Abstract
Objectives
The occurrence of pulmonary arterial hypertension (PAH) can greatly affect the prognosis of patients with chronic kidney disease (CKD). We aimed to construct a nomogram to predict the probability of PAH development in patients with stage 3–5 CKD to guide early intervention and to improve prognosis.
Methods
From August 2018 to December 2021, we collected the data of 1258 patients with stage 3–5 CKD hospitalized at the Affiliated Hospital of Xuzhou Medical University as a training set and 389 patients hospitalized at Zhongda Hospital as a validation set. These patients were divided into PAH and N-PAH groups with pulmonary arterial systolic pressure ≥ 35 mmHg as the cutoff. The results of univariate and multivariate logistic regression analyses were used to establish the nomogram. Then, areas under the receiver operating characteristic curve (AUC-ROCs), a calibration plot, and decision curve analysis (DCA) were used to validate the nomogram.
Results
The nomogram included nine variables: age, diabetes mellitus, hemoglobin, platelet count, serum creatinine, left ventricular end-diastolic diameter, left atrial diameter, main pulmonary artery diameter and left ventricular ejection fraction. The AUC-ROCs of the training set and validation set were 0.801 (95% confidence interval (CI) 0.771–0.830) and 0.760 (95% CI 0.699–0.818), respectively, which showed good discriminative ability of the nomogram. The calibration diagram showed good agreement between the predicted and observed results. DCA also demonstrated that the nomogram could be clinically useful.
Conclusion
The evaluation of the nomogram model for predicting PAH in patients with CKD based on risk factors showed its ideal efficacy. Thus, the nomogram can be used to screen for patients at high risk for PAH and has guiding value for the subsequent formulation of prevention strategies and clinical treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chronic kidney disease (CKD) is a life-threatening chronic disease which mainly manifests as kidney function impairment, and it is characterized by irreversible renal dysfunction and loss of homeostasis. The International Organization of Nephrology 2012 “Kidney Disease: Improving Global Outcomes” (KDIGO) produced CKD guidelines and proposed guiding recommendations for the definition, staging, diagnosis, treatment, and prevention of the disease [1]. CKD is a worldwide health problem and one of the leading causes of morbidity and mortality, affecting more than 10% of the world's population [2, 3]. With economic development and the accompanying changes in lifestyle and diet, the incidence of some nutritional metabolic diseases, such as hypertension, diabetes mellitus (DM), and obesity, has increased significantly. These metabolic diseases lead to an increase in the incidence of CKD [4, 5]. According to an epidemiological study, CKD replaced malnutrition and infection as the leading causes of mortality during the twentieth century [6].
Pulmonary arterial hypertension (PAH) is a small pulmonary artery disease characterized by vascular remodeling, and it can eventually lead to heart failure and death by increasing resistance in blood vessels in the lungs [7]. The gold standard for PAH diagnosis is right heart catheterization, but it is an invasive procedure with high risk. Therefore, it is not recommended for clinical monitoring [8]. Cardiac color Doppler ultrasound is a convenient and noninvasive examination method that can efficiently determine whether the heart tissue is abnormal in terms of anatomy and function. Therefore, it has been unanimously recognized by the medical community [9]. The early clinical symptoms of PAH are not atypical, so the early diagnosis rate is low.
PAH is common in patients with CKD and was found to occur in 56% of patients [10]. Investigations have shown that the incidence of PAH is related to the type of dialysis selected by patients with end-stage renal disease: the rate of PAH was 18.8–68.8% in maintenance hemodialysis patients [11] and 12–42% in peritoneal dialysis patients [12, 13]. However, the pathogenesis of PAH in patients with CKD has not been completely elucidated, and it may be related to anemia, diabetes mellitus, left ventricular structure and function, and dialysis mode [14,15,16]. At present, in patients with CKD, especially in patients with stage 3–5 chronic renal failure, the independent risk factors and the specific incidence of pulmonary hypertension are still unknown.
A nomogram is a simple, personalized visualization tool that has been widely used in diagnostic and prognostic determinations for cancer patients [17]. A study has shown that nomograms potentially represent an ideal model for predicting the prognosis of CKD patients [18]. However, no nomogram has been used to predict the risk of pulmonary hypertension in patients with CKD. In this study, we aimed to construct a nomogram to predict the probability of developing PAH in patients with stage 3–5 CKD to guide clinical diagnosis and early intervention and to improve prognosis.
Materials and methods
Study population and design
This retrospective study was based on data within an electronic medical record system. Patients who were hospitalized in the Department of Nephrology at the Affiliated Hospital of Xuzhou Medical University and Zhongda Hospital affiliated with Southeast University from August 2018 to December 2021 and diagnosed with stage 3–5 CKD according to the 2012 KDIGO guidelines were included in this study [1]. This study was approved by the Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (approval number XYFY2022-KL093-01). Because the study was retrospective, the review committee waived the requirement for written informed consent.
Inclusion criteria were as follows: (i) referring to the 2012 KDIGO CKD guideline, a diagnosis of stage 3–5 CKD; (ii) age ≥ 18 years, and for patients in the dialysis group, a maintenance hemodialysis (MHD) or peritoneal dialysis (PD) duration of at least 3 months; (iii) complete data for laboratory tests and examination indicators; and (iv) for patients in the dialysis group, the attainment of the dialysis adequacy standard before cardiac color Doppler examination. The exclusion criteria were as follows: (i) loss to follow-up; (ii) an MHD or PD duration < 3 months; (iii) MHD combined with PD; (iv) a history of cancer; (v) severe liver insufficiency; (vi) severe lung disease; and (vii) other reasons. Patients who met the inclusion criteria were examined by Doppler echocardiography. According to their pulmonary arterial pressure, they were divided into a PAH group and an N-PAH group. After admission, all patients with stage 3–5 CKD were received routine renoprotective therapy.
Echocardiographic detection
The clinical endpoint was defined as the occurrence of PAH in patients with stage 3–5 CKD, and pulmonary arterial systolic pressure (PASP) was calculated according to Bernoulli's formula after tricuspid regurgitation velocity was measured by an experienced color echocardiologist using a Philips EPIQ 7C color Doppler echocardiograph with a probe frequency of 1–5 MHz. According to the American Society of Echocardiography Guidelines for the Evaluation of Adult Right Heart Echocardiography, PASP > 35 mmHg was defined as PAH [19].
Predictor variables
Relevant literature was reviewed, and the factors that initially affected the grouping of patients included sex, age, body mass index (BMI), New York Heart Association (NYHA) function classification, smoking history, alcohol consumption history, hypertension, DM, cerebral infarction and coronary heart disease, dialysis way, etc. Laboratory indicators of patients in both groups, such as low density lipoprotein cholesterol (LDL-C), total cholesterol (TC), uric acid (UA), and serum albumin level, and Doppler echocardiography indicators, such as left ventricular ejection fraction (LVEF), left atrial diameter (LAD), left ventricular posterior wall diameter (LVPWD), left ventricular end diastolic diameter (LVDd), main pulmonary artery diameter (MPAD), and right ventricle diameter (RVD), were evaluated. For all patients, blood samples were obtained within 24 h of admission and were used for the determination of the above indicators, and patients underwent echocardiography within 48 h of admission. All patients were discharged, and follow-up records, regular outpatient visits, and telephone follow-up were established.
Statistical analysis
In this study, SPSS 22.0 statistical software was used to analyze the data. The Shapiro‒Wilk test and Levene test were used to evaluate the normality and homogeneity of measurement data variance. The measurement data conforming to a normal distribution are represented by the mean ± standard deviation, while the measurement data conforming to a nonnormal distribution are represented by the median (M) and interquartile range (M P25, P75). Count data are expressed as frequencies or percentages (%). For the measurement data conforming to a normal distribution, two independent-sample T tests were used for intergroup comparison. A nonparametric test (Mann‒Whitney U test) was used for between-group comparisons of the measurement data with a nonnormal distribution. The chi-square test or Fisher's exact probability method was used for between-group comparisons of the count data. Univariate and multivariate logistic regression methods were used to establish a predictive model represented by a nomogram. Variables with P < 0.05 in univariate analysis were included in the multivariate regression analysis. Based on the results of multivariate analysis, we established the nomogram. Then, we used the areas under the ROC and calibration plots to verify the nomogram. In addition, we also used a calibration plot to verify the model internally and DCA to determine the clinical usefulness of the nomogram.
Results
Baseline patient characteristics in the training set
Between August 2018 and September 2021, a total of 1258 CKD (stage 3–5) patients were referred to the affiliated Hospital of Xuzhou Medical University, and the data of 921 patients were selected as the training set for analysis according to the exclusion criteria. At the same time, we also selected 389 patients with CKD (stage 3–5) hospitalized in Zhongda Hospital affiliated with Southeast University and finally included the data of 276 patients as the validation set for analysis. The specific process is shown in Fig. 1.
Comparison of the general data of patients in the training set
Compared with the N-PAH group, patients in the PAH group were older and had a lower BMI. The histories of hypertension and DM were also significantly different between the two groups (P < 0.05). In other general data, there was no significant difference between the two groups. The general data characteristics of the PAH group and N-PAH group are shown in Table 1.
Comparison of the laboratory results of patients in the training set
Compared with the N-PAH group, the red blood cell (RBC) count, hemoglobin (Hb), and platelet (PLT) count were lower and serum creatinine (Scr), fasting blood glucose (FBG), and parathyroid hormone (PTH) were higher in the PAH group, and the differences were statistically significant (P < 0.05). Specific results are shown in Table 2.
Comparison of the Doppler echocardiography results of patients in the training set
The LAD, LVDd, LVPWD, RVD, and MPAD in the PAH group were higher than those in the N-PAH group, while LVEF was lower than that in the N-PAH group, and the differences were statistically significant (P < 0.05), as shown in Table 3.
Clinical features of the training and validation sets
To prevent the overfitting of the clinical prediction model in the analysis of influencing factors, we performed an analysis of the differences between the data in the training and validation sets. None of the differences in the features of the training and validation sets were significant, indicating that the dataset was reasonably divided. The basic information of the two groups was comparable, as shown in Supplementary Table S1.
Predictive nomogram development
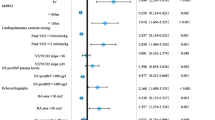
As shown in Table 4, univariate and multivariate logistic regression analyses were used to determine the risk factors for PAH. In univariate logistic regression analysis, age, hypertension, DM, Hb, PLT count, Scr, LVDd, LAD, MPAD, and LVEF were identified (P < 0.05). Then, we included the above factors in the multivariate analysis, and the independent risk factors for pulmonary hypertension were determined to be age (odds ratio (OR) 1.032; 95% confidence interval (CI) 1.020–1.043), DM (OR 1.942; 95% CI 1.317–2.863), Hb (OR 0.969; 95% CI 0.960–0.978), PLT count (OR 0.995; 95% CI 0.992–0.997), Scr (OR 1.001; 95% CI 1.000–1.002), LVDd (OR 1.043; 95% CI 1.020–1.067), LAD (OR 1.033; 95% CI 1.013–1.054), MPAD (OR 1.098; 95% CI 1.056–1.141) and LVEF (OR 0.946; 95% CI 0.931–0.962).
According to the results of multivariate logistic regression analysis, a nomogram was drawn to predict the occurrence of pulmonary hypertension in patients with stage 3–5 CKD, as shown in Fig. 2. The risk of PAH in patients with stage 3–5 CKD was estimated by calculating the scores corresponding to each risk factor (age, DM, Hb, PLT count, Scr, LVDd, LAD, MPAD, LVEF).
Validation of the nomogram
In the training set, the AUC for predicting PAH in CKD patients was 0.801 (95% confidence interval (CI) 0.771–0.830) (Fig. 3A), and the AUC was 0.760 (95% CI 0.699–0.818) in the validation set (Fig. 3B). This shows that the discriminative ability of this clinical predictive model is very good. Then, we used the bootstrap self-sampling method with B = 1000 repetitions and plotted the calibration curves for the training and validation sets of the nomogram. The results showed that the predicted results were in good agreement with the actual results (Fig. 4A, B). In addition, we also used DCA to evaluate the clinical validity of the nomogram (Fig. 5). The results showed that the range of the threshold probability of the nomogram was wide and thus that the model could be clinically useful.
Discussion
High rates of cardiovascular morbidity and mortality due to CKD place a serious burden on the health care system, and PAH is one of the common complications of CKD [20]. Despite the rapid development of medical treatment, the prognosis and quality of life of CKD patients with PAH are still poor. Therefore, early identification of PAH risk in patients with CKD and early intervention are of great significance. This is the first study to develop a nomogram to predict PAH in patients with CKD, and we found that age, DM, Hb, PLT count, Scr, LVDd, LAD, MPAD, and LVEF were independent risk factors for PAH.
The prevalence of CKD increases with age, and renal function gradually decreases so that 34.0% of people ≥ 65 years old have stage 3 or above CKD [21], which may be related to elderly individuals being more prone to chronic diseases such as hypertension, diabetes, and coronary heart disease. An epidemiological study showed that the prevalence of CKD in elderly individuals over 70 years old reached 47% [22]. Havlucu Y and his team revealed that the age of patients in a PAH group was significantly higher than that in an N-PAH group [23]. Currently, we know that the major risk factors for CKD are diabetes and hypertension [24]: approximately 80–85% of patients with CKD have hypertension, and more than 50% have diabetes [25, 26]. After multivariate logistic analysis, diabetes was identified as a risk factor for PAH in patients with CKD. Torkamani N et al. found that the presence of diabetes and higher HbA1c levels were strongly and independently associated with adverse renal outcomes in patients with CKD who were hospitalized ≥ 2 times. These patients were at high risk for the relatively rapid deterioration of kidney function [27]. DM can cause damage to the pulmonary vascular endothelium and decrease the release of vasodilators, thus aggravating the degree of atherosclerosis, further increasing blood pressure, and eventually leading to high pressure load in the right ventricle and increased pulmonary vascular resistance, resulting in PAH [28, 29]. Agarwal R et al. found that ambulatory systolic blood pressure (BP) was strongly associated with the progression of chronic kidney disease (CKD) and was an independent predictor of ESRD [30]. We found that hypertension was significant in univariate analysis (P = 0.031) but not in multivariate analysis (P = 0.641). Nevertheless, hypertension may have value for the prediction of PAH in patients with CKD, which can be further explored in future studies.
Regarding laboratory variables, we found that serum creatinine, hemoglobin, and the platelet count were independent risk factors for PAH in patients with CKD. Serum creatinine is a routine biomarker of CKD, which can lead to the late diagnosis of CKD [31]. A 2019 Korean study showed that low Hb levels and anemia were risk factors for ESRD incidence in the general population and CKD progression to ESRD [32]. The main features of PAH include pulmonary vasoconstriction, pulmonary vascular remodeling, and in situ thrombosis. PAH caused by anemia in patients with CKD may be related to the following mechanisms. Low Hb levels reduce the ability of RBCs to carry oxygen, resulting in hypoxemia, which leads to a corresponding increase in heart rate and cardiac output and the constriction of blood vessels in the lungs, eventually leading to PAH [11]. Studies have shown that platelets are involved in the formation and development of PAH and are associated with prognosis through the release of various cytokines that influence inflammatory processes, promote vascular remodeling, and ultimately lead to arterial stenosis [33]. Therefore, active control of serum creatinine, hemoglobin, and the platelet count is of positive significance to the prognosis of patients.
Our study found that in the PAH group, LAD, MPAD, and LVDd were higher than those in the N-PAH group, while LVEF was lower, and the differences were statistically significant. By further logistic analysis, we found that LVEF, left atrial volume (LAD), MPAD, and LVDd were independent risk factors for PAH in patients with CKD. Therefore, changes in cardiac structure and function can affect the occurrence of PAH in patients with CKD. LVEF is an indicator of left ventricular systolic function, and a previous study showed that PAH in patients with CKD is more likely to occur with a low LVEF, which is consistent with our findings [34]. LAD is an indicator of diastolic function [35]. An increase in LAD will increase the pressure of the left atrium, affect pulmonary venous return, and lead to pulmonary circulation congestion, further leading to increased pulmonary arteriole resistance and eventually PAH. Yang et al. confirmed that the occurrence of PAH in patients with CKD is closely related to an enlarged LAD [36]. It is generally believed that the inner diameter of the pulmonary artery in patients with PAH is wider than that in patients without PAH. When PAH occurs in patients with CKD, pulmonary artery pressure increases, and blood in the right ventricle cannot fully return to the left heart. Pulmonary artery vessels also dilate due to changes in pulmonary artery pressure, increasing the MPAD. With the prolongation of dialysis time and the decrease in dialysis adequacy, patients are prone to capacity overload, leading to cardiac systolic and diastolic dysfunction and increasing the risk of PAH. Therefore, to improve the prognosis and quality of life of patients, clinical attention should be given to the changes in cardiac structure and function in patients with CKD and timely treatment.
Conclusion
We developed a simple nomogram model to predict the risk of pulmonary hypertension in patients with CKD, and the evaluation of this nomogram showed its ideal efficacy. We determined that age, DM, Hb, PLT count, Scr, LVDd, LAD, MPAD, and LVEF were independent risk factors for PAH.
Limitations
There are some limitations to our study. First, this study is a retrospective study, and the data were obtained from the hospital medical record system. Some risk factors, such as hypervolemia, could not be included in the analysis because the data loss is greater than 30%. Second, when information on the dialysis status of the patients was collected in this study, the specific MHD or PD scheme of the patients was not analyzed in detail; therefore, further research is needed. Third, we did not follow up the CKD patients with PAH after discharge; therefore, information including drug use and all-cause mortality was not obtained.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving Global Outcomes 2012 Clinical Practice Guideline. Ann Intern Med 158(11):825–830
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B et al (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382(9888):260–272
Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D et al (2017) Assessment of global kidney health care status. JAMA 317(18):1864–1881
Grams ME, Chow EK, Segev DL, Coresh J (2013) Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis 62(2):245–252
Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF et al (2005) Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 365(9468):1398–1405
Zoccali C, Kramer A, Jager KJ (2010) Epidemiology of CKD in Europe: an uncertain scenario. Nephrol Dial Transplant 25(6):1731–1733
Sutendra G, Michelakis ED (2013) Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med. 5(208):208sr5
Gali N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A et al (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology ( ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37(1):67–119
Yang F, Chen X, Lin X, Chen X, Wang W, Liu B et al (2021) Automated analysis of doppler echocardiographic videos as a screening tool for valvular heart diseases. JACC Cardiovasc Imaging 15:551–563
Mukhtar KN, Mohkumuddin S, Mahmood SN (2014) Frequency of pulmonary hypertension in hemodialysis patients. Pak J Med Sci 30(6):1319–1322
Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A et al (2013) Pulmonary hypertension in CKD. Am J Kidney Dis 61(4):612–622
Nasri H (2013) Unexplained pulmonary hypertension in peritoneal dialysis and hemodialysis patients. Rev Port Pneumol 19(5):238–239
Oygar DD, Zekican G (2012) Pulmonary hypertension in dialysis patients. Ren Fail 34(7):840–844
Vlagopoulos PT, Tighiouart H, Weiner DE, Griffith J, Pettitt D, Salem DN et al (2005) Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol 16(11):3403–3410
Agarwal R (2012) Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant 27(10):3908–3914
He Y, Wang Y, Luo X, Ke J, Du Y, Li M (2015) Risk factors for pulmonary hypertension in maintenance hemodialysis patients: a cross-sectional study. Int Urol Nephrol 47(11):1889–1897
Balachandran VP, Gonen M, Smith JJ, DeMatteo RP (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16(4):e173–e180
You X, Gu B, Chen T, Li X, Xie G, Sang C et al (2021) Development of long-term cardiovascular disease risk prediction model for hemodialysis patients with end-stage renal disease based on nomogram. Ann Palliat Med 10(3):3142–3153
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF et al (2013) Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382(9889):339–352
Fernandez-Fernandez L et al (2021) Prevalence of chronic kidney disease in patients with diabetes in Extremadura (Spain) during the years 2012, 2013 and 2014: an observational study. J Clin Med 10(13):2886
Levey AS, Coresh J (2012) Chronic kidney disease. Lancet 379(9811):165–180
Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, Bayturan O et al (2007) Pulmonary hypertension in patients with chronic renal failure. Respiration 74(5):503–510
Satko SG, Sedor JR, Iyengar SK, Freedman BI (2007) Familial clustering of chronic kidney disease. Semin Dial 20(3):229–236
Kalaitzidis RG, Elisaf MS (2018) Treatment of hypertension in chronic kidney disease. Curr Hypertens Rep 20(8):64
Sonkar SK, Singh HP, Sonkar GK, Pandey S (2018) Association of vitamin D and secondary hyperparathyroidism with anemia in diabetic kidney disease. J Fam Med Prim Care 7(4):815–818
Torkamani N, Churilov L, Robbins R, Jerums G, Beik V, Radcliffe N et al (2020) Diabetes and higher HbA1c levels are independently associated with adverse renal outcomes in in patients following multiple hospital admissions. J Diabetes Complicat 34(1):107465
Chen WJ, Danad I, Raijmakers PG, Halbmeijer R, Harms HJ, Lammertsma AA et al (2014) Effect of type 2 diabetes mellitus on epicardial adipose tissue volume and coronary vasomotor function. Am J Cardiol 113(1):90–97
Kumar A, Bade G, Trivedi A, Jyotsna VP, Talwar A (2016) Postural variation of pulmonary diffusing capacity as a marker of lung microangiopathy in Indian patients with type 2 diabetes mellitus. Indian J Endocrinol Metab 20(2):238–244
Agarwal R, Andersen MJ (2006) Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 69(7):1175–1180
Cianciolo R, Hokamp J, Nabity M (2016) Advances in the evaluation of canine renal disease. Vet J 215:21–29
Yi SW, Moon SJ, Yi JJ (2019) Low-normal hemoglobin levels and anemia are associated with increased risk of end-stage renal disease in general populations: a prospective cohort study. PLoS ONE 14(4):e0215920
Kazimierczyk R, Kaminski K (2018) The role of platelets in the development and progression of pulmonary arterial hypertension. Adv Med Sci 63(2):312–316
Navaneethan SD, Roy J, Tao K, Brecklin CS, Chen J, Deo R et al (2016) Prevalence, predictors, and outcomes of pulmonary hypertension in CKD. J Am Soc Nephrol 27(3):877–886
Paulus WJ, Tschpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE et al (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550
Yang QM, Bao XR (2014) Pulmonary hypertension in patients with stage 1–3 chronic kidney disease. Genet Mol Res 13(3):5695–5703
Funding
This work was supported by grants from the Applied Basic Research Project of Xuzhou to Defeng Pan [Grant Number: KC20097].
Author information
Authors and Affiliations
Contributions
YH developed the analysis plan and the writing of the paper. SX, XW, and XJ undertook the data analysis. HW, CH, TX, MG, AL, JW collected the dataset and provided advice on its analysis. DP guided the analysis and made substantial improvements to the paper. HZ supervised the study and contributed to the data analysis plan. YH, XW, SX and HW contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, Y., Wang, X., Xiao, S. et al. Development and validation of a risk nomogram model for predicting pulmonary hypertension in patients with stage 3–5 chronic kidney disease. Int Urol Nephrol 55, 1353–1363 (2023). https://doi.org/10.1007/s11255-022-03431-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03431-x