Abstract
Background
The feasibility and efficacy of low-protein diets (LPD) treatment in chronic kidney disease (CKD) is controversial. Based on the characteristics of the Chinese diet, we observe the qualification rates and short-term clinical effects of LPD for CKD patients in our center.
Methods
This is a retrospective cohort study. CKD stages 3–5 patients who were regularly followed up 5 times (over 2 years) and treated with LPD were included. We collected clinical data to observe the changes in LPD qualification rates and divided patients into LPD and non-LPD group according to the average dietary protein intake (DPI) of 5 follow-up time points and compared the changes in primary and secondary outcome measures between the two groups.
Results
We analyzed data from 161 eligible CKD stages 3–5 patients. From baseline to the 5th follow-up time point, the LPD qualification rates of all patients were 11.80%, 35.40%, 47.82%, 53.43% and 54.04%, respectively. For primary outcome measures, the urine protein/creatinine ratio (UPCR) decreased more in the LPD group than in the non-LPD group [Median (interquartile range, IQR) of the difference between the 5th follow-up time point and baseline: 0.19 (− 0.01–0.73) vs. 0.10 (− 0.08–0.27), P < 0.001]. We constructed three classes of mixed linear models (model I, II, III). The UPCR slopes were all negative in the LPD group and positive in the non-LPD group (P < 0.001). Meanwhile, in model I, the estimate glomerular filtration rate(eGFR) decline slope in the LPD group was lower than that in the non-LPD group [slope (standard error): − 1.32 (0.37) vs. − 2.35 (0.33), P = 0.036]. For secondary outcome measures, body mass index (BMI) triglycerides (TG), body weight, and fat free mass (FFM) showed stable statistical differences in the comparison of LPD and non-LPD groups, with greater declines in the former.
Conclusion
The results of this study suggest that LPD treatment can reduce UPCR in patients with CKD stages 3–5, and may also delay the decline in eGFR. Meanwhile, it also reduces BMI, TG, body weight, and FFM, thus the need to prevent malnutrition in clinical implementation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chronic kidney disease (CKD) has a global prevalence of around 8–16%. It can diminish quality of life, and increase the risk of cardiovascular disease and mortality. As such, it has been recognized as a global public health problem [1, 2]. In recent years, nutrition management has been considered an important approach for conservative treatment for CKD patients, and accordingly, it is gaining attention [3, 4]. Effective nutritional treatment not only can ensure that CKD patients’ nutritional requirements are met, but also can reduce metabolite accumulation, therefore contributing to the control of uremic symptoms and other complications, such as electrolyte disturbances and acid–base imbalances, sodium and water retention, and mineral and bone disorder (CKD-MBD) syndrome [3].
Low-protein diets (LPD) have long been controversial in nutritional management. The initial results of the modification of diet in renal disease (MDRD) study showed that LPD had little benefit in the short-term, and did not delay CKD progression [5]. However, other scholars have arrived at different conclusions after reanalyzing the MDRD study, arguing that inconclusive evidence should not be mistaken for evidence supporting null hypotheses [6]. In 2011, the Clinical Practice Guidelines issued by the British Renal Society (BRS) recommended a protein intake of 0.75 g/kg ideal body weight (IBW)/day for CKD stage 4–5 non-dialysis patients [7]. In 2019, it was changed to 0.8–1.0 g/kg IBW/day, and the BRS declared that they believed there was insufficient evidence to recommend LPD therapy (1C) [8]. In contrast, the 2020 update of the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for Nutrition in CKD pointed out that a protein-restricted diet could reduce the risk of end-stage renal disease (ESRD) or death (1A), and improve quality of life (2C). For patients with CKD stages 3–5 and without diabetes, LPD should be limited to 0.55–0.60 g dietary protein/kg body weight (BW)/day, and for patients with CKD stage 3–5 and diabetes, LPD should be limited to 0.60–0.80 g dietary protein/kg BW/day [9].
However, perhaps due to differences in dietary habits, LPD has been recommended for nutritional therapy for CKD in China since the 2005 Consensus on Protein Nutritional Therapy for Chronic Kidney Disease [10]. The Health Industry Standard of the People's Republic of China—Dietary Guide for Chronic Kidney Disease Patients WS/557–2017 promulgated in 2017 also provides detailed guidance on LPD [11]. The latest Clinical Practice Guidelines for Nutritional Therapy of Chronic Kidney Disease in China (2021 Edition) also still recommends protein diet restriction [12].
Another problem pertaining to LPD is that its implementation in clinical settings is difficult. Results from a US survey showed that average daily protein intakes were 1.3 g/kg/day [13]. Thus, to meet LPD treatment standards, it would be necessary to reduce the total protein intake by more than half, which in turn would affect patients' dietary satisfaction and motivation [14]. Coupled with patients’ varying education levels and diet patterns, the lack of effective monitoring and feedback mechanisms in medical institutions, as well as patients’ minimal understanding of nephropathy, insufficient doctor-patient communication, insufficient family support, and other reasons, LPD treatment compliance is often compromised [15]. Moreover, many nephrologists lack training and experience in LPD; they fear malnutrition and are unable to develop detailed LPD regimens. As such, they do not implement the LPD treatment [16].
Our center emphasizes LPD therapy for CKD, and has established a professional nutrition management team to oversee patients’ dietary intake, and to collect clinical data. The Chinese Nutrition Society established the current protein dietary reference intakes (DRIs) in China in 2013, which include the estimated averaged requirement (EAR) and recommended nutrient intake (RNI). The EAR for protein is 60 g/day for adult males and 50 g/day for adult females (0.9 g/kg/day). The RNI for protein is 65 g/day for adult males and 55 g/day for adult females (1.0 g/kg/day). A study showed that the total dietary protein intake of the Chinese population would vary with the seasons, with an average estimate of 68.48 ± 22.07 g/day [17, 18]. However, due to the specificity of the Chinese diet, which consists of primarily mixed foods, there are few international reports on LPD in Chinese CKD patients. With this in mind, how is LPD adherence among CKD patients with Chinese dietary habits? What effect does LPD have on CKD prognosis? Can restricting dietary protein affect nutritional status? We conducted a retrospective study to observe the LPD qualification rates among patients with CKD stages 3–5, based on our data. We then analyzed the short-term effects of LPD on CKD progression.
Methods
Study setting and study design
This is a retrospective cohort study. It was conducted in the Chronic Disease Management Center at the Nephrology Department of Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, Guangdong Province, China. This study has been registered on the Chinese Clinical Trial Registry, registration number: ChiCTR1900024633.
Diagnostic and staging criteria
CKD diagnosis and clinical staging was based on the KDOQI clinical practice guidelines [19]. We estimated GFR using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI 2009 Serum creatinine) [20].
Inclusion and exclusion criteria
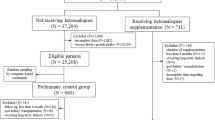
The inclusion criteria were: (I) patients aged 18 years and older; (II) estimated (e)GFR < 60 mL/min/1.73 m2; (III) patients who had signed written informed consent to receive self-management and were willing to share clinical data; (IV) results of routine blood tests, routine urine tests, biochemical tests, urinary protein/creatinine ratio (UPCR), 24-h urine analysis, and body composition analysis needed to have been obtained every 6 months over the previous 2 years; The exclusion criteria were: (I) patients with psychiatric illness or other reasons which prevented their cooperation; (II) patients undergoing renal replacement therapy; (III) Patients with acute, subacute or chronic inflammatory conditions; (IV) patients with cancer; (V) patients who are ingesting steroids.
Dietary restriction of protein intake
This cohort study’s nutrition management team consisted of 2 nephrologists, 3 nurses, 1 registered dietitian, and several clinical graduate students. After patients had established a complete record file at the chronic disease management center, physicians would prescribe nutritional prescriptions, and nurses would provide dietary education according to the patients’ disease conditions.
For detailed diet management steps, see WS/557–2017 [11]: Step 1: Calculate energy intake and food exchange portion. WS/557–2017 uses the Broca-Katsura formula (Katsura method) to calculate the standard body weight (SBW) for Asians and recommends daily caloric and protein intakes for patients with CKD based on the SBW [21, 22], (male) SBW = (height cm-100) *0.9 (kg); (female) SBW = (height cm-100) *0.9 (kg) -2.5 (kg); for patients with CKD stages 3–5, energy intake needed to be maintained at 35 kcal/kg SBW/day (age ≤ 60 years) or 30 kcal to 35 kcal/kg SBW/day (age > 60 years).
Step 2: Calculate protein intake based on the nephropathy food exchange portion in China. This method, based on the renal exchange list, is a practical tool for dietary planning and facilitates nutritional management of kidney disease in developing countries or regions [23, 24]. It classifies foods into 3 levels according to their protein content: 0–1 g, 4 g, and 7 g. 0–1 g foods included oils, starches, melon vegetables, and some fruits; 4 g foods included grains and yams, green leafy vegetables; 7 g foods included beans, meat, eggs and dairy. Every food portion had a unique weight, but all could provide calories in 90 kcal or multiples of 90 kcal (with the exception of vegetables). The total calories that a patient needed to consume per day divided by 90 kcal was how many food portions that patient needed per day. Not only was this method conducive to rapid LPD calculation, but it also ensured sufficient energy intake.
Step 3: Allocate food. At least 50% of total protein intake needed to be high-quality protein. First, allocate the high-quality protein foods (7 g foods) to guarantee the proportion of high-quality protein, and then allocate the non-high-quality protein food (4 g foods) and consider the rationality of food pairings at the same time. The remaining calories could be provided by the low-protein foods (0–1 g foods) since they contained little or no protein.
Step 4: Develop specific recipes. Pair specific foods according to the results of the above food exchange portion, and according to taste.
Personalized adjustment
In actual application, the above steps would be fine-tuned based on age, weight, physical activity type, comorbidities, stress conditions, and the specific protein content of the food. As for the intake of minerals and vitamins, we would select appropriate foods according to patient needs. We measured the 24-h urinary urea excretion to assess the patients’ actual protein intake. A professional dietitian from the team would handle any cases with serious conditions or complex nutritional management needs, and nurses would give ordinary patients routine nutritional guidance.
Assessing nutritional status
We followed up with patients recruited for the study at least every 6 months. At each follow-up, patients would be required to fill out the Subjective Global Assessment (SGA) scale and perform laboratory tests including serum albumin (ALB), triglycerides (TG), total cholesterol (TC), hemoglobin (Hb), and body composition analysis based on bioelectrical impedance.
Grouping
We measured the 24-h urinary urea excretion to calculate the normalized protein equivalent of nitrogen appearance rate (nPNA) according to the Maroni-Mitch formula. In this way, we could evaluate the patients’ actual protein intake [25]. Next, we calculated the patients’ dietary protein intake (DPI), and then we averaged the DPI of the 5 follow-up time points within 2 years. According to WS/557–2017, we considered patients with DPI < 0.8 g/kg SBW/day qualified, and included them in the LDP group. Otherwise, they were included in the non-LDP group. The specific indicators we used to observe LPD’s effect on kidney-related indicators were as follows.
Primary and secondary outcome measures
This study’s primary outcome measures were UPCR and estimated glomerular filtration rate (eGFR), where UPCR is a spot (random) urine protein creatinine ratio (P/C ratio), an alternative, rapid and simple method for detecting and estimating quantitative proteinuria assessment with good reliability in a wide range of disease states [26, 27]. The secondary outcome measures were serum creatinine (SCR), blood urea (UREA), serum uric acid (UA), carbon dioxide combining power (CO2CP), TG, TC, ALB, Hb and the related indicators of nutrition assessment as measured by a Body Composition Analyzer (Ver. LookinBody120, InBody, South Korea). This included body mass index (BMI), total body water (TBW), extracellular water ratio (EWR), waist-hip ratio (WHR), fat free mass (FFM), arm circumference (AC) and arm muscle circumference (AMC).
We performed all laboratory testing in Guangdong Provincial Hospital of Chinese Medicine’s laboratory, and collected the data from the hospital’s Hospital Information System (HIS).
Statistical analysis
Continuous variables conforming to a normal distribution are presented as mean ± standard deviation (SD), and we analyzed them with a t-test. Continuous variables not conforming to the normal distribution are presented as medians (interquartile range, IQR), and we compared them using a Mann–Whitney U-test. Categorical variables are presented as frequencies (percentage), and we compared them using either a chi-square test or Fisher’s exact test. We used mixed linear model (MLM) for repeated measures to calculate the slope changes in the two groups’ indicators. We constructed three classes of random intercepts and random slope models (I, II, III). Then, we entered group, time, and the interaction between group and time into Model I. Model II adjusted the demographic data based on Model I [including age, sex, height, weight, SBW, BMI, education level, retirement status, marital status, comorbidities, systolic blood pressure (SBP) and diastolic blood pressure (DBP)]. We assessed comorbidities using the Charlson comorbidity index, which could predict the risk of death in patients with CKD [28]. Model III adjusted for all covariates in Model II and for body composition analysis indicators (including TBW, EWR, BMI, WHR, FFM, AC and AMC), DPI, dietary energy intake (based on a three-day diet diary) and laboratory test indicators (including SCR, UREA, UA, CO2CP, eGFR, TG, TC, ALB, Hb and UPCR). We used Little's test to validate whether the missing data was a random sample of the total data. We determined that the missing data in this study were indeed randomly missing, and that the missing rate was below 10%. The missing values can be supplemented automatically when using mixed linear model analysis [29]. The study’s significance level was set at P ≤ 0.05, and all data was analyzed using SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Ethical issues
All patients included in the study signed an informed consent that allowed their clinical data to be used for medical research. Additionally, this study was approved by the Ethics Committee at Guangdong Provincial Hospital of Chinese Medicine (Approval notice: AF/04-06.1/10.0, 4, July 2019, ZF2019-153-01).
Results
Patient selection and grouping results
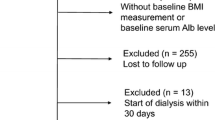
A total of 218 patients with CKD stages 3–5 were enrolled from October 01, 2019 to October 31, 2021, and each patient was followed up at least every 6 months. We retrospectively collected clinical data for these 218 patients at 5 follow-up points (2 years). 204 patients completed CKD routine laboratory tests and 189 patients had the results of 24-h urine analysis; 161 patients received regular nutritional status assessment via body composition analyzers. Therefore, 161 patients were eligible for inclusion in the final analysis. Finally, according to the grouping criteria, we included 69 patients from the study in the LPD group, and 92 patients in the non-LPD group.
Baseline data after grouping
We compared the two groups’ baseline characteristics. Most baseline characteristics showed no significant differences in baseline characteristics. This included demographics, laboratory tests, and body composition analysis between the two groups. However, there were statistically significant differences in height, SBW, primary disease, and DPI between the 2 groups (Tables 1 and 2). The LPD group had higher height, higher SBW, and lower DPI than the non-LPD group. Additionally, patients in the LPD group had a greater proportion of primary glomerulopathy, while patients in the non-LPD group had a greater proportion of diabetic kidney disease and unknown primary disease. In the MLM, we adjusted any of the above variables which had statistically significant differences.
DPI and its qualification rate
Compared with the baseline, most patients’ DPI declined gradually from the second follow-up time point onward, and the qualification rate also gradually increased (Figs. 1 and 2). The median DPI for all patients decreased from 0.99 (0.90–1.21) g/kg SBW/day to 0.77 (0.67–0.95) g/kg SBW/day from baseline to the fourth follow-up time point, with only a slight rebound at the fifth follow-up time point. The median DPI for the fifth follow-up time point was 0.79 (0.66–0.96) g/kg SBW/day. The DPI qualification rate showed a trend of gradual improvement. The DPI qualification rate was 11.80% at baseline and 54.40% at the fifth follow-up point. Meanwhile, DPI in the two patient groups gradually decreased along ensuing follow-up time points. However, DPI decreased more in the LPD group than in the non-LPD group [0.95 (0.83–1.13) to 0.66 (0.61, 0.73) vs. 1.08 (0.93–1.23) to 0.92 (0.82–1.13)]. Among patients with qualified DPI intake at each follow-up time point, the LPD group accounted for a greater proportion than the non-LPD group (Table 3 and Supplementary material, Table 1).
Dietary protein intake for 161 patients at 5 follow-up time points. DPI dietary protein intake, SBW standard body weight. Each boxplot contains 5 nodes for each dataset, from top to bottom are the maximum value, the 75th percentile, the median, the 25th percentile, and the minimum value. All 161 patients received continuous nutrition education. Most patients had their highest DPI at baseline, and gradually decreased on the 2nd–4th follow-up time points. Compared with the 4th follow-up time point, some patients’ DPI at the 5th follow-up time point increased slightly
Qualified dietary protein intake rate among 161 patients at 5 follow-up time points. DPI dietary protein intake. From baseline to the 5th follow-up time point, the 161 patients’ DPI qualification rate gradually increased and the disqualification rate gradually decreased. The highest increase in qualification rate was at the second follow-up time point, while the lowest increase in qualification rate was at the 5th follow-up time point
Primary outcomes
There was no occurrence of renal replacement therapy in this study, from baseline to the 5th follow-up time point. For the primary outcome measures, at the 5th follow-up point, the UPCR of the LPD group was lower than that of the non-LPD group [0.52 (0.23–0.97) vs. 0.95 (0.22–2.00), P = 0.050], and from baseline to the 5th follow-up time point, UPCR decline in the LPD group exceeded that of the non-LPD group [0.19 (− 0.01–0.73) vs. − 0.11 (− 0.05–0.03), P < 0.001]. Meanwhile, there was no statistically significant difference in eGFR between the two groups at the fifth follow-up time point. There was also no statistically significant difference between the two groups in the difference in eGFR between the baseline and the 5th follow-up time point (Supplementary material, Table 2).
The data obtained in this study were had been measured repeatedly. We used a mixed linear model to compare the differences with regards to various indicators’ trends between the two groups. In this study, we set various indicators (BMI, SBP, DBP, Hb, PCR, ALB, UREA, SCR, CO2CP, UA, TG, TC, eGFR, TBW, WHR, FFM, EWR, AC and AMC) as outcome variables, and estimated the varied intercept differences and varied slope differences of various outcome variables over time. Therefore, according to the factors and covariates, 3 classes of models (I, II, and III) were fitted, and models II and III adjusted for factors that had statistically significant differences at baseline. The models’ accuracy could be determined by comparing the − 2log-likelihood. The smaller the value, the more errors were explained, the more reliable the estimated results, and the more accurate the models; the − 2log-likelihoods of most indicators in Model III were superior to those of Model II; Model II also was superior to Model I (supplementary materials). The differences in the variation trend for each indicator could be determined by comparing the slope of the curve. The positive and negative values of the slope indicated the upward and downward varied trend for each indicator, while the magnitude of the slope indicated the degree of the varied trend.
In all three MLMs, the UPCR slopes in the LPD group were negative, presenting an overall downward trend; the UPCR slopes in the non-LPD group were all positive, presenting an overall upward trend [Model I: Slope (standard error, SE): − 0.21 (0.05) vs. 0.17 (0.04), P < 0.001. Model II: − 0.21 (0.05) vs. 0.14 (0.05), P < 0.001. Model III: − 0.32 (0.06) vs. 0.003 (0.05), P < 0.001]. In Model I, there was a statistically significant difference in slope between the two groups, and the decrease in eGFR in the LPD group was less than that in the non-LPD group [− 1.32 (0.37) vs. − 2.35 (0.33), P = 0.036]. In Models II and III, there was no statistically significant difference in slope between the two groups (Supplementary material, Table 2). The data distribution trends of the primary outcome measures for the 5 follow-up time points are shown in Fig. 3.
Boxplot of primary outcome measures at 5 follow-up time points. In the boxplot, some data are reflected as outliers. The circles in the middle of the box with the gray fill color are the averages, while the short red underlined lines connect the medians. The 75%th and 25%th quartiles of the five box data are connected by gray line segments, and they form a trend graph over time with the median connecting line. In the primary outcome measures, the urinary protein/creatinine ratio gradually decreased in the LDP group, while the opposite was true for the Non-LPD group. Estimated glomerular filtration rate also showed an opposite trend in both groups, but it was not significant enough
Secondary outcomes
We compared secondary outcome measures in the LPD and Non-LPD groups, and the trends in the data at the 5 follow-up time points are shown in Supplemental Figs. 1–4. Specific data comparing the two groups are shown in Supplemental Tables 3–6. In terms of renal function, there were no significant differences in SCR, urea, UA or CO2CP between the two groups. In terms of lipids, TG and TC were lower in the LPD group than in the non-LPD group, but only TG had a statistically difference between the two groups, as well as in the comparison of Model I and Model II [difference comparison: 0.11 (− 0.29–0.50) vs. − 0.09 (− 0.55–0.28), P = 0.035. Model I: − 0.12 (0.05) vs. 0.09 (0.05), P = 0.006. Model II: − 0.09 (0.06) vs. 0.08 (0.05), P = 0.024]. Comparison of body composition analysis between the two groups showed that in terms of body weight, the LPD group had lower body weight than the non-LPD group, and the LPD group lost more weight compared to the baseline [difference comparison: 1.69 (− 0.41–4.26) vs. 0.69 (− 0.96–2.55), P = 0.050]. The BMI of the two patient groups showed a downward trend during the follow-up period, while BMI showed an even greater decrease in the LPD group. [5th follow-up time point: 21.65 (19.58–23.30) vs. 22.40 (20.45–24.35)), P = 0.035. Difference comparison: 0.75 (− 0.07–1.57) vs. 0.27 (− 0.39–0.95), P = 0.041. Model I: − 0.32 (0.06) vs. − 0.15 (0.05), P = 0.032. Model II: − 0.17 (0.04) vs. − 0.04 (0.03), P = 0.013. Model III: − 0.17 (0.05) vs. − 0.04 (0.04), P = 0.032].
The TBW loss in the LPD group was greater than that in the non-LPD group, but the observed change trend was unstable when we used the MLM to evaluate it [Difference comparison: 0.55 (0–1.28) vs. 0 (− 0.60–0.90), P = 0.026. Model I: − 0.25 (0.07) vs. − 0.06 (0.06), P = 0.039. Model II: − 0.14 (0.07) vs. 0.06 (0.06), P = 0.013. Model III: 0.02 (0.005) vs. − 0.001 (0.004) P = 0.003]. During the follow-up period, there was a statistically significant difference between the two patient groups’ WHR slopes, but because the slope value was too small, this might not have had clinical significance [Model I: 0.001 (0.002) vs. − 0.004 (0.001), P = 0.026. Model II: 0.003 (0.002) vs. − 0.003 (0.002), P = 0.007]. The decrease in FFM in the LPD group was greater than that in the non-LPD group, and all three classes models suggested a downward trend in the LPD group during the follow-up period, while in Models II and III, the non-LPD group showed an upward trend [difference: 0.75 (0–1.78) vs. 0.10 (− 0.80–1.20), P = 0.018. Model I: − 0.36 (0.10) vs. − 0.07 (0.08), P = 0.025. Model II: − 0.20 (0.09) vs. 0.10 (0.08), P = 0.008. Model III: − 0.02 (0.007) vs. 0.003 (0.006), P = 0.003]. In addition, the output from Model III suggested that the changing Hb trend in the LPD group might have been an improvement over that of the non-LPD group [2.15 (0.70) vs. 0.43 (0.59), P = 0.035].
Discussion
In this study, we observed the qualification rate and short-term clinical benefits of LPD for patients at our center with stages 3–5 CKD.
The results showed that the LPD qualification rate was only about 50% at the 5th follow-up time point (the 24th month), which is similar to the results from studies in other countries. An Italian cross-sectional study showed that adherence to dietary prescriptions in children and adolescents was 56% [30]. Another retrospective study from Brazil included 321 non-dialysis CKD patients, 158 of whom had adhered to LPD, and the adherence was 49% [31]. There are many reasons for the low LPD qualification rate. In this study, the proportion of diabetic kidney disease patients in the non-LPD group (19.6%) exceeded that in the LPD group (7.2%) (Table 2). Previous studies have shown that implementing LPD is more complicated when patients have diabetic nephropathy. Blood glucose management is more difficult for them as well, and their adherence to management is lower. Therefore, this may be one of the reasons for the low LPD qualification rate in the non-LPD group [32, 33].
Meanwhile, according to the characteristics of the patients in the center, we considered that the reasons for the low qualification LPD rate may have included: (1) The LPD implementation process was indeed cumbersome; (2) The patients in our study were middle-aged or elderly, and the dietary structure was difficult to change; (3) cooking methods and food-pairing for Chinese cuisine is rich and varied, and it is not easy for patients to follow an LPD alone when they are accustomed to eating with family. Therefore, clinicians could improve CKD patients’ low LPD qualification rates in two ways. First, for patients of means, low-protein or protein-free products could be recommended, and energy supplements and keto acid preparations should be taken as needed [34, 35]. Second, personalized nutrition prescriptions could be formulated based on patients' dietary habits and disease conditions. In China, low-protein foods are recommended for CKD patients, including wheat starches, sweet potato starches, cassava starches, mung beans, pea starches and products derived from them; recipes could also be recommended according to patients’ unique situations, to diversify diets. This would not only meet patients’ tastes, but also increase the diversity of patient choices, thereby improving their compliance with LPD [11]. However, the ideal situation would be for patients need to keep in regular communication with the clinical team. Throughout chronic disease management, patients’ dietary habits and dietary structure would gradually approach the LPD standards due to continuous precise nutritional guidance and dietary education. This could improve the compliance of patients with CKD stages 3–5 to LPD [36]. In addition, there is still a lack of reports on whether the nutrient intake qualification rate for patients receiving dietary guidance has continued to improve, or has reversed [37]. The results of this study showed that the DPI qualification rate of 161 patients increased from 11.80% at baseline to 54.40% at the 5th follow-up time point. Therefore, we believe that although the dietary structure in China is complex, LPD is still feasible, and also would optimize the diet of CKD patients through better management.
The results of this study also indicate that LPD could offer short-term benefits for patients with CKD stages 3–5. We performed statistical analysis using a parametric test, a non-parametric test, and a mixed linear model. The primary outcome measure results showed that LPD could reduce urinary protein excretion, reduce UPCR in CKD stages 3–5 patients, and may also slow eGFR decline. The secondary outcome measures showed that LPD reduced TG and decreased body weight, BMI and FFM; it may also have an effect on WHR, TBW and Hb.
CKD patients in Japan, another country with East Asian cultural roots, have also been treated with LPD under comprehensive management. Based on the pooled results of meta-analyses from several clinical studies, Japanese scholars also believed that LPD could reduce urinary protein excretion, protect renal function and alleviate subjective symptoms [38]. The mechanism through which LPD reduces urine protein excretion may be a factor in inhibiting the renin-angiotensin system (RAS) [39]. In clinical practice, the application of angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs) reduces proteinuria, and delays CKD progression in patients with large amounts of proteinuria. Studies have also shown that the overexpression of transforming growth factor-β (TGF-β) influences CKD progression and proteinuria occurrence, and that the combined treatment of ACEI/ARB and LPD reduces TGF-βexpression [40, 41].
The results of this study also suggest that LPD both causes weight loss, and lowers BMI and TG. Obesity and hyperlipidemia are the most prevalent independent risk factors for CKD [42]. Meanwhile, high BMI and lipid metabolism disorders were both risk factors for CKD co-occurring with cardiovascular disease [43]. A previous study has revealed that kidney disease progression is correlated with obesity and high BMI (> 30 kg/m2), and has a U-shaped relationship with mortality [44]. In addition to body weight, a cross-sectional study in China has also shown that hypertriglyceridemia is correlated with increased urinary albumin-to-creatinine ratios (ACR) [45]. The results of the meta-analysis also showed that LPD could reduce proteinuria, cause weight loss, adjust lipid metabolism, and reduce the onset of azotemia, thereby delaying the patient’s time to end-stage renal disease [46, 47]. It is important to note the “obesity paradox” in CKD patients: a study has shown that obesity is correlated with a lower risk of death in patients with CKD, especially ESRD [48]. This is because obesity may represent the body's nutritional reserves, and studies have suggested that adipose tissue may help chelate uremia toxins in CKD patients [49]. Therefore, in nutrient management, we need to distinguish between the pathological state of abnormal lipid accumulation and the good nutritional state of body fullness, according to patients’ varying conditions, and differentiate between the effects of lipid packing, dyslipidemia, and obesity on patients during different periods. We also must avoid abnormal lipid distribution, lipid loss, and muscle consumption, and provide the most beneficial nutritional guidance for patients [50, 51].
LPD is a safe treatment for CKD patients; it causes few adverse reactions, and few studies have reported protein-energy waste (PEW) during LPD [46, 52]. There were no cases of PEW in this study, which may be related to the absence of patients included in hemodialysis or peritoneal dialysis. In addition, we effectively avoided PEW by several methods, including (I) prescribing nutritional supplements such as keto acids to appropriate patients; (II) recommending low-protein staple foods to increase dietary energy intake; (III) providing ongoing nutritional counseling to optimize dietary nutritional intake; (IV) enhancing management of chronic kidney disease comorbidities; and (V) instructing patients to perform appropriate exercise. Such LPD treatment also does not increase the phosphorus burden, and the data at the beginning and end of this study showed that the median serum phosphorus of patients was about 1.2 mmol/L. At the 5th follow-up time point, we observed no statistically significant differences between ALB and other nutritional monitoring indicators such as AMC and AC, between the two groups. However, the results of the comparison of the difference between the two patient groups and the MLM in this study suggested that during the LPD implementation, patients tended to decrease their body weight and FFM. Although the decline was not large, it still reminds us that we should regularly monitor patient nutrition, follow patients’ nutritional status, and adjust nutrition programs in real-time according to actual situations, so as to prevent lipid loss and muscle waste.
This study has its weaknesses. In addition to the common limitations of non-randomized methods, the follow-up period was not long enough. Moreover, this was a single-center retrospective study, and we did not investigate the specific reasons for LPD non-compliance. We were also unable to perform Kaplan-Mayer and Cox analysis of the primary outcome indicators because no patients had entered the endpoint event by the time data collection was complete. To ensure the integrity of the 24-h urinary urea excretion results and other data, the patients included in the study were screened. Therefore, the sample size of this cohort is small, the patients are younger, and there are fewer comorbidities, and their follow-up adherence may have been better than patients who were not included. The results and limitations of this study highlight the need for further study, and we hope to further verify the therapeutic effects of LPD with prospective and/or randomized studies in the future.
Conclusion
The results of this study suggest that LPD treatment can reduce UPCR in patients with CKD stage 3–5, and may delay the decline in eGFR. Meanwhile, it also reduces BMI, TG, body weight, and FFM. We found no adverse reactions during LPD implementation, yet we still recommend regular nutritional status assessment to prevent PEW. However, with Chinese dietary habits, the LPD qualification rate for patients with CKD stages 3–5 is still low. Yet guidance and education offer room for additional improvement, and thus, further studies are needed to improve LPD qualification rates according to the characteristics of the Chinese diet.
Availability of data and materials
Data used and/or analyzed in the current study are available upon reasonable request to the corresponding author.
References
Drawz P, Rahman M (2015) Chronic kidney disease. Ann Intern Med 162:ITC1–ITC16
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B et al (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382:260–272
Kalantar-Zadeh K, Fouque D (2017) Nutritional management of chronic kidney disease. N Engl J Med 377:1765–1776
Downer S, Berkowitz SA, Harlan TS, Olstad DL, Mozaffarian D (2020) Food is medicine: actions to integrate food and nutrition into healthcare. BMJ 369:m2482
Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW et al (1994) The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 330:877–884
Levey AS, Greene T, Beck GJ, Caggiula AW, Kusek JW, Hunsicker LG et al (1999) Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J Am Soc Nephrol 10:2426–2439
Wright M, Jones C (2011) Renal Association Clinical Practice Guideline on nutrition in CKD. Nephron Clin Pract 118(Suppl 1):c153–c164
Wright M, Southcott E, MacLaughlin H, Wineberg S (2019) Clinical practice guideline on undernutrition in chronic kidney disease. BMC Nephrol 20:370
Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W et al (2020) KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis 76:S1–S107
Nephrology ECGotAoA-KAFi (2005) Consensus on protein nutrition therapy for chronic kidney disease. Chinese J Nephrol 421–424
National Health and Family Planning Commission (2017) Hygiene standard. Dietary guide for chronic kidney disease patients. WS/557-2017[S]
Chinese Nephrologist Association ECGoNTGotnCotCAoIM (2021) Clinical practice guidelines for the nutritional treatment of chronic kidney disease in China, 2021 edn. Natl Med J China. 101:539–559
Moore LW, Byham-Gray LD, Scott Parrott J, Rigassio-Radler D, Mandayam S, Jones SL et al (2013) The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int 83:724–732
Piccoli GB, Di Iorio BR, Chatrenet A, D’Alessandro C, Nazha M, Capizzi I et al (2020) Dietary satisfaction and quality of life in chronic kidney disease patients on low-protein diets: a multicentre study with long-term outcome data (TOrino-Pisa study). Nephrol Dial Transplant 35:790–802
Milas NC, Nowalk MP, Akpele L, Castaldo L, Coyne T, Doroshenko L et al (1995) Factors associated with adherence to the dietary protein intervention in the Modification of Diet in Renal Disease Study. J Am Diet Assoc 95:1295–1300
Kalantar-Zadeh K, Moore LW, Tortorici AR, Chou JA, St-Jules DE, Aoun A et al (2016) North American experience with Low protein diet for Non-dialysis-dependent chronic kidney disease. BMC Nephrol 17:90
Ouyang Y, Tan T, Song X, Huang F, Zhang B, Ding G et al (2021) Dietary protein intake dynamics in elderly Chinese from 1991 to 2018. Nutrients 13
Huang K, Yu D, Guo Q, Yang Y, Wei X, Zhao L et al (2022) Validation of the MSM and NCI method for estimating the usual intake of nutrients and food according to four seasons of seven consecutive daily 24 hour dietary recalls in Chinese adults. Nutrients 14:445
National KF (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1-266
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Urata H, Tahara Y, Nishiyama K, Fukuyama Y, Tsunawake N, Moji K (2001) Validity of various indices of obesity calculated from height and weight data for adult males use of the underwater-weighing method as a reference. Nihon Koshu Eisei Zasshi 48:560–567
Nishida M, Funahashi T (2009) Validity of indices (BMI, Rohrer index, Broca method) for assessment of obesity. Nihon Rinsho 67:301–306
Ziegler VS, Sucher KP, Downes NJ (1989) Southeast Asian renal exchange list. J Am Diet Assoc 89:85–92
Ameh OI, Cilliers L, Okpechi IG (2016) A practical approach to the nutritional management of chronic kidney disease patients in Cape Town, South Africa. BMC Nephrol 17:68
Maroni BJ, Steinman TI, Mitch WE (1985) A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27:58–65
Chen YT, Hsu HJ, Hsu CK, Lee CC, Hsu KH, Sun CY et al (2019) Correlation between spot and 24h proteinuria: derivation and validation of equation to estimate daily proteinuria. PLoS ONE 14:e0214614
Kaminska J, Dymicka-Piekarska V, Tomaszewska J, Matowicka-Karna J, Koper-Lenkiewicz OM (2020) Diagnostic utility of protein to creatinine ratio (P/C ratio) in spot urine sample within routine clinical practice. Crit Rev Clin Lab Sci 57:345–364
Hemmelgarn BR, Manns BJ, Quan H, Ghali WA (2003) Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42:125–132
Peters SA, Bots ML, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR 3rd et al (2012) Multiple imputation of missing repeated outcome measurements did not add to linear mixed-effects models. J Clin Epidemiol 65:686–695
Cotugno G, Nicolo R, Cappelletti S, Goffredo BM, Dionisi Vici C, Di Ciommo V (2011) Adherence to diet and quality of life in patients with phenylketonuria. Acta Paediatr 100:1144–1149
Rizzetto F, Leal VO, Bastos LS, Fouque D, Mafra D (2017) Chronic kidney disease progression: a retrospective analysis of 3-year adherence to a low protein diet. Ren Fail 39:357–362
Betonico CC, Titan SM, Correa-Giannella ML, Nery M, Queiroz M (2016) Management of diabetes mellitus in individuals with chronic kidney disease: therapeutic perspectives and glycemic control. Clinics (Sao Paulo) 71:47–53
Williams AF, Manias E, Walker R (2008) Adherence to multiple, prescribed medications in diabetic kidney disease: a qualitative study of consumers’ and health professionals’ perspectives. Int J Nurs Stud 45:1742–1756
D’Alessandro C, Rossi A, Innocenti M, Ricchiuti G, Bozzoli L, Sbragia G et al (2013) Dietary protein restriction for renal patients: don’t forget protein-free foods. J Ren Nutr 23:367–371
Cupisti A, Bolasco P (2017) Keto-analogues and essential aminoacids and other supplements in the conservative management of chronic kidney disease. Panminerva Med 59:149–156
Laddu D, Hauser M (2019) Addressing the nutritional phenotype through personalized nutrition for chronic disease prevention and management. Prog Cardiovasc Dis 62:9–14
Palmer SC, Maggo JK, Campbell KL, Craig JC, Johnson DW, Sutanto B et al (2017) Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev 4:CD011998
Watanabe S (2017) Low-protein diet for the prevention of renal failure. Proc Jpn Acad Ser B Phys Biol Sci 93:1–9
Zhang JY, Yin Y, Ni L, Long Q, You L, Zhang Q et al (2016) Low-protein diet supplemented with ketoacids ameliorates proteinuria in 3/4 nephrectomised rats by directly inhibiting the intrarenal renin-angiotensin system. Br J Nutr 116:1491–1501
Peters H, Border WA, Noble NA (2000) Angiotensin II blockade and low-protein diet produce additive therapeutic effects in experimental glomerulonephritis. Kidney Int 57:1493–1501
Koppe L, Fouque D (2019) The role for protein restriction in addition to renin-angiotensin-aldosterone system inhibitors in the management of CKD. Am J Kidney Dis 73:248–257
Gai Z, Wang T, Visentin M, Kullak-Ublick GA, Fu X, Wang Z (2019) Lipid accumulation and chronic kidney disease. Nutrients 11:722
Global Burden of Metabolic Risk Factors for Chronic Diseases C (2014) Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol 2:634-647
Lu JL, Molnar MZ, Naseer A, Mikkelsen MK, Kalantar-Zadeh K, Kovesdy CP (2015) Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol 3:704–714
Su W, Wang J, Mu Y (2020) Association between hypertriglyceridemic waist phenotype and increased urinary albumin-creatinine ratio in Chinese adults: the REACTION study. Diabetes Metab Syndr Obes 13:2965–2974
Rhee CM, Ahmadi SF, Kovesdy CP, Kalantar-Zadeh K (2018) Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. J Cachexia Sarcopenia Muscle 9:235–245
Li XF, Xu J, Liu LJ, Wang F, He SL, Su Y et al (2019) Efficacy of low-protein diet in diabetic nephropathy: a meta-analysis of randomized controlled trials. Lipids Health Dis 18:82
Vashistha T, Mehrotra R, Park J, Streja E, Dukkipati R, Nissenson AR et al (2014) Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. Am J Kidney Dis 63:612–622
Kovesdy CP, Anderson JE, Kalantar-Zadeh K (2007) Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 49:581–591
Guebre-Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D et al (2013) Ectopic lipid accumulation: a potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 95:1971–1979
Wahl P, Ducasa GM, Fornoni A (2016) Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol 310:F433–F445
Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G (2016) Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol 27:2164–2176
Funding
This work was supported by the National Key Research and Development Program of China (Project No. 2019YFE0196300), and the Double First-Class Project at Guangzhou University of Chinese Medicine.
Author information
Authors and Affiliations
Contributions
XZ participated in the design of the study, the analysis and interpretation of the data, and drafting the article. MZ and NL participated in the data analysis and preparation of the manuscript. WO provided guidance on the statistical analysis. HC, YX and BL participated in analysis and interpretation of the data and drafting the article. FT and LF proofread the content of the article. YW and XL were responsible for the design of the study and the critical revision of the article’s important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Ethics approval and consent to participate
The authors are accountable for all aspects of this work in ensuring that questions related to the accuracy or integrity of any part of the work are investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All included patients provided written informed consent, and the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine approved the study protocol (no. ZF2019-153-01). This study has been registered at the Chinese Clinical Trial Registry (ChiCTR1900024633).
Consent for publication
Not applicable. This manuscript does not include any identity revealing information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Xl., Zhang, M., Lei, N. et al. An investigation of low-protein diets’ qualification rates and an analysis of their short-term effects for patients with CKD stages 3–5: a single-center retrospective cohort study from China. Int Urol Nephrol 55, 1059–1070 (2023). https://doi.org/10.1007/s11255-022-03390-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03390-3