Abstract
Introduction
Patients under renal replacement therapy are at an increased risk of severe infection with SARS-CoV-2, and have been known to have impaired response to standard vaccination. This systematic review and meta-analysis aims at evaluating the efficacy of booster dose vaccination in this population.
Methods
A systematic review has been conducted to find trials on the booster dose vaccination in kidney transplant recipients (KTRs) or patients under dialysis. Data of seroconversion rates at different timepoints, especially 1 month prior and post-booster dose vaccination have been collected and analyzed. Effects of different factors including type of renal replacement therapy (RRT), vaccine type and brands, magnitude of response to the standard vaccination, and immunosuppression drugs on the response rates have been investigated. Meta-analyses were performed using software Stata v.17.
Results
Overall 58 studies were included. Both RRT patient subgroups represented significant seroconversion, post- (versus pre-) booster dose vaccination, but only in KTRs the booster dose seroconversion surpassed that of the standard protocol. T-cell response was also significantly augmented after booster vaccination, with no difference between the RRT subgroups. mRNA and vector vaccine types had comparable immunogenicity when employed as boosters, both significantly higher than the inactivated virus vaccine, with no significant disparity regarding the vaccine brands. Patients with poor response to standard vaccination had a significant response to booster dose, with dialysis patients having stronger response. The differential effects of vaccine types and brands in the poor responders was similar to that of the overall RRT population. No rejection episodes or graft failure post-booster vaccination was reported.
Conclusion
In patients under RRT, booster dose vaccination against SARS-CoV-2 is safe and efficacious determined by significant seroconversion, and therefore, it should be considered to be implemented in all these patients. Since in the KTR patients, the third dose vaccination significantly increased the seroconversion rates even beyond that of the standard protocol, three dose vaccine doses is recommended to be recognized as the standard vaccination protocol in this population. The same recommendation could be considered for dialysis patients, due to their augmented risk of breakthrough infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ongoing SARS-CoV-2 infection pandemic had been associated with huge morbidity, mortality and economic burden. By 23 October 2022, over 624 million people have had documented COVID-19 infection worldwide, and more than 6.5 million died [1]. The level of the pandemic impact on almost every aspect of human life was extremely high necessitating extended lockdowns, strict precaution measures and restrictions of local and international transportations. These unprecedented effects were of such magnitude as to prompt extraordinary attempts at producing potent vaccines at a speed that is now considered a historical record. After the introduction of the vaccines, overwhelming data from different studies indicated that vaccines were capable of inducing high rates of humoral and cellular responsiveness besides providing clinical protection against the infection, hospitalizations and mortality [2, 3].
Subsequently, reports from multiple large-scale researches indicated that certain people, most notably the elderly, the immunocompromised and those with certain comorbidities (e.g. hypertension, lung disorders, etc.) are significantly at higher risk of infection and specially its sequelae [4]. One of these at risk populations are chronic kidney disease patients with reports indicating high rates of hospitalizations and deaths due to COVID-19 infection. On the other hand, controversial reports have been published on the efficacy of COVID-19 vaccinations in this and other subgroups of patients with immunocompromised states indicating suboptimal responses to standard vaccination in either laboratorial assays or clinical settings [4]. Nevertheless, subsequent introduction of booster vaccinations has substantially changed the current knowledge with reports of higher protection levels offered by booster vaccination [5]. In this study, the aim had been to investigate the effects of booster vaccination on the humoral and cellular immune response to SARS-CoV-2 spike antigen in patients under renal replacement therapy (RRT) including patients undergoing conventional dialysis (hemodialysis (HD) or peritoneal dialysis (PD)) and kidney transplant recipients (KTR).
Methodology
Literature search strategy
A systematic review of the literature has been conducted on August 2022 in the Medline/Pubmed and Europe PMC search engines in quest of studies reporting their experience with the efficacy of booster vaccination against SARS-CoV2 infection in patients under RRT. The literature search had been lastly updated on September 26, 2022 to include the potential new publications or finding the potential missing ones. The found studies have been reinvestigated for their citations through Google Scholar searches to find more studies potentially missed by the original method. Search terms mainly included “Third dose + COVID-19 vaccination + dialysis”, “Booster + COVID-19 vaccine + kidney transplant”, and different combination of terms including word-level substitutions of terminologies (e.g. SARS-CoV-2 for COVID-19, renal for kidney, hemodialysis, “peritoneal dialysis” or “renal replacement therapy” for dialysis, “third dose” or “forth dose” for booster, mRNA-1273 for Moderna, and so forth). Due to the subspecial target population to the systematic review, no restriction was set for inclusion regarding the studies’ publication dates, study design (prospective or retrospective), language (to the extent they could be comprehended by the author—finally all the reviewed reports were in English), inclusion of a control group (or not), vaccine type/formula, vaccine brands, laboratory assays employed, booster order (third, fourth, etc.), status regarding peer-reviewing (pre-print studies have also been included) was applied. Sample size had a restriction criterion: to be included, studies should have had at least ten reported cases. As well, in studies reporting heterogeneous patient populations (e.g. solid organ transplants), data were included into the analysis only if the regarded information for the study target populations (HD, PD or KTR) could be discretely retrieved.
Study selection
The literature screening was initially conducted reviewing the article titles. Studies with relevant titles were further reviewed by their abstracts, and in case the abstracts implicated data of interests, the fulltexts were reviewed. Finally, meta-analysis of data were performed from the studies meeting the following criteria: human participants with end-stage renal disease under RRT methods (HD, PD, KTR) receiving standard SARS-CoV-2 vaccination who had received at least one booster dose from any type or any manufacturer. Studies containing data from RRT patients were included into the meta-analyses irrespective of the inclusion of healthy control groups.
Data extraction
Supplementary tables1 and 2 summarize the data of interest that have been sought and collected for each study on the KTR and dialysis patients, respectively. Data were extracted according to a designed checklist in Microsoft Excel. The key information were re-stratified according to the checklist, and reviewed twice by the author. The extracted data included the RRT setting (KTR, HD/PD), study design (retrospective or prospective), country of study, vaccine type (i.e. mRNA, viral vector and whole inactivated virus) and brands (i.e. BNT162b2 (Pfizer), mRNA-1273 (Moderna), ChAdOx1, Ad26.COV2.S (J&J), and CoronaVac), study population, immunosuppressant employed, predefined threshold levels of antibody response (cutoff for seroconversion), poor response to the standard vaccination (including non-responders (no seroconversion) and suboptimal responders); data for each extracted separately as well), time of antibody testing after booster dose, assays employed for the antibody testing, T cell reaction, seroconversion before and after the booster dose (one study employed data from a control group in spite of pre-booster dose measure [12]), any rejection episodes or graft failure after booster vaccination, baseline serum creatinine levels for responders and non-responders, and breakthrough infections after booster dose vaccination. Wherever possible, only naïve patients were included into the analyses and those with a history of infection were excluded. Studies that had only included non- or suboptimal-responders to the standard two-dose vaccination were excluded from the epidemiological reports of booster dose immunogenicity and were included into separate meta-analyses of patients with non- and suboptimal-response to the conventional two-dose schedules. Moreover, data from patients under highly potent monoclonal antibodies especially belatacept, were not included into the main epidemiological analyses wherever possible, but were secured for inclusion into the meta-analyses of the immunosuppressant effects.
Outcomes’ definition
Primary outcomes
The main primary outcomes of interest in this study were the differential rates of breakthrough infections, hospitalizations and death due to COVID-19 infection after standard- versus booster dose vaccinations in patients on RRT. Although individual studies had occasional data on some of the above-mentioned factors, none of which met the criteria required for inclusion into meta-analyses. The only data existing at an enough volume for meta-analysis was the breakthrough infection rates just after booster vaccination. Besides, seropositivity rates post-booster vaccination were analyzed for the renal patients compared to that in healthy controls. Seroconversion rates early after a booster dose (about 4 weeks after the third or fourth dose) was the next primary outcome index. For this purpose, serum antibody levels or reports of seropositivity status just before the booster doses and one month after had been collected for the efficacy analysis. Whenever data were unavailable on the seropositivity before the booster dose, the epidemiology of post-booster seropositivity were pooled and meta-analyzed separately. As well, data of seroconversion 1 month after the standard (2-dose) vaccination were collected for comparing with the post-booster data to evaluate the potential cumulative effect of the booster doses on inducing seroconversion (versus only compensating for the waning immunity). Another measure of primary outcome was T cell-specific immunity rates in response to booster dose vaccination against SARS-CoV-2.
Secondary outcomes
In the secondary outcomes, factors affecting the response to booster vaccination were sought. The differential impact of booster vaccination in KTR patients versus those under dialysis, the disparity in response to the booster dose regarding the type (mRNA versus vector vaccines) and brands (BNT162b2 vs. mRNA-1273) of the administered vaccines in different contexts (regarding the study participants’ response to the standard vaccinations) were some of the predefined secondary outcomes. Finally, the differential impact of the immunosuppressant types, which were employed for preventing kidney allograft rejection, on the seroconversion rates was also investigated.
Data synthesis and statistical analysis
DerSimonian and Laird random effects model was used to estimate the pooled Odds ratios and corresponding 95% confidence intervals for the primary and secondary outcomes of interest. An Odds ratio > 1 indicates that the RRT participants had a higher rate of achieving response (i.e. seroconversion or T cell response) post-booster vaccination compared to the control (pre-booster, post-dose 2 vaccine or healthy controls). Only in one case (RRT patients versus healthy controls), meta-analyses of randomized controlled trials could be achieved, and in the other subgroup analyses, the comparisons were made in the same subjects between measurements at different timepoints (i.e. 1 month after booster COVID-19 vaccination compared with own serostatus before booster vaccination at two timepoints: right prior to the booster dose or one month post-dose 2 of the standard protocol). In one study, the comparisons were between post-booster cases and controls’ measures at the same timepoint [12]. Statistical heterogeneity of the meta-analyses was assessed using χ2 test and I2 statistic, with p values < 0.10, or the I2 statistic ≥ 50% as definitions for significant heterogeneity. To avoid biases, studies containing significant bias in their included populations or approaches were excluded in each subsection meta-analysis, regarding the analysis purpose. For example, in the overall response to the booster doses, studies including only patients with impaired response to the standard vaccination protocol or those including only patients under highly potent monoclonal immunosuppression were excluded. That same criteria were applied to other subsection analyses including the vaccine type or brand, and immunosuppression effect meta-analyses.
Subgroup analyses were conducted to determine whether the results were influenced by the type of RRT, vaccine types or brands, and the levels of response to the standard vaccination (i.e. normal, weak- or non-responders). Stratifications were then used to estimate effect sizes in different subgroups, and to compare the differences between the respective estimates. Since the serum anti-SARS-CoV-2 antibody levels had been measured through different methods and assays, they were considered to be amenable to statistical pooling for meta-analysis.
In subgroup analyses where there was no pre-booster results for comparisons (e.g. breakthrough infections, or overall post-booster seroconversion rates irrespective of the existence of pre-booster measurements), the absolute risk was measured as a proportion from 0 to 100%. All statistical analyses were conducted using STATA vs.17 (StataCorp.) and p value of < 0.05 were considered statistically significant.
Results
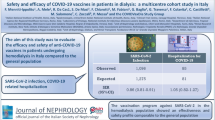
Figure 1 illustrates flowchart of the study selection and review process. Overall 58 studies [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63] with a total number of 8596 participants were finally selected and included into the systematic review and meta-analysis. The included trials had been conducted in Austria, Brazil, Canada, Denmark, France, Germany, Israel, Italy, Japan, Norway, Poland, Spain, Thailand, and USA. Characteristics of the included trials are summarized in supplementary tables 1 and 2. No rejection episodes or graft failure after booster dose was reported by any of the reviewed studies.
Primary outcome
Figure 2 illustrates the forest plot of the meta-analysis of the seroconversion rates in response to booster vaccination in renal patients versus healthy controls. Compared to the healthy controls, the KTR patients represented significantly lower rates of seroconversion for SARS-CoV-2 post-booster vaccination (Odds ratio (95% CI), 0.05 (0.01–0.28; I2 = 0%), while the associated rates were not significant for patients under dialysis (0.68 (0.06–7.13; I2 = 0%); the between group difference (dialysis vs KTRs) did not reach significance level (p = 0.08).
As shown in Fig. 3, compared to the pre-booster measurements, seropositivity was significantly augmented 1 month after booster vaccination for both the RRT subgroups (Odds ratio (95% CI) of 3.99 (3.16–5.04; I2 = 55.8%) and 6.53 (2.32–18.39; I2 = 69.6%) for the KTR and dialysis patients, respectively), with no significant difference between the two groups (p = 0.28). However, the overall post-booster dose seropositivity rate (irrespective of the pre-booster status) was significantly higher in the dialysis population than the KTR patients (0.88 (0.84–0.93; I2 = 86.4%) vs. 0.69 (0.64–0.73; I2 = 83.6%), respectively; p < 0.01) (Fig. S2). On the other hand, compared to the rates 1 month after the second dose, third dose vaccination was associated with significantly higher seropositivity at 1 month only in the KTR subgroup, with no significant difference detected for the dialysis patients (odds ratio (95% CI), 3 (1.82–4.95; I2 = 74.7%) and 1.42 (0.66–3.04; I2 = 0%), respectively) (Fig. S3).
Due to data scarcity on reports of breakthrough infections post-standard (two-dose) vaccination, only reports of breakthrough infections post-booster vaccination were included into meta-analysis. Using the DerSimonian–Laird method, the overall estimated incidence [95% confidence interval (CI)] of breakthrough infection with SARS-CoV-2 infection was 0.06 (95% CI 0.04–0.08; I2 = 93.2%). As illustrated in Fig. S4, compared to the KTR patients, the respective estimates for patients under dialysis had a higher trend of breakthrough infections [0.05 (95% CI 0.02–0.08; I2 = 92.3% vs. 0.10 (95% CI 0.05–0.16; I2 = 70.5%); though significance levels was not achieved, p = 0.09)]. T cell-specific response rates post-booster vaccination were very diverse in different trials with an overall 1.65 (0.98–2.77) and 2.89 (1.47–5.72) times larger rates compared to pre-booster status for the KTR and dialysis patients, respectively (p = 0.2, figure S5). Since some of the studies had no reports of cellular response prior to the third dose, the overall rate of T-cell specific reactivity post-booster vaccination were pooled and meta-analyzed which returned an overall rate (95% CI) of 0.53 (0.31–0.76) with no significant difference between the KTR and dialysis patients (p = 0.24, Fig. S6). Other predefined primary outcome indices could not be achieved due to the lack of reported data.
Secondary outcome
Although there are large body of evidence on the magnitude of the effects imposed by different vaccine types or brands on the seroconversion rates, the issue in RRT patients has not been broadly discussed in the literature. Figure 4 illustrates the meta-analysis of response to booster dose vaccination compared to the pre-booster serostatus stratified by the vaccine type, indicating that both vector and mRNA vaccine types when administered as the booster could significantly augment seropositivity with no significant difference, while both are superior to the inactivated virus vaccine. Similar findings were reached when vaccine brands of the booster dose were investigated, representing no significant difference between different vaccine brands (notably between BNT162b2 (Pfizer) & mRNA-1273 (Moderna) for mRNA vaccines) (Fig. S7). Results were reproduced when the meta-analysis included stratification by the RRT types (i.e. KTR patients taking BNT162b2 vs. mRNA-1273, etc.; Fig. S8).
Poor response to the standard vaccination and its effect on the immunogenicity of the booster dose was the next subject of investigation. As illustrated in figure S9, primarily poor responders to the standard vaccination seroconverted at a rate (95% CI) of 0.77 (0.65–0.89; I2 = 77.9%) and 0.41 (0.36–0.47; I2 = 87%) for dialysis and KTR patients, respectively, with dialysis patients representing a significantly higher seroconversion rates than KTRs (p < 0.01). The significance of the vaccine types as the booster dose on seroconversion in this subpopulation was also investigated; mRNA and vector-based vaccine types were equally efficacious to induce seroconversion (0.51 (0.49–0.54; I2 = 0%) and 0.52 (0.23–0.82; I2 = 86.6%), respectively) while both were significantly more efficacious than inactivated virus vaccine (0.20 (0.16–0.23; I2 = 0%) (Fig. S10). Reanalysis after stratifying the data based upon vaccine brands did retuned no significant effect (Fig. S11).
Then poor-responders’ data were substratified again to investigated booster dose effects, separately for non-responders and suboptimal responders of the standard 2-doses. Dialysis patients in either group were significantly more likely to seroconvert in response to boosters compared to the KTRs (0.66 (0.58–0.73; I2 = 0%) vs. 0.42 (0.36–0.48; I2 = 52.1%), p < 0.01 Fig. S12, and 0.88 (0.83–0.94; I2 = 0%) and 0.33 (0.31–0.34; I2 = 0%), p < 0.01 Fig. S13, respectively).
Demographic data
No significant gender bias was found regarding the response to boosting vaccination (Fig. S1). Attempts on pooling data of other demographic factors including the baseline serum creatinine/eGFR levels, age, vintage on dialysis (or transplantation), and BMI failed because of either the inconsistencies in the measurement units (i.e. mg/dL versus µM for creatinine and different time units (i.e. years/months/days) for vintage statistics) or different statistical indices for reports [i.e. mean (SD) or median (IQR/range)]. Yet attempts have been made to provide an overview. In case of baseline serum creatinine levels, except for one study [17], all other reports indicated trends of lower serum creatinine values in seroresponders than in non-responders [53, 58, 61], with some of them reaching significance [18, 21, 60]. Vintage on RRT, however, tended to be longer in responders in all [17, 21, 32, 53, 61], but one [13] study. Likewise, in only one study [32], the BMI was higher in the non-responders, and in the remaining reports, seroconverters characterized with higher BMI trends [13, 17, 21, 61]. Age effect on the seroresponse was very inconsistent in different studies. While in three studies responders were inclined towards older ages [17, 32, 61], in three others, advanced age was associated with non-responsiveness [13, 21, 53].
Immunosuppressant effect
Figures S14-S33 summarize meta-analyses investigating differential effects of the immunosuppressant drugs on their serological response to vaccination. As is evident in the figures, the lowest booster vaccination response belonged to patients under belatacept, significantly lower than that under mycophenolate mofetil (MMF), tacrolimus, calcineurin inhibitors (CNi, i.e. tacrolimus and cyclosporine), Mammalian target of rapamycin inhibitors (mTORi, i.e. sirolimus and everolimus), and steroids, while the best response was detected for patients under treatment with mTORi significantly higher than that for MMF, tacrolimus or CNi, steroids, basiliximab, and belatacept. No other significant difference was detected regarding the type of immunosuppressant.
Discussion
This systematic review of 58 studies showed significant cellular and humoral immune response in patients under either type of RRT (i.e. dialysis, kidney transplants) after booster dose vaccination against SARS-CoV-2 infection. Although cellular or serological response does not essentially mean protection against infection, however, due to overwhelming epidemiological data on the prevalence of infection in patients with or without immune reactivity, findings of this study put high priority on boosting vaccination for these patients.
The detected rise in the seropositivity rates after boosting vaccination can be interpreted in two ways: compensating for the waning immunity after the standard two-dose vaccination, and/or through intensification of the response to the standard protocol. The literature suggests lower seroconversion rates after vaccination against SARS-CoV-2 in both dialysis and KTR patients than that for the healthy controls (27%, 88% and 100%, respectively), with a highly significant difference between the RRT methods [64]. In the current study, the post-booster seropositivity rates were 41%, 77% and 100% for the KTR, dialysis and healthy controls with patients in both RRT subgroups significantly augmenting seroconversion rates compared to pre-boosting measurements, yet it was only the KTR and not dialysis patients whose seroconversion rates went significantly beyond the levels measured after the standard two doses (Fig. S3). That means, booster vaccination in the dialysis patients well compensates for the waning immunity after standard dose vaccination, while in the KTRs, it actually surpasses counteracting the waning immunity and significantly intensifies the seroconversion provided by the standard protocol at the first place. This warrants consideration of the third dose for all the KTR patients as part of their standard vaccination protocol, while more doses (i.e. fourth or more) could be considered as boosters.
Indeed, the most important measures in the evaluation of the vaccine efficacy against SARS-CoV-2 infection are hospitalizations and deaths. However, due to data scarcity on these indices in the reviewed reports, the next important milestone achievable through this systematic review was breakthrough infection. Although compared to the KTRs, patients under dialysis therapy represented significantly superior humoral response to vaccine boosting, and also they were the only RRT subgroup that experienced significant augmentation of T cell response post-booster dose, the incidence of breakthrough infection in these patients was almost twice as high as that of the KTRs. This might undermine the general concept that humoral or cellular response can be interpreted to clinical benefits, however, a plausible explanation for this seemingly controversy is the disparity in the exposure, with dialysis (especially hemodialysis) patients being at higher risk of exposure to the virus, due to their compelling frequent attendance to the dialysis facilities. Moreover, the epidemiology of breakthrough infections should be interpreted with caution, because the screening methodologies were not consistent among different studies, with disparities in the detection methods as well as their approaches with some of them systematically testing the patients for asymptomatic infections, while some others only sought to test and report the symptomatic individuals. Follow-up duration was also significantly different between the studies ranging from less than 2 to over 8 months post-booster vaccination [7, 51].
The SARS-CoV-2 vaccine constructs have also been proposed to play major roles in the seroconversion rates, with the mRNA vaccines significantly eliciting higher immunogenicity than the other types of vaccines, most notably the viral vectors [65], although there are reports indicating heterogeneous mRNA-vector vaccination could be as immunogenic as homologous mRNA vaccination [66]. The current study on patients under RRT is consistent with the latter, where homologous or heterologous booster dose vaccination with the vector or mRNA vaccines were equally effective in inducing seroconversion in RRT patients, whereas inactivated virus vaccine was less effective. Regarding the brands of the vaccines, the literature indicates superiority in effectiveness for mRNA-1273 versus BNT162b2 [67]. Although due to the shortage of evidence, the current systematic review could only investigate the seroconversion rates after booster vaccination, no vaccine brand predilection was found in this regard.
Besides the overall responsiveness to booster dose vaccination in immunocompromised patients, the idea of how the non-(or weak-) responders to the standard vaccination would respond to boosting is a distinct and critical idea. As had been observed for the overall analyses, while both the RRT subgroups with poor-response to the standard vaccine protocol significantly augmented seroconversion, the dialysis patients were more likely to respond to the booster vaccination than the KTRs (Fig. S9), and this disparity remained significant even after excluding the two studies that had employed inactivated virus vaccines for their KTR population [42, 47] (data not shown). As had been observed in baseline analyses, the efficacy of booster vaccination in the poor responders was equivalent with respect to the vaccine types or brands, except for the inactivated virus vaccine which represented relatively less effectiveness (Fig. S8).
The immunosuppressive agents have also been shown to significantly impact response to vaccination [68]. In the current study, most of the immunosuppressant drugs represented no different immunogenicity in response to booster vaccination in the KTRs with two exceptions: compared to all the other drugs, mTORi agents offered the best vaccine response while using belatacept was associated with the least seroconversion rates. This finding has clinical implication with giving priority to specific immunosuppressant drugs to be employed or avoided at the time of vaccination.
The current study is associated with a lot of limitations. Most notably, the review was not on the randomized controlled trials. Although a number of the studies had used healthy controls, but the main body of the review included observational trials in which the seropositivity rates were investigated at pre- versus post-booster dose vaccination timepoint. There might be more confounding factors regarding the outcomes of interest. For example, not essentially every patient who received the primary vaccination also received boosters, and there might be some selection biases towards boosting vaccination in the less responsive and/or more vulnerable subjects. That same limitation might apply to the differential effects of vaccine types and brands: There might be skewed selection in favor of the particular vaccine types/brands that have been previously shown to be more reactogenic in the general population, to be employed in the most vulnerable and immunocompromised patients. But since the majority of the studies were consistently using one vaccine brand in all their patients, this limitation is not likely to significantly confound the results. There was also different time intervals between the standard and booster doses in different studies which could confound the comparisons. On the other hand, different studies were using different assays for antibody detection, and the antibody thresholds set to determine seropositivity was also inconsistent between the different studies, although it is noteworthy that the same outcome indices had been essentially used for both the measurements (i.e. pre- and post-boosting) in each study. Limitations are also applicable to the meta-analyses of the immunosuppressant effects on the seroconversions: besides the inconsistencies in dosing schedules, the investigated immunosuppressant drugs were not used in isolation and mostly were used in combination to other drugs. So confounding effects of the other drugs should also be considered while interpreting the results.
In conclusion, this study signifies the safety and utmost importance of the booster vaccination in patients under renal replacement therapy and suggests a third vaccine dose to be included as the standard vaccination protocol to the kidney transplant patients. As well, since dialysis patients represented higher breakthrough infections compared to the KTRs despite higher seroconversion rates, the same idea (standardization of booster dose vaccination) is also reasonable for application in this patient population. Except for the inactivated virus vaccines, no priority was found regarding the vaccine types or brands to be used as booster doses in this patient population. Future studies targeting hard outcomes, including the SARS-CoV-2-associated hospitalizations and mortality are recommended.
References
World Health Organization (2022) COVID-19 weekly epidemiological update, 115th edn. https://covid19.who.int. Accessed 26 Oct 2022
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383(27):2603–2615. https://doi.org/10.1056/NEJMoa2034577
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, COVE Study Group (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384(5):403–416. https://doi.org/10.1056/NEJMoa2035389
Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, Adhikari UD, Winkler ML, Mueller AA, Hsu TY, Desjardins M, Baden LR, Chan BT, Walker BD, Lichterfeld M, Brigl M, Kwon DS, Kanjilal S, Richardson ET, Jonsson AH, Alter G, Barczak AK, Hanage WP, Yu XG, Gaiha GD, Seaman MS, Cernadas M, Li JZ (2020) Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 383(23):2291–2293. https://doi.org/10.1056/NEJMc2031364
Efros O, Anteby R, Halfon M, Meisel E, Klang E, Soffer S (2022) Efficacy and safety of third dose of the COVID-19 vaccine among solid organ transplant recipients: a systemic review and meta-analysis. Vaccines (Basel) 10(1):95. https://doi.org/10.3390/vaccines10010095
Cassaniti I, Gregorini M, Bergami F, Arena F, Sammartino JC, Percivalle E, Soleymaninejadian E, Abelli M, Ticozzelli E, Nocco A, Minero F, Pattonieri EF, Lilleri D, Rampino T, Baldanti F (2022) Effect of a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine on humoral and cellular responses and serum anti-HLA antibodies in kidney transplant recipients. Vaccines (Basel) 10(6):921. https://doi.org/10.3390/vaccines10060921.PMID:35746528;PMCID:PMC9227063
Grupper A, Rabinowich L, Ben-Yehoyada M, Katchman E, Baruch R, Freund T, Hagin D, Shlomo SB, Schwartz D, Schwartz IF, Shashar M, Bassat OK, Halperin T, Turner D, Saiag E, Goykhman Y, Shibolet O, Levy S, Houri I, Katchman H (2022) Humoral response to the third dose of Sars-Cov-2 vaccine in kidney transplant recipients. Transplant Proc 54(6):1439–1445. https://doi.org/10.1016/j.transproceed.2022.02.011
Bruminhent J, Setthaudom C, Phornkittikorn P, Chaumdee P, Prasongtanakij S, Srisala S, Malathum K, Boongird S, Nongnuch A, Assanatham M, Nakgul L, Sanmeema N, Phuphuakrat A, Kiertiburanakul S, Ramathibodi Transplant Infectious Diseases (RTID) Study Group (2022) An additional dose of viral vector COVID-19 vaccine and mRNA COVID-19 vaccine in kidney transplant recipients: a randomized controlled trial (CVIM 4 study). Am J Transplant 22(11):2651–2660. https://doi.org/10.1111/ajt.17151
Crane C, Phebus E, Ingulli E (2022) Antibody response to 2- and 3-dose SARS-CoV-2 mRNA vaccination in pediatric and adolescent kidney transplant recipients. Pediatr Nephrol 27:1–4. https://doi.org/10.1007/s00467-022-05661-8
Tylicki L, Dębska-Ślizień A, Muchlado M, Ślizień Z, Gołębiewska J, Dąbrowska M, Biedunkiewicz B (2021) Boosting humoral immunity from mRNA COVID-19 vaccines in kidney transplant recipients. Vaccines (Basel) 10(1):56. https://doi.org/10.3390/vaccines10010056
Stumpf J, Tonnus W, Paliege A, Rettig R, Steglich A, Gembardt F, Kessel F, Kröger H, Arndt P, Sradnick J, Frank K, Tonn T, Hugo C (2021) Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation 105(11):e267–e269. https://doi.org/10.1097/TP.0000000000003903
Quiroga B, Soler MJ, Ortiz A, Orero E, Tejedor S, Mantecón CJJ, Gómez Pérez VO, Marín Franco AJ, Alfaro Sánchez C, Puerta Carretero M, Jaldo Rodríguez MT, Carnerero Di Riso MA, Martínez S, González CC, Cervienka M, Macías Carmona N, Arroyo D, Pérez Del Valle KM, de Arriba G, Mazuecos A, Cazorla JM, Pereira M, González Parra E, Sánchez Márquez MG, Lancho Novillo C, Toyos Ruiz C, Aguilar Cervera MC, Muñoz Ramos P, Sánchez Horrillo A, Jimeno Martín I, Toapanta N, Cigarrán Guldris S, Folgueiras López M, Valero San Cecilio R, Villacorta Linaza B, Minguela Pesquera I, Santana Estupiñán R, Zamora R, Soriano S, Muñoz de Bustillo E, Pizarro Sánchez MS, Martínez Puerto AI, Yugueros A, Muñiz Pacios L, Leyva A, Rojas J, Gansevoort RT, de Sequera P, SENCOVAC collaborative network (2020) Humoral response to third dose of SARS-CoV-2 vaccines in the CKD spectrum. Clin J Am Soc Nephrol 17(6):872–876. https://doi.org/10.2215/CJN.01770222
McEvoy CM, Hu Q, Abe KT et al (2022) Humoral responses in the omicron era following three-dose SARS-CoV-2 vaccine series in kidney transplant recipients. medRxiv. https://doi.org/10.1101/2022.06.24.22276144
Abedon AT, Alejo JL, Kim JD, Thomas L, Mitchell J, Chiang TPY, Avery RK, Tobian AAR, Levan ML, Warren DS, Massie AB, Garonzik-Wang JM, Segev DL, Werbel WA (2022) Six-month antibody kinetics and durability after 3 doses of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation 106(5):e281–e283. https://doi.org/10.1097/TP.0000000000004069
Bertrand D, Hamzaoui M, Lemée V, Lamulle J, Laurent C, Etienne I, Lemoine M, Lebourg L, Hanoy M, Le Roy F, Nezam D, Farce F, Plantier JC, Boyer O, Guerrot D, Candon S (2021) Antibody and T-cell response to a third dose of SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Kidney Int 100(6):1337–1340. https://doi.org/10.1016/j.kint.2021.09.014
Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, Segev DL (2021) Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 174(9):1330–1332. https://doi.org/10.7326/L21-0282
Benotmane I, Bruel T, Planas D, Fafi-Kremer S, Schwartz O, Caillard S (2022) A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int 101(5):1073–1076. https://doi.org/10.1016/j.kint.2022.02.011
Hod T, Ben-David A, Olmer L, Scott N, Ghinea R, Mor E, Levy I, Indenbaum V, Lustig Y, Grossman E, Rahav G (2022) BNT162b2 third booster dose significantly increases the humoral response assessed by both RBD IgG and neutralizing antibodies in renal transplant recipients. Transpl Int 35:10239. https://doi.org/10.3389/ti.2022.10239
Stumpf J, Schwöbel J, Karger C, Schirutschke H, Mauer R, Klimova A, Tonn T, Hugo C (2022) Anti-SARS-CoV-2 revaccination success in kidney transplant recipients with no initial humoral response is linked to primary vaccine type. Front Med (Lausanne) 9:910987. https://doi.org/10.3389/fmed.2022.910987
Medina-Pestana J, Almeida Viana L, Nakamura MR, Lucena EF, Granato CFH, Dreige YC, Amorim LVP, Chow CYZ, Demarchi Foresto R, Roberto Requião-Moura L, Tedesco-Silva H, Cristelli MP (2022) Immunogenicity after a heterologous BNT262b2 versus homologous booster in kidney transplant recipients receiving 2 doses of CoronaVac vaccine: a prospective cohort study. Transplantation 106(10):2076–2084. https://doi.org/10.1097/TP.0000000000004260
Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, Martzloff J, Perrin P, Pszczolinski R, Moulin B, Fafi-Kremer S, Caillard S (2022) Prediction of vaccine response and development of a personalized Anti-SARS-CoV-2 vaccination strategy in kidney transplant recipients: results from a large single-center study. J Pers Med 12(7):1107. https://doi.org/10.3390/jpm12071107
Al Jurdi A, Gassen RB, Borges TJ, Lape IT, Morena L, Efe O, Solhjou Z, El Fekih R, Deban C, Bohan B, Pattanayak V, Kotton CN, Azzi JR, Riella LV (2022) Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int 101(6):1282–1286. https://doi.org/10.1016/j.kint.2022.04.009
Alejo JL, Mitchell J, Chiang TP, Abedon AT, Boyarsky BJ, Avery RK, Tobian AAR, Levan ML, Massie AB, Garonzik-Wang JM, Segev DL, Werbel WA (2021) Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation 105(12):e280–e281. https://doi.org/10.1097/TP.0000000000003934
Espi M, Charmetant X, Barba T, Mathieu C, Pelletier C, Koppe L, Chalencon E, Kalbacher E, Mathias V, Ovize A, Cart-Tanneur E, Bouz C, Pellegrina L, Morelon E, Juillard L, Fouque D, Couchoud C, Thaunat O, REIN Registry (2022) A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int 101(2):390–402. https://doi.org/10.1016/j.kint.2021.10.040
Beilhack G, Monteforte R, Frommlet F, Reindl-Schwaighofer R, Strassl R, Vychytil A (2022) Humoral response to mRNA-1273 SARS-CoV-2 vaccine in peritoneal dialysis patients: is boostering after six months adequate? Front Med (Lausanne) 9:905798. https://doi.org/10.3389/fmed.2022.905798
Biedunkiewicz B, Tylicki L, Ślizień W, Lichodziejewska-Niemierko M, Dąbrowska M, Kubanek A, Rodak S, Polewska K, Tylicki P, Renke M, Dębska-Ślizień A (2022) Waning humoral response after COVID-19 mRNA vaccination in maintenance dialysis patients and recovery after a complementary third dose. Vaccines (Basel) 10(3):433. https://doi.org/10.3390/vaccines10030433
Kitamura M, Takazono T, Yamaguchi K, Tomura H, Yamamoto K, Harada T, Funakoshi S, Mukae H, Nishino T (2022) Favorable humoral response to third dose of BNT162b2 in patients undergoing hemodialysis. J Clin Med 11(8):2090. https://doi.org/10.3390/jcm11082090
Yahav D, Rahamimov R, Mashraki T, Ben-Dor N, Steinmetz T, Agur T, Zingerman B, Herman-Edelstein M, Lichtenberg S, Ben-Zvi H, Bar-Haim E, Cohen H, Rotem S, Elia U, Margalit I, Zvi BR (2022) Immune response to third dose BNT162b2 COVID-19 vaccine among kidney transplant recipients-a prospective study. Transpl Int 35:10204. https://doi.org/10.3389/ti.2022.10204
Schimpf J, Davidovic T, Abbassi-Nik A, Sprenger-Mähr H, Lhotta K, Zitt E (2022) Enhanced SARS-CoV-2 antibody response after a third heterologous vector vaccine Ad26COVS1 dose in mRNA vaccine-primed kidney transplant recipients. Transpl Int 36:10357. https://doi.org/10.3389/ti.2022.10357
Massa F, Cremoni M, Gérard A, Grabsi H, Rogier L, Blois M, Couzin C, Hassen NB, Rouleau M, Barbosa S, Martinuzzi E, Fayada J, Bernard G, Favre G, Hofman P, Esnault VLM, Czerkinsky C, Seitz-Polski B, Glaichenhaus N, Sicard A (2021) Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine 73:103679. https://doi.org/10.1016/j.ebiom.2021.103679
Cirillo L, Citera F, Mazzierli T, Becherucci F, Terlizzi V, Lodi L, Buti E, Romagnani P (2022) Response to third dose of vaccine against SARS-CoV-2 in adolescent and young adult kidney transplant recipients. Transplantation 106(8):e386–e387. https://doi.org/10.1097/TP.0000000000004199
Bensouna I, Caudwell V, Kubab S, Acquaviva S, Pardon A, Vittoz N, Bozman DF, Hanafi L, Faucon AL, Housset P (2022) SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis 79(2):185-192.e1. https://doi.org/10.1053/j.ajkd.2021.08.005
Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C (2021) Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int 100(3):702–704. https://doi.org/10.1016/j.kint.2021.06.025
Westhoff TH, Seibert FS, Anft M, Blazquez-Navarro A, Skrzypczyk S, Zgoura P, Meister TL, Pfaender S, Stumpf J, Hugo C, Viebahn R, Roch T, Stervbo U, Babel N (2021) A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int 100(5):1135–1136. https://doi.org/10.1016/j.kint.2021.09.001
Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S, Caillard S (2021) Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 326(11):1063–1065. https://doi.org/10.1001/jama.2021.12339
Kamar N, Abravanel F, Marion O, Esposito L, Hebral AL, Médrano C, Guitard J, Lavayssière L, Cointault O, Nogier MB, Bellière J, Faguer S, Couat C, Del Bello A, Izopet J (2022) Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am J Transplant 22(5):1467–1474. https://doi.org/10.1111/ajt.16950
Del Bello A, Abravanel F, Marion O, Couat C, Esposito L, Lavayssière L, Izopet J, Kamar N (2022) Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant 22(1):322–323. https://doi.org/10.1111/ajt.16775
Becker M, Cossmann A, Lürken K, Junker D, Gruber J, Juengling J, Ramos GM, Beigel A, Wrenger E, Lonnemann G, Stankov MV, Dopfer-Jablonka A, Kaiser PD, Traenkle B, Rothbauer U, Krause G, Schneiderhan-Marra N, Strengert M, Dulovic A, Behrens GMN (2022) Longitudinal cellular and humoral immune responses after triple BNT162b2 and fourth full-dose mRNA-1273 vaccination in haemodialysis patients. Front Immunol 13:1004045. https://doi.org/10.3389/fimmu.2022.1004045
Benning L, Klein K, Morath C, Bartenschlager M, Kim H, Buylaert M, Reineke M, Töllner M, Nusshag C, Kälble F, Reichel P, Schnitzler P, Zeier M, Süsal C, Bartenschlager R, Schaier M, Speer C (2022) Neutralizing antibody activity against the B.1.617.2 (delta) variant before and after a third BNT162b2 vaccine dose in hemodialysis patients. Front Immunol 13:840136. https://doi.org/10.3389/fimmu.2022.840136
Benning L, Morath C, Bartenschlager M, Kim H, Reineke M, Beimler J, Buylaert M, Nusshag C, Kälble F, Reichel P, Töllner M, Schaier M, Klein K, Benes V, Rausch T, Rieger S, Stich M, Tönshoff B, Weidner N, Schnitzler P, Zeier M, Süsal C, Hien Tran T, Bartenschlager R, Speer C (2022) Neutralizing antibody response against the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am J Transplant 22(7):1873–1883. https://doi.org/10.1111/ajt.17054
Masset C, Ville S, Garandeau C, Le Borgne F, Letellier T, Cantarovich D, Meurette A, Guillot-Gueguen C, Bentoumi-Loaec M, Giral M, Dantal J, Blancho G (2022) Observations on improving COVID-19 vaccination responses in kidney transplant recipients: heterologous vaccination and immunosuppression modulation. Kidney Int 101(3):642–645. https://doi.org/10.1016/j.kint.2021.11.024
Cristelli MP, Nakamura MR, Viana LA, Tedesco-Silva H, Medina-Pestana J (2022) The fourth dose of CoronaVac vaccine results in a small increase of seroconversion and antibody values among kidney transplant recipients. Transplantation 106(9):e420–e421. https://doi.org/10.1097/TP.0000000000004219
Schrezenmeier E, Rincon-Arevalo H, Stefanski AL, Potekhin A, Straub-Hohenbleicher H, Choi M, Bachmann F, Pross V, Hammett C, Schrezenmeier H, Ludwig C, Jahrsdörfer B, Lino A, Eckardt KU, Kotsch K, Dörner T, Budde K, Sattler A, Halleck F (2021) B and T Cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J Am Soc Nephrol 32(12):3027–3033. https://doi.org/10.1681/ASN.2021070966
Longlune N, Nogier MB, Miedougé M, Gabilan C, Cartou C, Seigneuric B, Del Bello A, Marion O, Faguer S, Izopet J, Kamar N (2021) High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant 36(9):1704–1709. https://doi.org/10.1093/ndt/gfab193
Balsby D, Nilsson AC, Möller S, Lindvig SO, Davidsen JR, Abazi R, Poulsen MK, Holden IK, Justesen US, Bistrup C, Johansen IS (2022) Determinants of antibody response to a third SARS-CoV-2 mRNA vaccine dose in solid organ transplant recipients: results from the prospective cohort study COVAC-Tx. Vaccines (Basel) 10(4):565. https://doi.org/10.3390/vaccines10040565
Mitchell J, Alejo JL, Chiang TPY, Kim J, Chang A, Abedon AT, Avery RK, Tobian AAR, Massie AB, Levan ML, Warren DS, Garonzik-Wang JM, Segev DL, Werbel WA (2022) Antibody response to a fourth dose of SARS-CoV-2 vaccine in solid organ transplant recipients: an update. Transplantation 106(7):e338–e340. https://doi.org/10.1097/TP.0000000000004137
Medina-Pestana J, Covas DT, Viana LA, Dreige YC, Requião-Moura LR, Nakamura MR, Lucena EF, Foresto RD, Tedesco-Silva H, Cristelli MP (2022) Homologous third dose of inactivated whole-virion vaccine fails to elicit a robust immune response among kidney seronegative transplant recipients. Transplantation 106(5):e284–e285. https://doi.org/10.1097/TP.0000000000004029
Heinzel A, Schrezenmeier E, Regele F, Hu K, Raab L, Eder M, Aigner C, Jabbour R, Aschauer C, Stefanski AL, Dörner T, Budde K, Reindl-Schwaighofer R, Oberbauer R (2022) Three-month follow-up of heterologous vs. homologous third SARS-CoV-2 vaccination in kidney transplant recipients: secondary analysis of a randomized controlled trial. Front Med (Lausanne) 9:936126. https://doi.org/10.3389/fmed.2022.936126
Karakizlis H, Agarwal V, Aly M, Strecker K, Csala B, Esso I, Chen J, Nahrgang C, Wolter M, Slanina H, Schüttler CG, Jessen S, Ronco C, Seeger W, Weimer R, Sester M, Birk HW, Husain-Syed F (2022) Humoral and cellular immune responses to the mRNA-1273 SARS-CoV-2 vaccine booster in patients on maintenance dialysis. J Nephrol 22:1–4. https://doi.org/10.1007/s40620-022-01371-4
Osmanodja B, Ronicke S, Budde K, Jens A, Hammett C, Koch N, Seelow E, Waiser J, Zukunft B, Bachmann F, Choi M, Weber U, Eberspächer B, Hofmann J, Grunow F, Mikhailov M, Liefeldt L, Eckardt KU, Halleck F, Schrezenmeier E (2022) Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J Clin Med 11(9):2565. https://doi.org/10.3390/jcm11092565.PMID:35566691;PMCID:PMC9105533
Housset P, Kubab S, Hanafi L, Pardon A, Vittoz N, Bozman DF, Caudwell V, Faucon AL (2022) Humoral response after a fourth “booster” dose of a Coronavirus disease 2019 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int 101(6):1289–1290. https://doi.org/10.1016/j.kint.2022.04.006
Cheng CC, Platen L, Christa C, Tellenbach M, Kappler V, Bester R, Liao BH, Holzmann-Littig C, Werz M, Schönhals E, Platen E, Eggerer P, Tréguer L, Küchle C, Schmaderer C, Heemann U, Renders L, Protzer U, Braunisch MC (2022) Improved SARS-CoV-2 neutralization of delta and omicron BA.1 variants of concern after fourth vaccination in hemodialysis patients. Vaccines (Basel) 10(8):1328. https://doi.org/10.3390/vaccines10081328
Charmetant X, Espi M, Barba T, Ovize A, Morelon E, Mathieu C, Thaunat O (2022) Predictive factors of a viral neutralizing humoral response after a third dose of COVID-19 mRNA vaccine. Am J Transplant 22(5):1442–1450. https://doi.org/10.1111/ajt.16990
Charmetant X, Espi M, Barba T et al (2021) Predictive factors of response to 3rd dose of COVID-19 mRNA vaccine in kidney transplant recipients. medRxiv. https://doi.org/10.1101/2021.08.23.21262293
Caillard S, Thaunat O, Benotmane I, Masset C, Blancho G (2022) Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med 175(3):455–456. https://doi.org/10.7326/L21-0598
Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, Jabbour R, Hofbauer TM, Merrelaar A, Eder M, Regele F, Doberer K, Spechtl P, Aschauer C, Koblischke M, Paschen C, Eskandary F, Hu K, Öhler B, Bhandal A, Kleibenböck S, Jagoditsch RI, Reiskopf B, Heger F, Bond G, Böhmig GA, Strassl R, Weseslindtner L, Indra A, Aberle JH, Binder M, Oberbauer R (2022) Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med 182(2):165–171. https://doi.org/10.1001/jamainternmed.2021.7372
Marlet J, Gatault P, Maakaroun Z, Longuet H, Stefic K, Handala L, Eymieux S, Gyan E, Dartigeas C, Gaudy-Graffin C (2021) Antibody responses after a third dose of COVID-19 vaccine in kidney transplant recipients and patients treated for chronic lymphocytic leukemia. Vaccines (Basel) 9(10):1055. https://doi.org/10.3390/vaccines9101055
Charmetant X, Espi M, Benotmane I, Barateau V, Heibel F, Buron F, Gautier-Vargas G, Delafosse M, Perrin P, Koenig A, Cognard N, Levi C, Gallais F, Manière L, Rossolillo P, Soulier E, Pierre F, Ovize A, Morelon E, Defrance T, Fafi-Kremer S, Caillard S, Thaunat O (2022) Infection or a third dose of mRNA vaccine elicits neutralizing antibody responses against SARS-CoV-2 in kidney transplant recipients. Sci Transl Med 14(636):eabl6141. https://doi.org/10.1126/scitranslmed.abl6141
Tillmann FP, Figiel L, Ricken J, Still H, Korte C, Plassmann G, von Landenberg P (2021) Evolution of SARS-CoV-2-neutralizing antibodies after two standard dose vaccinations, risk factors for non-response and effect of a third dose booster vaccination in non-responders on hemodialysis: a prospective multi-centre cohort study. J Clin Med 10(21):5113. https://doi.org/10.3390/jcm10215113
Kantauskaite M, Müller L, Hillebrandt J, Lamberti J, Fischer S, Kolb T, Ivens K, Koch M, Andree M, Lübke N, Schmitz M, Luedde T, Orth HM, Feldt T, Schaal H, Adams O, Schmidt C, Kittel M, Königshausen E, Rump LC, Timm J, Stegbauer J (2022) Immune response to third SARS-CoV-2 vaccination in seronegative kidney transplant recipients: possible improvement by mycophenolate mofetil reduction. Clin Transplant 23:e14790. https://doi.org/10.1111/ctr.14790
Tylicki L, Biedunkiewicz B, Ślizień Z, Muchlado M, Dębska-Ślizień A (2022) Heterologous high dose SARS-CoV-2 mRNA vaccine booster may improve immune response in seronegative kidney transplant recipients. Arch Med Sci 18(4):1100–1102. https://doi.org/10.5114/aoms/150000
Midtvedt K, Vaage JT, Heldal K, Munthe LA, Lund-Johansen F, Åsberg A (2022) Fourth dose of the SARS-CoV-2 vaccine in kidney transplant recipients with previously impaired humoral antibody response. Am J Transplant 22(11):2704–2706. https://doi.org/10.1111/ajt.17091
Thümmler L, Gäckler A, Bormann M, Ciesek S, Widera M, Rohn H, Fisenkci N, Otte M, Alt M, Dittmer U, Horn PA, Witzke O, Krawczyk A, Lindemann M (2022) Cellular and humoral immunity against different SARS-CoV-2 variants is detectable but reduced in vaccinated kidney transplant patients. Vaccines (Basel) 10(8):1348. https://doi.org/10.3390/vaccines10081348
Akyol M, Çevik E, Ucku D et al (2021) Immunogenicity of SARS-CoV-2 mRNA vaccine in dialysis and kidney transplant patients: a systematic review. Tuberkuloz ve Toraks 69(4):547–560. https://doi.org/10.5578/tt.20219612
Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, Visser LG, Geers D, Schmitz KS, Garcia Garrido HM, Koopmans MPG, Dalm VASH, Kootstra NA, Huckriede ALW, Lafeber M, van Baarle D, GeurtsvanKessel CH, de Vries RD, van der Kuy PHM, SWITCH Research Group (2022) Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N Engl J Med 386(10):951–963. https://doi.org/10.1056/NEJMoa2116747
Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, Abu-Omar A, Ziegler L, Guckelmus C, Urschel R, Schneitler S, Becker SL, Gärtner BC, Sester U, Sester M (2021) Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med 27(9):1530–1535. https://doi.org/10.1038/s41591-021-01464-w
Dickerman BA, Gerlovin H, Madenci AL, Kurgansky KE, Ferolito BR, Figueroa Muñiz MJ, Gagnon DR, Gaziano JM, Cho K, Casas JP, Hernán MA (2022) Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med 386(2):105–115. https://doi.org/10.1056/NEJMoa2115463
Caillard S, Thaunat O (2021) COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol 17(12):785–787. https://doi.org/10.1038/s41581-021-00491-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interests exists regarding this study. The author received no grants and has no financial interest in the study results. All the study processes were performed by the author entirely.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taheri, S. Efficacy and safety of booster vaccination against SARS-CoV-2 in dialysis and renal transplant patients: systematic review and meta-analysis. Int Urol Nephrol 55, 791–802 (2023). https://doi.org/10.1007/s11255-023-03471-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03471-x