 0.17–1.09, p = 0.076), and Fried frailty cumulative score (OR = 0.74, 95% CI 0.52–1.05, p = 0.09) did not reach statistical significance. Age did not affect seroconversion in our cohort (OR = 1.07, 95% CI 0.95–1.2, p = 0.267) (Table 3).
Next, a multivariable binary logistic regression model was calculated. Pre-frail (OR = 0.27, 95% CI 0.07–1.00, p = 0.050) and frail (OR = 0.14, 95% CI 0.03–0.73, p = 0.019) status were associated with an increased risk of unresponsiveness to SARS-CoV-2 vaccination. The model was adjusted for eGFR (OR = 1.05, 95% CI 1.02–1.08, p < 0.001), MMF-free immunosuppression (OR = 10.14, 95% CI 2.83–36.25, p < 0.001), longer time from transplant (OR = 2.92, 95% CI 1.19–7.18, p = 0.019), and gender (Fig. 2). The stability of confidence intervals of all variables in the multivariable binary logistic regression model was checked internally using bootstrap resampling (n = 1000; Supplementary Table 2). Furthermore, an additional sensitivity analysis was performed where all statisticaly significant variables in univariable regression were also included in the multivariable model. This approach did not alter the study results (Supplementary Table 3).
Furthermore, Fried frailty components were separately analyzed in univariable regression (Table 4). The most significant negative impact on seroconversion was observed for physical inactivity (OR 0.36, 95% CI 0.14–0.95, p = 0.039). Weakness (hand grip strength) alone did not affect seroconversion; however, better seroconversion was observed in KTR with a higher absolute value of maximal hand grip strength (OR = 1.05, CI 95% 1.01–1.11, p = 0.045).
Discussion
In this study, we aimed to evaluate the association between frailty and an impaired humoral response to SARS-CoV-2 mRNA vaccination in elderly virus-naïve KTR. We observed a better seroconversion rate in the non-frail group, compared to the pre-frail and frail KTR groups. Furthermore, of the Fried frailty components, physical inactivity had the most negative impact on seroconversion.
Frailty was assessed using Fried frailty criteria, which were previously validated by McAdams-DeMarco in KTR [14]. The prevalence of frailty syndrome was studied in kidney transplant candidates, but less is known about the long-term frailty prevalence in KTR. The group of McAdams-DeMarco observed that 23.7% kidney transplant candidates 65–75 years old and 22.7% transplant candidates older than 75 years old are frail at the time of transplant [15]. Although frailty might be reversible after transplant, it’s incidence rises later in the follow-up period after transplantation [16]. These results support our observation that every fourth elderly KTR was assessed as frail.
Among Fried frailty components, physical inactivity and exhaustion were most frequently observed in our cohort. The group of McAdams-DeMarco described poor grip strength and low physical activity as the most frequent frailty components in KTR at time of transplant, exhaustion was present in 25% of patients [15]. Since the majority of our patients described deconditioning and exhaustion during the pandemic lockdown, we hypothesize that prolonged social isolation due to the COVID-19 pandemic might have a negative impact on physical activity in this vulnerable population [17].
In our study, low physical activity showed the best correlation with humoral response among all the Fried frailty components. This observation is in line with previous reports that studied humoral responses to the flu vaccination and suggested better humoral responses among more physically active older adults [18] and among healthy adults that exercised prior to vaccination [19].
The seroconversion rate in our KTR cohort was only 33% as opposed to the 45.8% observed in the whole cohort in the primary study [5]. While age had a significant impact on seroconversion in the primary study (adults 18 +), we have not found any link between age and seroconversion rates in SARS-CoV-2 naïve KTR older than 70 years of age. The impact of age on vaccination response is poorly understood; however, lower antibody production and T-cell response after the first dose of BNT162b2 vaccine in 80 + years old individuals was previously reported [20]. While number of studies indicated that seroconversion rate in older KTR after SARS-COV-2 vaccination was impaired, our study suggests that the impact of age is no longer relevant among KTR older than 70 years [2, 4, 5].
Similarly to previous studies, we identified an association between MMF therapy and an impaired humoral response to vaccination in frail KTR [21]. Therefore, when analyzing frailty effects on seroconversion, the adjustment for immunosuppression was necessary. Interestingly, all four KTR, who were not frail and did not receive MMF, mounted antibodies after SARS-COV-2 mRNA vaccination. Conversely, seroconversion rate of just 17% was observed in frail KTR treated with MMF. The effect of frailty on the response to flu vaccination was studied in the general population with inconclusive results [11, 22], and data regarding the response to SARS-CoV-2 vaccines in frail subjects are scarce. Ríos et al. [12] showed no effect of frailty on post-vaccination antibody levels among nursing homes residents. However, a major limitation of said study is that majority of the subjects had previously experienced COVID-19 infection and were not virus naïve, which, in turn, biased the observation regarding vaccine-induced antibody production. To the best of our knowledge, our study is the first to assess the effect of frailty on mRNA vaccination response in solid organ recipients.
Based on the findings of our study, we suggest that geriatric assessment might be a useful tool to identify the most vulnerable KTRs at risk of insufficient vaccine response. These patients might, therefore, benefit from interventions such as application of additional vaccine booster doses or passive immunization.
The limitation of our study is the lack of validation cohort and the lack of data concerning the cellular response, as the measurement was not performed.
In conclusion, our study shows a significantly impaired humoral response to SARS-CoV-2 mRNA vaccination in frail elderly KTR who were naïve to SARS-CoV-2.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Abbreviations
- AU:
-
Arbitrary units
- BMI:
-
Body mass index
- CNI:
-
Calcineurin inhibitor
- COVID-19:
-
Coronavirus disease 2019
- eGFR:
-
Estimated glomerular filtration rate
- IgG:
-
Immunoglobulin G
- IS:
-
Immunosuppression
- IU:
-
International units
- KTR:
-
Kidney transplant recipients
- PCR:
-
Polymerase chain reaction
- MMF:
-
Mycophenolate mofetil
- mRNA:
-
Messenger ribonucleic acid
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SPSS:
-
Statistical package for the social sciences
References
Hilbrands LB, Duivenvoorden R, Vart P et al (2020) COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 35(11):1973–1983. https://doi.org/10.1093/ndt/gfaa261
Marion O, Del Bello A, Abravanel F et al (2021) Predictive factors for humoral response after 2-dose SARS-CoV-2 vaccine in solid organ transplant patients. Transplant Direct. 8(1):e1248. https://doi.org/10.1097/txd.0000000000001248
Grupper A, Rabinowich L, Schwartz D et al (2021) Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 21(8):2719–2726. https://doi.org/10.1111/ajt.16615
Benotmane I, Gautier-Vargas G, Cognard N et al (2021) Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 99(6):1498–1500. https://doi.org/10.1016/j.kint.2021.04.005
Magicova M, Zahradka I, Fialova M et al (2022) Determinants of immune response to anti-SARS-CoV-2 mRNA vaccines in kidney transplant recipients: a prospective cohort study. Transplantation. https://doi.org/10.1097/TP.0000000000004044
Hariharan S, Israni AK, Danovitch G (2021) Long-term survival after kidney transplantation. N Engl J Med 385(8):729–743. https://doi.org/10.1056/nejmra2014530
Demaret J, Corroyer-Simovic B, Alidjinou EK et al (2021) Impaired functional T-cell response to SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine in older people. Front Immunol. https://doi.org/10.3389/fimmu.2021.778679
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: Evidence for a phenotype. Journals Gerontol Ser A Biol Sci Med Sci. https://doi.org/10.1093/gerona/56.3.m146
Yao X, Hamilton RG, Weng N et al (2011) Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine 29(31):5015–5021. https://doi.org/10.1016/j.vaccine.2011.04.077
Moehling KK, Nowalk MP, Lin CJ et al (2018) The effect of frailty on HAI response to influenza vaccine among community-dwelling adults ≥ 50 years of age. Hum Vaccines Immunother 14(2):361–367. https://doi.org/10.1080/21645515.2017.1405883
Loeb N, Andrew MK, Loeb M et al (2020) Frailty is associated with increased hemagglutination- inhibition titers in a 4-year randomized trial comparing standard- And high-dose influenza vaccination. Open Forum Infect Dis. https://doi.org/10.1093/OFID/OFAA148
Salmerón Ríos S, Mas Romero M, Cortés Zamora EB et al (2021) Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J Am Geriatr Soc 69(6):1441–1447. https://doi.org/10.1111/jgs.17153
Magicova M, Fialova M, Zahradka I et al (2021) Humoral response to SARS-CoV-2 is well preserved and symptom dependent in kidney transplant recipients. Am J Transplant 21(12):3926–3935. https://doi.org/10.1111/ajt.16746
McAdams-DeMarco MA, Law A, Salter ML et al (2013) Frailty and early hospital readmission after kidney transplantation. Am J Transplant 13(8):2091–2095. https://doi.org/10.1111/ajt.12300
McAdams-DeMarco MA, Ying H, Olorundare I et al (2017) Individual frailty components and mortality in kidney transplant recipients. Transplantation 101(9):2126–2132. https://doi.org/10.1097/TP.0000000000001546
Chu NM, Ruck J, Chen X et al (2022) Long-term trajectories of frailty and its components after kidney transplantation. J Gerontol Ser A. https://doi.org/10.1093/gerona/glac051
Dor-Haim H, Katzburg S, Revach P, Levine H, Barak S (2021) The impact of COVID-19 lockdown on physical activity and weight gain among active adult population in Israel: a cross-sectional study. BMC Public Health. https://doi.org/10.1186/s12889-021-11523-z
Schuler PB, Leblanc PA, Marzilli TS (2003) Effect of physical activity on the production of specific antibody in response to the 1998–99 influenza virus vaccine in older adults. J Sports Med Phys Fitness 43(3):404. http://www.ncbi.nlm.nih.gov/pubmed/14625524.
Edwards KM, Burns VE, Allen LM et al (2007) Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun 21(2):209–217. https://doi.org/10.1016/J.BBI.2006.04.158
Collier DA, Ferreira IATM, Kotagiri P et al (2021) Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 596(7872):417–422. https://doi.org/10.1038/s41586-021-03739-1
McAdams-Demarco MA, Law A, Tan J et al (2015) Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation 99(4):805–810. https://doi.org/10.1097/TP.0000000000000444
Moehling KK, Zhai B, Schwarzmann WE et al (2020) The impact of physical frailty on the response to inactivated influenza vaccine in older adults. Aging (Albany NY) 12(24):24633–24650. https://doi.org/10.18632/aging.202207
Acknowledgements
The authors would like to thank Marie Kolarova for administrative help, Olga Kaucka for the collection of the biological material, and Hedvika Cacarova for proofreading.
Funding
Open access publishing supported by the National Technical Library in Prague. This study was supported by the Ministry of Health of the Czech Republic, Grant no. NV19-06-00031, NU22-C-126 and NU21-06-00021, and by the Charles University research program Cooperation 34 -Internal disciplines and by conceptual development of research organizations (Institute for Clinical and Experimental Medicine-IKEM, IN 00023001).
Author information
Authors and Affiliations
Contributions
MS, HV, and SRB performed the frailty assessment; PH and JG performed statistical analyses; MF and IS antibody detection; MM and IZ clinical data collection; MS, HV, SRB, IZ, and OV wrote the manuscript. All authors reviewed and edited the final manuscript. MS, HV, and SRB contributed equally to this work.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethics approval
The local ethical board approved this study under No. G-21-07. Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmalz, M., Vankova, H., Rajnochova-Bloudickova, S. et al. The impact of frailty syndrome on humoral response to SARS-CoV-2 mRNA vaccines in older kidney transplant recipients. Int Urol Nephrol 55, 2959–2965 (2023). https://doi.org/10.1007/s11255-023-03557-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03557-6