Abstract
Purpose
Continuous oxygen therapy to compensate for decreased oxygen saturation in the blood is a life-saving treatment used in case lung involvement. Excess oxygen delivery was reported to be a common situation, in which about 50% of the patients showed hyperoxemia and 4% in severe hyperoxemia. In this work, we investigated the effects of hyperoxia on the rat kidneys and whether tadalafil has an effect to reduce this damage.
Materials and methods
Three groups of 8 male rats each weighing 300–350 g were formed. The groups were divided into the control group, hyperoxia group, and hyperoxia and tadalafil administered group for 10 days. At the end of the 10th day, blood and kidney samples were taken for biochemical analysis (SOD and NO levels) and histopathological examination.
Results
While our findings showed that SOD levels were significantly different among the control and experimental groups and within the experimental groups, no statistical difference was found in terms of NO levels among the groups (Table 1).
While the glomerular and tubular injury was higher in the Hyperoxia group and the Hyperoxia + Tadalafil group than in the control group (p < 0.001), as a result of the rate of severe glomerular and tubular injury in the hyperoxia group, was 62.5% and 43.8% and in the group given tadalafil was 43.8% and 31.3%, respectively (Table 2).
Conclusions
Exposure to hyperoxia condition causes renal glomerular and tubular damage, and tadalafil does not show a protective effect on this damage according to this study’s dose and exposure time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many patients have been exposed to oxygen therapy due to their decreased saturation depending on lung involvement because of some diseases, such as chronic obstructive pulmonary disease, cancer, some respiratory disorders in newborns, and some infectious diseases, especially, currently COVID-19 [1, 2]. Continuous oxygen therapy to compensate for decreased oxygen saturation in the blood is a life-saving treatment commonly used in critically ill patients [3, 4]. High-flow oxygen therapy was recommended for patients with acute respiratory failure and who do not respond to conventional therapy [5]. Excess oxygen delivery was reported to be a common situation, in which about 50% of the patients showed hyperoxemia and 4% in severe hyperoxemia [4].
Some animal studies in the literature show that the state of hyperoxia causes histopathological injuries in some organs, such as the lung, brain, cardiac and vascular system, pampiniform plexus, and testes, and may affect biological systems such as antioxidant enzymes and cytokine production through excessive production of reactive oxygen species (ROS) and generate free radical-mediated damages in various organs [1,2,3, 6,7,8] and thus may cause the affected organ failure [1,2,3, 6,7,8].
ROS are metabolites of oxygen and hence are produced under hyperoxic conditions. All living aerobic cells are normally exposed to some ROS that is toxic to living organisms [9, 10]. In fact, the body tries to eliminate the harmful effects of ROS with its antioxidant defense systems such as superoxide dismutase (SOD) and catalase that interact with them and protect the organism against these metabolites [1, 11, 12]. As a result of normal living conditions, the living organism manages to survive in a healthy way with a delicate balance between the positive and negative effects of ROS products to which it is constantly exposed [13]. The antioxidant defense mechanism achieves these processes endogenously in an enzymatic way (such as SOD, glutathione peroxidase (GPx), catalase (CAT) and exogenously in a non-enzymatic way [ascorbic acid (Vitamin C), -tocopherol (Vitamin E), glutathione (GSH), carotenoids, flavonoids, and other antioxidants] [1, 14].
Exogenous antioxidants are needed when the endogenous enzymatic system is insufficient to reduce ROS-related damage. In the literature, it is possible to find many studies using tadalafil as an exogenous antioxidant to reduce various organ damage due to ROS. However, these studies differ from each other in terms of their results [1, 15,16,17,18]. With this study model, we investigated the effects of hyperoxia on the rat kidneys and whether tadalafil has an effect to reduce this damage, to shed some light on this issue.
Materials and methods
All experiments were performed in accordance with the guidelines for animal research from the National Institutes of Health and were approved by our Animal Local Ethics Committee with the number of 2022–01/10. B.30.2.ULU.0.8Z.00.00/168).
Animal model
Twenty-four mature male Sprague–Dawley rats were 6 months and weighing 300–350 g kept in standard conditions with free access to food and water. The rats were randomly divided into three groups with an equal number of 8. The groups were formed as the control (C), the hyperoxia (H), and the hyperoxia + tadalafil (H + T) groups, respectively.
Groups
Control group (C) Eight adult male rats were kept in room conditions (FiO2 = 0.21), humidity rate of 60 ± 5%, and room temperature of 21 ± 1 °C. Surgery was performed under general anesthesia. Hyperoxia group (H) Eight male rats were exposed to hyperoxia (FiO2 = 0.96) in a hyperoxia cabinet for 8 h/day for 10 days. All rats were removed from the oxygen cabinet and placed in separate cages. Each rat was fed with an amount of 10–12 ml/100 g of water and sufficient food, daily. Hyperoxia + tadalafil (HT) group Eight adult male rats were exposed to hyperoxia in a hyperoxia cabinet for 8 h/day for 10 days. While the rats are in the hyperoxia cabin, the rats were fed food and water. After the rats were removed from the cabin, tadalafil was dissolved in water and the amount of a dose of 10 mg/kg was given to the rats. Ten days later, having taken some blood samples for biochemical analysis from each group, surgery was performed under general anesthesia to take out the kidneys for histopathological examination.
Creation of hyperoxia
The oxygen was given to the rats in a cabin via an inlet hole at one end and exhaled air was expelled from the outlet hole from the other end. While the exit hole of the cabin was left open, the entrance hole was connected to the oxygen cylinder with an extension hose. Oxygen was continuously supplied to the cabin, so that the FiO2 = 0.96 (96%) value of the air inside the cabin remained constant. The FiO2 value in the exit hole of the cabin was checked every 2 h with an oxygen monitor (Datex-Ohmeda™ 5120 oxygen monitor and oxygen sensor; BOC Healthcare, Chalfont St. Giles, UK) calibrated with the help of a cannula.
Surgical method
Sevoflurane (Sevorane, Abbott USA) was administered at 1–2% volume concentration in 4 lt/min O2 flow using Dräger Titus (Lübeck, Germany) brand device to provide anesthesia. First, a thoracotomy incision was made to access the heart. After anesthesia was achieved, both kidneys were removed and the heart was reached by thoracotomy incision for biochemical analysis, and the rats were sacrificed after blood samples were taken. The excised tissues were kept for histopathological examination in a 10% formaldehyde solution.
Biochemical examination
The samples were centrifuged at 2500 g for 10 min, and serum aliquots were stored at − 20 °C until the analysis. Serum nitric oxide (NO) levels were measured by an enzyme-linked immunosorbent assay (ELISA) kit for rats (Bioassay Technology Laboratory, Shangai, China) in accordance with the manufacturer’s instructions. Samples were placed into wells coated with a rat NO antibody used as a catcher. Biotinylated rat NO antibody was added and bound to NO in the sample. Then, streptavidin–horseradish peroxidase was added. Following the sandwich formation, the excess unbound conjugate was removed and substrate solution was added. The absorbances were determined with an ELISA reader at 450 nm (FLASH Scan S12, Analytic Jena AG, Germany). Levels of serum NO concentrations were shown as µmol/L. Rat serum SOD1 levels were determined by ELISA kit (Bioassay Technology Laboratory, China) according to the principle mentioned above. SOD1 levels were expressed as ng/mL.
Histopathologic examination
The kidneys were fixed in 10% neutral buffered formalin for 24 h. Tissues were paraffinized following graded ethyl alcohol dehydration. Paraffin blocks were cut to obtain 4-micron ribbons. Ribbons were transferred to slides and stained with routine hematoxylin and eosin staining technique after deparaffinization. Each slide was examined and graded under a light microscope (Olympus CX41) semi-quantitatively. The sections were photographed directly using a stereomicroscope at 400 × high-power fields.
The glomeruli and convoluted tubules in the cortex of the kidneys were examined. Endothelial and epithelial cell proliferation of the capillaries cluster in the glomerulus was scored between 0 and 3 (0: no, 1: mild, 2: moderate, 3: severe). Parenchymal degeneration of the tubules was evaluated. It was scored according to the degree of narrowing of the tubular lumen and swelling of the epithelial cell tissue in the tubule (0: no, 1: mild, 2: moderate, 3: severe). Fifteen glomeruli and tubules were examined in the cortical region, and the average was taken. The mean of the scores given by evaluating 15 glomeruli in each rat was calculated.
Statistical analysis
Data were analyzed using SPSS-22 for Windows (SPSS, Inc., Chicago, IL, USA) software. The continuous data were not normally distributed. Statistical analysis of the medians of continuous variables was performed with the Kruskal–Wallis test. Results were given as median, minimum and maximum values. Categorical variables were analyzed with a Chi-square test, and p < 0.05 was considered statistically significant.
Results
Table 1 shows the biochemical data and analysis results of the study groups (Table 1). While our findings showed that SOD levels were significantly different among the control and experimental groups and within the experimental groups, no statistical difference was found in terms of NO levels among the groups (Table 1). According to our findings, the SOD level was the highest in the hyperoxia group and the lowest in the hyperoxia + tadalafil group (Table 1).
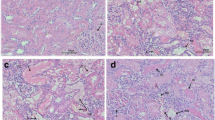
Analysis results of histopathological findings in study groups are summarized in Tables 2 and 3. Glomerular and tubular injuries were higher in Group H and H + T than in Group C. The photo of the histopathologic findings related to normal structures of the glomeruli and tubule for the control group is shown in Fig. 1. In Fig. 2A, B pathologic findings related to the proliferation of endothelial and epithelial cells and filling of the Bowman’s space in glomeruli for the hyperoxia group (H) and tadalafil group (H + T) are shown and in Fig. 3A, B hydropic degeneration of the tubular epithelium and the enlargement of the tubule.
While the glomerular and tubular injury was higher in the H group and the H + T group than in the control group (p < 0.001), as a result of the rate of severe glomerular and tubular injury in the hyperoxia group (H), was 62.5% and 43.8% and in the group given tadalafil (H + T) was 43.8% and 31.3%, respectively (Table 2).
Discussion
Oxygen therapy is required in 15–20% of hospitalized patients and hyperoxia occurs in approximately half of them [19], and also currently, the high prevalence of hyperoxia was shown during the COVID-19 pandemic, as oxygen therapy was given to 75% of patients hospitalized for Covid 19 infection [20]. Damiani and Girardi reported that more oxygen therapy without hypoxia causes oxidative stress (OS) (which is defined as the deterioration of the balance between ROS and antioxidants in favor of ROS [21]) and inflammation, especially in patients with myocardial infarction (MI), stroke, cardiac arrest, and sepsis [22]. Increased OS can trigger oxidative changes that can lead to molecular and cellular dysfunction by damaging proteins, lipids, and nucleic acids which provide intracellular signaling in the cell thereby disrupting intracellular signaling pathways [23] and inducing proinflammatory responses [24].
Based on our research, we can say that among the studies reporting that exposure to hyperoxia causes damage to various organs by increasing oxidative stress, and exposures related to the kidneys, especially in the neonatal period [2]. Human and animal studies have shown that neonatal hyperoxia increases OS and can adversely affect glomerular and tubular maturity, leading to renal corpuscle enlargements, renal tubular necrosis, interstitial inflammation, and fibrosis. [2]. According to studies conducted on rats in the neonatal period, it has been reported that this situation may pave the way for hypertension in adult life [2]. However, we can say that there is only one study related to the adult kidney [25], and according to the conclusion of this study, among critically ill patients with 2 or more SIRS criteria, treatment with a low-normal Pao2 target compared with a high-normal Pao2 target did not result in a statistically significant reduction in organ dysfunction. However, the authors have mentioned that their study may have had limited power to make more clear this issue more, as well [25].
Normally, the body's defense and protection system aims to protect the integrity of the cell for normal function of the cell by activating the antioxidant mechanism against ROS up to a certain threshold. Therefore, the cell tries to deal with the OS in this way [1]. Once this threshold point is exceeded in favor of ROS, the cell needs exogenous antioxidants to maintain its integrity and thus function, and this suggests that the balance between SOD and ROS is achieved by some kind of feedback mechanism [1]. The increase in SOD, which is one of the antioxidant markers that increase in response to ROS, can be used as a biochemical indicator of the increased OS [21].
In this experimental study, we aimed to show the effects of hyperoxia in adult rat kidneys by increasing OS with the hyperoxia model, both histopathologically and by measuring SOD, which is one of the antioxidant biochemical markers. In addition, we aimed to investigate the contradictory results about the effect of tadalafil, which has been used as an exogenous antioxidant in many studies, with the same method.
In our study, based on the analysis of the studied biochemical parameters, while there was a difference in SOD levels between the hyperoxia group and the control group, this difference disappeared with the use of tadalafil in the hyperoxia + tadalafil group. In this work, while OS increases with the hyperoxia method, SOD increases to compensate for its formed damage, and when an exogenous antioxidant (which in our study is tadalafil) is used, the SOD level decreases in this group as the need for SOD will also decrease. In their experimental study examining the relationship between hyperoxia–ischemic reperfusion and SOD, Cai et al. reported that the SOD level was higher in the normobaric hyperoxia + ischemia + reperfusion group than in the ischemic–reperfusion group alone [26]. And thus, the authors concluded that normobaric hyperoxia intervention may have protective effects on rats’ kidneys having a renal ischemia–reperfusion injury, probably by means of the increased SOD level in that group [26]. The difference between our study and the study of Cai et al. was that the level of SOD was measured in serum, while they measured it in kidney tissue. However, we found that the NO level was not affected by the increased OS level and its damage. This may be related to the duration and/or amount of exposure to hyperoxia and thus to the level of OS damage. To further clarify this issue experimental and clinical randomized controlled studies are needed.
Tubular damage rate, which is one of the histopathological findings of the hyperoxia effect in our study (Figs. 1, 2 and 3), was also shown in some studies in the literature [2, 27,28,29] in neonatal rat kidneys, but unlike these studies, we preferred adult rats in our study. Xu et al. stated that inhibited expression of tight junction proteins (claudin-4, occludin, and ZO-1) in proximal tubules might be a potential mechanism of neonatal hyperoxia-induced nephrogenic disorders [28]. In the analysis of the histopathological findings of our study, we see that the state of hyperoxia causes glomerular and tubular damage due to increased oxidative stress, and this damage rate does not improve much with the use of tadalafil (Tables 2 and 3). Although the difference in both glomerular and tubular damage rates was not significant in the experimental groups in total, when we analyzed according to the degree of damage, we found that tadalafil reduced the degree of severe damage in the tadalafil-treated group. In our study, we attributed the different pathological findings in both kidneys of a rat due to hyperoxia, to the fact that these two kidneys may have different anatomical structures and this structure may give different responses to rat metabolism. In a similar study in the literature, but in the rat ovaries, it was reported that tadalafil at a dose of 5 mg/kg decreased the rate of ovarian damage and accelerated the recovery rate [15]. In the current study, the insignificance of the difference in the degree of injury may be related to the tadalafil dose and treatment duration in the tadalafil-treated group, and an important difference from the study by Arikan et al. [15] is that it was a study on kidneys. In the study of Sugimoto et al. [17] investigating the effect of tadalafil at a dose of 10 mg/kg for 30 days in the rat prostate in a nonbacterial prostatitis model, it was reported that tadalafil showed anti-inflammatory and stromal anti-proliferative effects at this dose and time. In addition these, in the study of Özmerdiven et al., it was reported that tadalafil had positive effects on post-ESWL renal damage [18].
Since the histopathological findings of oxidative stress induced by hyperoxia and the analysis results of biochemical parameters show the parallelism between the groups, they confirm each other in terms of their results.
Study Limitation
To investigate the significance of only SOD and NO parameters first as the antioxidant markers due to the cost-effective approach, taking into account the budget of our clinic without measuring the levels of other antioxidant markers and failure to investigate ROS levels are the limitation of our study.
Conclusion
Based on our study results, exposure to hyperoxia condition causes renal glomerular and tubular damage, and tadalafil does not show a protective effect on this damage according to the dose and exposure time of our study. Hence, there is a need for randomized controlled experimental and clinical studies to determine the effect of tadalafil on this issue and, if any, at what dose and duration.
References
Yeni S, Demir A, Kilicarslan N, Cicek MC, Saricetin A, Dirican M et al (2022) Tadalafil against hyperoxia-induced oxidative stress; an experimental study. Andrologia 54:e14494
Jiang JS, Chou HC, Yeh TF, Chen CM (2015) Neonatal hyperoxia exposure induces kidney fibrosis in rats. Pediatr and Neonatal 56(4):235–241
Esteban A, Anzuento A, Alia I, Gordo F, Apezteguia C, Palizas F, Cide D et al (2000) How is mechanical ventilation employed in the intensive care unit? an international review. Am J Respir Crit Care Med 61(5):1450–1458
Ni YN, Wang YM, Liang BM, Liang ZA (2019) The effect of hyperoxia on mortality in critically ill patients: a systematic review and meta analysis. BMC Pulm Med 19:53
Nielsen JK, Bonnesen B, Hansen EF, Jensen JS, Lapperre TS, Weinreich UM, Hilberg O (2020) Guideline for the management of COVID-19 patients during hospital admission in a non-intensive care setting. Eur Clin Respir J 7:1761677. https://doi.org/10.1080/20018525.2020.1761677
Bhandari V, Maulik N, Kresch M (2000) Hyperoxia causes an increase in antioxidant enzyme activity in adult and fetal rat type II pneumocytes. Lung 178(1):53–60
Barazzone C, White CW (2000) Mechanisms of cell injury and death in hyperoxia: role of cytokines and Bcl-2 familiy proteins. Am J Respir Cell Mol Biol 22(5):517–519
Verratti V, Di Giulio C, Berardinelli F, Tiboni GM, Pellicciotta M, Brunetti L, Ferrante C et al (2008) Pampiniform plexus and oxidative stress during chronic hypoxia and hyperoxia. Int J Immunopathol Pharmacol 21(2):353–357
Mostafa T, Anis TH, Ghazi S, El-Nashar AR, Imam H, Osman IA (2006) Reactive oxygen species and antioxidants relationship in the internal spermatic vein blood of infertile men with varicocele. Asian J Androl 8(4):451–455. https://doi.org/10.1111/j.1745-7262.2006.00172.x
de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PHJ, Bosman RJ et al (2008) Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care 12(6):R156
Freeman BA, Crapo JD (1982) Free radicals and tissue injury. Lab Invest 47(5):412–426 (PMID: 6290784)
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4(8):118–126. https://doi.org/10.4103/0973-7847.70902
Dröge W (2022) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. https://doi.org/10.1152/physrev.00018.2001
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological funcitons and human disease. Int J Biochem Cell Biol 39(1):44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Arikan DC, Bakan V, Kurutas EB, Sayar H, Coskum A (2010) Protective effect of tadalafil on ischemia/reperfusion injury of rat ovary. J Pediatr Surg 45(11):2203–2209. https://doi.org/10.1016/j.jpedsurg.2010.07.011
Morelli A, Comeglio P, Filippi S, Sarchielli E, Vignozzi L, Maneschi E, Cellai I et al (2013) Mechanism of action of phosphodiesterase type 5 inhibition in metabolic syndrome-associated prostate alterations: an experimental study in the Rabbit. Prostate 73:428–441
Sugimoto M, Zhang X, Ueda N, Tsunemori H, Taoka R, Hayashida Y, Hirama H et al (2019) A phosphodiesterase 5 inhibitor, tadalafil, suppresses stromal predominance and inflammation in a rat model of nonbacterial prostatitis. BMC Urol 19:99
Ozmerdiven G, Vuruskan BA, Kaygisiz O, Vuruskan H (2017) Protective effects of diltiazem and tadalafil on shock wave-induced kidney injury in rats. Bratisl Lek Listy 118(4):228–232. https://doi.org/10.4149/BLL_2017_045
Hale KE, Gavin C, O’Driscoll BR (2008) Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J 25(11):773–776
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A Review. JAMA 324(8):782–793
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35(5):1147–1150
Damiani E, Donati A, Girardis M (2018) Oxygen in the critically ill: friend or foe? Curr Opin Anaesthesiol 31(2):129–135
Lopez-Alarcona C, Denicola A (2013) Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal Chim Acta 763:1–10
Xu S, Touyz RM (2006) Reactive oxygen species and vascular remodeling in hypertension: still alive. Can J Cardiol 22(11):947–951
Gelissen H, de Grooth HJ, Smulders Y, Wils EJ, de Rujter W, Vink R, Smit B et al (2021) Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA 326(10):940–948
Cai SY, Pei J, Yan B, Liu ZY, Chen Y, Sima CY, Su CJ et al (2021) Effects of normobaric hyperoxia intervention on renal ischemia-reperfusion injury in rats. Zhonghua Yi Xue Za Zhi 101(14):1036–1040
Chen CM, Chou HC (2019) Maternal inflammation exacerbates neonatal hyperoxia-induced kidney injury in rat offspring. Pediatr Res 86(2):174–180
Xu X, Zhang X, Gao L, Liu C, You K. (2020) Neonatal hyperoxia downregulates claudin-4, occludin, and ZO-1 expression in rat kidney accompanied by impaired proximal tubular development. Oxid Med Cell Longev, p 2641461. https://doi.org/10.1155/2020/2641461
Ali MF, Venkatarayappa SKB, Benny M, Rojas C, Yousefi K, Shehadeh KA, Kulandavelu S et al (2020) Effects of Klotho supplementation on hyperoxia-induced renal injury in a rodent model of postnatal nephrogenesis. Pediatr Res 88(4):565–570
Funding
This study was not funded by any grant.
Author information
Authors and Affiliations
Contributions
Contributed substantially to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: NK, AD, SY, OK, AS, MD Drafted the work or revised it critically for important intellectual content: NK, AD, MCC, SY and OK.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kilicarslan, N., Demir, A., Yeni, S. et al. The danger of hyperoxia on the rat kidneys: is tadalafil a real shield?. Int Urol Nephrol 55, 241–247 (2023). https://doi.org/10.1007/s11255-022-03416-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03416-w