Abstract
Objective
Chronic kidney disease (CKD) is often complicated by anemia, which seriously affects the quality-of-life and prognosis of patients. These patients usually need iron replacement therapy. Oral iron affects the composition and abundance of intestinal flora by increasing intestinal iron concentration.
Methods
We undertook an interventional study to investigate the effects of oral versus intravenous iron therapy on the gut microbiota. Oral ferrous succinate tablets (n = 14) or intravenous iron sucrose (n = 14) was administered to anemic maintenance hemodialysis (MHD) patients for 2 months.
Results
Oral and intravenous iron treatments had different effects on gut microbial composition and diversity. After oral iron treatment, the α-diversity was decreased, while at the phylum level, the abundance of Firmicutes was reduced and the abundance of Bacteroides was increased. At the genus level, the abundance of Blautia and Coprococcus was decreased, and the abundance of Bacteroidetes was increased. Oral iron therapy was associated with a higher abundance of Lactobacillus compared with that measured in intravenous iron-treated patients. According to metagenome function prediction analysis, oral iron increased the metabolic processes of phenylalanine, valine, leucine, and isoleucine. These changes may increase uremic toxin levels, thereby increasing the progression of renal disease.
Conclusion
Iron therapy affects the diversity and composition of gut flora in MHD patients. Oral iron affects the number of bacteria and increases amino acid metabolism compared with intravenous iron. These results indicate that intravenous iron may be more appropriate for MHD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human gastrointestinal (GI) tract contains over 100 trillion bacteria with the balance of the gut microbiota related closely to human health. Recently, the theory of the gut–kidney axis has become increasingly important. It has been shown that gut microbes of CKD patients are significantly altered [1], changes that may lead to a deterioration in renal function. Many non-antibiotic drugs, such as iron preparations and proton-pump inhibitors, may also cause changes in the composition of the gut microbiome [2]. In addition, the interaction between drugs and the gut microbiota may be complex and bidirectional [2].
Iron is crucial for numerous physiological and cellular processes, such as mitochondrial respiration, DNA synthesis, oxygen transport, and some metabolic pathways. MHD patients usually require oral iron preparations to supplement iron storage. However, iron supplementation may have bad effects on the gut microbiota in MHD patients, because it increases luminal iron content which will be used by some iron affinity pathogens that are detrimental to the health of patients. Some researchers have suggested that intravenous iron may have different effects on the gut microbiome than oral iron. This study in MHD patients, therefore, compared the influence of oral and intravenous iron administration on the gut microbiota and evaluated whether intravenous iron had better effects on the gut microbiome.
Materials and methods
Study design
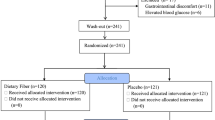
This research is a non-blind randomized controlled trial which enrolled 28 patients maintained on regular HD in the Fifth Clinical Medical College of Shanxi Medical University for more than 3 months. Patients who were diagnosed with CKD5 (maintenance hemodialysis) complicated by iron deficiency (iron serum saturation <20% with or without a ferritin level <200 mg/L) were included in the study. The age inclusion criteria were >18 years and <75 years, while the exclusion criteria were blood transfusions, antibiotic usage, diarrhea, the combination of folate or vitamin B12 deficiency in the last 3 months, gastric ulcer, gastric hemorrhage, additional probiotics, hormone use, pre-existing hematological disease, and cancer. Recruited patients were randomized to receive either oral iron (200mg of ferrous succinate tablet, once a day) or intravenous iron (100 mg of iron sucrose, 3 times a week), which was used in previous trials [3, 4] and according to KDIGO clinical practice guideline for anemia in chronic kidney disease. Oral iron treatments were continued for at least 2 months [3, 4]. Intravenous iron was infused at least ten times. Follow-up iron treatment depends upon hemoglobin and ferritin levels.
Sample collection, DNA extraction, and 16S rRNA gene amplicon sequencing
Stool specimens were collected at baseline and 2 months after iron treatment. A stool collection kit and a pamphlet with sampling instructions were given to each participant. The stool samples were collected in the morning, frozen within 30 min, and then stored at − 80 °C until sequencing was completed. The OMEGA Soil DNA Kit (D5625-01) (Omega Bio-Tek, Norcross, GA, USA) was used to extract total community genomic DNA from 50 mg of feces. Cleavage and digest of the DNA were carried out in a lysis solution containing protease and the DNA and then adsorbed in a binding solution containing magnetic particles. The purified DNA was used for 16SrRNA gene sequencing using primers targeted to the V3–V4 region (338F(5’-barcode + ACTCCTACGGGAGGCAGCA-3’) and (806R (5’-GGACTACHVGGGTWTCTAAT-3’)). The 16S rRNA genes were amplified in technical triplicates. The Illumina NovaSeq (NovaSeq 6000 SP reagent kit (500cycles)) was used to perform paired-end sequencing (2 × 250 base pairs) in a single batch.
Statistical Analysis
The QIIME 2 microbial analysis platform was used to analyze and map the microbial data. The survival feature sequence ASVs were denoised using DADA2. The Mann–Whitney U test was used to compare variance in the Chao1, observed species, Shannon diversity index, Simpson diversity index, Faith's phylogenetic diversity, Pielou's evenness, and Good’s coverage in intra-sample comparisons (α-diversity). Intergroup comparisons (β-diversity) were assessed using principal coordinate analysis (PCoA). The PCoA analysis was performed by calculating Jaccard and Bray–Curtis distance matrices [5]. The α-diversity index and PCoA diagram were drawn using the R software package “ggplot2”. LEfSe analysis was used to identify different species between the two treatment groups. The LDA effect was calculated and the group with LDA score >2 and P < 0.05 was considered statistically significant [6]. We used PICRUSt2 to predict the metagenomes. The metagenome was then divided into three layers and abundance clustering performed on the functions of multiple samples according to the KEGG database annotation that was then used to construct a pathway clustering heat map. Differences in results between the two groups with P < 0.05 were considered statistically significant (Supplementary Material 1).
Results
A total of 28 MHD patients (10 females, 18 males) were enrolled in the study, with 14 receiving oral iron and 14 receiving intravenous iron (Fig. 1). As shown in Table 1, the oral and intravenous groups were matched for age, gender, BMI, serum ferritin, and mean iron saturation level.
The continuous variables are expressed as means ± standard deviations, while categorical variables are expressed as mean percentages. BMI, body mass index. Hb, Hemoglobin. HD, hemodialysis.
Bacterial diversity following iron therapy
A rarefaction curve was constructed to evaluate the depth of OTU clustering. The data showed that the curves of each sample tended to be flat, indicating that the current sequencing depth reflected the diversity of intestinal flora (supplemental materials 2). Species accumulation curves were used to estimate whether the sample size is sufficient. The curve keeps an upward trend, which indicates that the sample size is not sufficient (supplemental materials 3). A comparison of the α-diversity statistics showed that patients treated with oral iron had less Chao1 and Observed species compared to the IV group (Fig. 2, P < 0.05). The Shannon and Simpson diversity indices were similar between the two groups. The Chao1 and Observed species are established indicators of species richness, and therefore, our data indicate that oral iron therapy reduces the abundance of bacterial richness without affecting diversity.
The α-diversity of the oral iron group was altered following iron therapy. Estimation of α-diversity using Chao1, Faith’s phylogenetic diversity, Goods coverage, Shannon diversity, Simpson diversity, Pielou’s evenness, and observed species was considerably lower in the oral group than in the intravenous group. B, after oral iron therapy and D, after intravenous iron therapy. *P < 0.05
Principle coordinate analysis (PCoA) was used to examine differences in the fecal metabolomes between the oral and IV groups. Figure 3 shows that oral administration of iron resulted in different gut microbiome clusters compared to those formed with intravenous iron (Fig. 3, P < 0.05). This suggests that oral and intravenous iron therapy has different effects on bacterial diversity, with oral iron therapy reducing bacterial richness. The β-diversity of the MHD patients also differed with oral and intravenous iron treatment.
We examined the composition of the gut microbiota in the MHD patients from phylum to genus level and also analyzed the data to identify significant differences in taxonomic composition between the oral and intravenous groups (Fig. 4A and B). Firmicutes were the dominant phylum in MHD patients. Compared to intravenous iron therapy, oral iron therapy decreased the relative abundance of Firmicutes and significantly increased the abundance of Bacteroidetes at the phylum level (P < 0.01, Fig. 4C). At the genus level, the abundance of the Blautia and Coprococcus was significantly decreased, while the abundance of Bacteroidetes was increased significantly in the oral group compared to that measured in the intravenous group (P < 0.05, Fig. 4D).
Average bacterial community compositions at the phylum A and genus B levels in both groups. Relative abundance of dominant bacterial taxa at the phylum C and genus D levels with significant differences between the two treatment groups. *P < 0.05, **P < 0.01. B, after oral iron therapy and D, after intravenous iron therapy
Bacterial communities in oral and intravenous iron-treated patients differ
We use LEfSe to determine differences in the bacterial taxa between oral and intravenous iron treatment. The microbiota of the IV group showed a greater abundance of the Verrucomicrobiae class, Verrucomicrobiales, Actinomycetales, Oceanospirillales, Rhizobiales, and Gemellales orders, Enterococcaceae, Peptostreptococcaceae, Verrucomicrobiaceae, Leuconostoaceae, Micrococcaceae, Camobateriaceae, Halomonadaceae, Actinomycetaceae, Hyphomicrobiaceae, and Gemellaceae families, and Enterococcus, Ruminococcus, VadinHB04, Akkermansia, Chelativorans, Rothia, Halomonas, Actinomyces, and Devosia genus. The Lactobacillaceae family, and the lactobacillus and vagococcus species were found in greater abundance in the oral group (P < 0.01, Fig. 5). These results suggest that the gut microbiome in MHD patients responds differently to oral and intravenous iron therapy.
LEfSe to identify bacterial taxa that shows significant differences in enrichment between the two treatment groups. The abundance of the taxa from phylum to genus levels is included. A Taxonomic cladogram of the LEfSe analysis; B histogram of the linear discriminant analysis scores; B, after oral iron therapy and D, after intravenous iron therapy. A LDA > 2 and P < 0.05 were considered statistically significant
Metagenomes function prediction analysis
We used PICRUSt2 software to perform a metagenomic prediction analysis on the 16S rRNA data. This analysis showed 48 gene categories were significantly regulated. The oral group had 36 upregulated genes and 12 downregulated genes when compared to the IV group. Mapping the gene family to KEGG databases was used to obtain abundance data of each metabolic pathway in the samples. By exploring the KEGG pathway or module, we found that amino acid metabolism-related genes increased in the oral group, such as those involved in phenylalanine, alanine, aspartate, and glutamate metabolism, and valine, leucine, and isoleucine degradation. The IV group was associated with an increased abundance of genes for ABC transporters, degradation of nitrotoluene, glycosaminoglycan, and bisphenol, metabolism of glycerolipid and methane, resistance to beta-lactam, biosynthesis of ansamycins and unsaturated fatty acids, the two-component system, yeast meiosis, and the pentose phosphate pathway (Fig. 6).
Discussion
Anemia is a common complication in CKD patients, especially in those with advanced CKD. Anemia usually results from absolute iron deficiency or a functional deficiency [7]. People with these abnormalities can choose oral or IV iron for treatment. There are many GI side effects caused by oral iron, such as nausea, vomiting, upper abdominal pain, diarrhea, and constipation [7, 8]. Excess luminal iron can induce oxidative stress and lipid peroxidation which damages intestinal epithelial cells, resulting in destruction of the mechanical barrier [9]. In addition, oral iron can change the composition of the gut microbiota and gut metabolome, which may have adverse effects on kidney function by producing more uremic toxins [3, 10]. Therefore, we collected fecal samples of MHD patients to analyze the influence of oral (PO) and intravenous (IV) iron therapy on the gut microbiota by comparing bacterial diversity and composition in the two groups.
In recent years, an increasing number of studies have reported an association between the gut microbiome and CKD. The composition and diversity of the gut microbiome in CKD patients is changed significantly, with studies showing that compared to healthy controls, the relative abundance of 190 bacterial operational taxonomic units (OTUs) was altered, and bacterial diversity was reduced [11, 12]. Plasma trimethylamine-N-oxide (TMAO) levels were also elevated due to dysbiosis of the gut microbiome, which would be harmful to the long-term survival of CKD patients [13, 14]. Several recent studies have confirmed that iron supplementation has an effect on the intestinal flora of living animals, increasing the abundance of other pathogens, such as Escherichia coli and Streptococcus, and decreasing the prevalence of Bifidobacterium and Lactobacillus [15, 16], changes that may have adverse effects on kidney function.
Our study compared the influence of different irons on the gut microbiome using 16sRNA sequencing. We found that oral and intravenous iron supplements had different effects on gut microbiota diversity, bacterial taxa, and predictive metagenomics in patients with MHD. These findings were consistent with those of the following earlier studies. Similar results were found by Lee [3] in patients with inflammatory bowel disease (IBD) who showed that oral and IV iron therapies differentially affected bacterial communities. The presence of particular molecular bacterial species was changed by oral iron treatment, which resulted in elevation of luminal iron concentrations. Based on these results, IV iron therapy was considered to have beneficial effects on the gut microbiota [3]. Our study also found that bacterial diversity and composition changed markedly after iron therapy. The microbial diversity of the oral group was significantly different from that of the IV group, while the microbial richness of the oral group was significantly reduced compared to that measured in the IV group. This suggested that IV iron therapy may be a healthier option [17] and result in a more diverse microbiota compared with that achieved by oral iron therapy. There is also evidence that gut microbial diversity is decreased significantly in patients with CKD compared to that in healthy controls [11]. These results support the possibility that oral administration of iron may have adverse effects in MHD patients.
In the oral group, the abundance of the phylum Firmicutes was decreased significantly, while Bacteroidetes was increased significantly. This resulted in a reduction in the Firmicutes/Bacteroidetes (F/B) ratio. It has been reported that obesity is associated with an increased F/B ratio, while IBD is associated with a decreased F/B ratio, with the genus Lactobacillus being the most studied probiotic for adjusting the ratio [18]. However, opinions differ on these associations, and it is currently difficult to relate the F/B ratio to a specific health condition and even obesity [19]. At the genus level, the abundance of Bacteroides was increased significantly in the oral group in our study. Similarly, it has been reported that mice fed different concentrations of iron had an increased abundance of Bacteroides [20]. A previous study in humans showed that Bacteroides played a complex role, with some subclasses of Bacteroides having beneficial humancial effects, while others were harmful [21]. There is also evidence that Bacteroides dorei and Bacteroides vulgatus reduce the production of lipopolysaccharides by gut microbiota and alleviate endotoxemia, changes which are both beneficial for preventing atherosclerosis [22].
In contrast, we found short-chain fatty acid (SCFA) producing bacteria, including species of Coprococcus. Blautia, and Lachnospiraceae, were less abundant in the oral group. SCFAs are the main bacterial metabolites produced by specific anaerobic bacteria that ferment non-starch polysaccharides (NSP) and resistant starch (RS) in the colon. This mainly includes acetic acid, propionic acid, and butyric acid. SCFAs are signaling molecules that mediate interactions between the diet, gut microbiota, and the host, and play an important role in immunity, metabolism, and the endocrine system [23]. SCFAs also have an anti-inflammatory function by regulating immune cell chemotaxis, the release of reactive oxygen species (ROS) and cytokines, and there is also evidence that they help preserve gut health by preventing and improving a range of ailments, including cancer [24].
Butyrate is an SCFA that is regarded as a source of colon energy. Butyrate can greatly improve intestinal barrier function while also reducing the inflammatory response. In addition, butyrate maintains intestinal immune balance and has a potential protective effect against IBD pathogenesis [25]. Interestingly, a recent study conducted in mice with diabetic nephropathy showed that butyrate can reduce skeletal muscle atrophy in diabetic nephropathy by stimulating the FFA2-mediated PI3K/AKT/mTOR signaling pathway [26]. A decrease in Coprococcus also has adverse effects on health. A recent study reported that Coprococcus plays an important role in health by producing vitamin B and SCFAs [27]. A systematic review showed that Coprococcus was decreased in patients with CKD and led to a decrease in butyrate [28], which is known to protect kidneys by GPR109a- and epigenetic-mediated mechanisms [29]. A previous study also showed an increase in the abundance of Lachnospiraceae in the IV iron group [30], which was reduced in patients with CKD [28]. Earlier research reported that Blautia was reduced significantly in people with CKD [11]. However, another study suggested that Blautia may be a new functional genus with potential probiotic properties [31], a finding inconsistent with previous research results. It is reported that Blautia is positively correlated with SCr and BUN [11]. These results indicate that a decrease in SCFA-producing bacteria in gut flora associated with oral administration of iron is damaging to the health of CKD patients.
Our study showed that there were differences in two bacterial taxa between the oral and IV groups. This result was similar to that reported in a previous study that showed IBD patients who developed different microbial taxa after receiving various iron therapies [3]. According to our results, the abundance of Lactobacillus was higher in the oral group compared to that measured in the IV group. Lactobacillus is one of the probiotics, and has been shown to correlate negatively with SCr and BUN levels [11]. When compared to intravenous iron treatment, Phipps et al. demonstrated that the abundance of Lactobacillus was increased after oral iron treatment in colorectal cancer patients [4]. Taken together, these results support our conclusion that patients receiving oral and intravenous iron treatment had diverse bacterial communities. Functional potential analysis using PICRUSt2 showed that oral iron supplementation increased the metabolic processes of phenylalanine, valine, leucine, and isoleucine. Amino acids are the precursors of uremic toxins. The spectrum of intestinal amino acid metabolism becomes disordered as CKD progresses, a change which is linked to intestinal microbiota dysbiosis and metagenomic alterations [32]. In addition, protein and amino acid metabolism produces precursors of uremic toxins, such as indole and p-cresol, which are further metabolized to indoxyl sulfate (IS) and p-cresyl sulfate (pCS) in the intestine and liver [33]. IS induces fibrosis-, inflammatory-, and obesity-promoting effects through the nuclear factor-Κb (NF-κB) and mitogen-activated protein kinase (MAPK) pathways [34]. IS also decreases the viability of endothelial and vascular smooth muscle cells, thereby increasing the risk of cardiovascular disease [35, 36]. PCS causes cardiomyocyte apoptosis and diastolic dysfunction through activation of NADPH oxidase and production of ROS [36]. Because iron affects amino acid metabolism, CKD patients are at a disadvantage. In the future, regulating intestinal amino acid metabolism could be a potential technique to slow the progression of CKD.
Conclusions
In conclusion, we analyzed the effects of iron administration on intestinal flora in maintenance hemodialysis patients and compared the impacts of ferrous succinate and iron sucrose. The findings showed that the species composition and diversity of gut microflora changed significantly after iron therapy. Oral iron reduced alpha diversity and the abundance of SCFAs-producing bacteria in patients with MHD when compared to the changes caused by intravenous iron. However, the Lactobacillus genus was more abundant in patients on oral iron therapy, which is beneficial for patients with MHD. However, it is necessary to validate this finding in vitro. PICRUSt2 function prediction analysis showed that oral iron increased amino acid metabolism, which may increase the progression of renal disease. This could be linked to cardiovascular disease and all-cause mortality. As a result, oral iron therapy may be more hazardous to intestinal bacteria, while intravenous iron therapy may be more appropriate for patients with renal anemia. The current study had several limitations in that various factors such as nutrition may interfere with intestinal flora, and also, the sample size was relatively small. Furthermore, we only observed changes in flora over the short term and it is possible that different intervention times may provide different microbiological findings. Further metabolomics analysis is therefore required to find a clinical correlation between different microbial communities after iron therapy and all-cause mortality and cardiovascular disease risk in patients with MHD.
Data availability
All data in this study can be obtained with the permission of the author.
References
Wu IW, Gao SS, Chou HC, Yang HY, Chang LC, Kuo YL, Dinh MCV, Chung WH, Yang CW, Lai HC, Hsieh WP, Su SC (2020) Integrative metagenomic and metabolomic analyses reveal severity-specific signatures of gut microbiota in chronic kidney disease. Theranostics 10(12):5398–5411. https://doi.org/10.7150/thno.41725
Weersma RK, Zhernakova A, Fu J (2020) Interaction between drugs and the gut microbiome. Gut 69(8):1510–1519. https://doi.org/10.1136/gutjnl-2019-320204
Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, Lucio M, Michalke B, Schmitt-Kopplin P, Fedorak R, Haller D (2017) Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 66(5):863–871. https://doi.org/10.1136/gutjnl-2015-309940
Phipps O, Al-Hassi HO, Quraishi MN, Dickson EA, Segal J, Steed H, Kumar A, Acheson AG, Beggs AD, Brookes MJ (2021) Oral and intravenous iron therapy differentially alter the on- and off-tumor microbiota in anemic colorectal cancer patients. Cancers. https://doi.org/10.3390/cancers13061341
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62(2):142–160. https://doi.org/10.1111/j.1574-6941.2007.00375.x
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60. https://doi.org/10.1186/gb-2011-12-6-r60
Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI (2020) Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol 31(3):456–468. https://doi.org/10.1681/asn.2019020213
Fishbane S, Spinowitz B (2018) Update on anemia in ESRD and earlier stages of CKD: core curriculum 2018. Am J Kidney Dis 71(3):423–435. https://doi.org/10.1053/j.ajkd.2017.09.026
Qi X, Zhang Y, Guo H, Hai Y, Luo Y, Yue T (2020) Mechanism and intervention measures of iron side effects on the intestine. Crit Rev Food Sci Nutr 60(12):2113–2125. https://doi.org/10.1080/10408398.2019.1630599
Kortman GAM, Reijnders D, Swinkels DW (2017) Oral iron supplementation: Potential implications for the gut microbiome and metabolome in patients with CKD. Hemodial Int Symp Home Hemodial 21(Suppl 1):S28-s36. https://doi.org/10.1111/hdi.12553
Ren Z, Fan Y, Li A, Shen Q, Wu J, Ren L, Lu H, Ding S, Ren H, Liu C, Liu W, Gao D, Wu Z, Guo S, Wu G, Liu Z, Yu Z, Li L (2020) Alterations of the human gut microbiome in chronic kidney disease. Adv Sci 7(20):2001936. https://doi.org/10.1002/advs.202001936
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83(2):308–315. https://doi.org/10.1038/ki.2012.345
Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, You C, Nie J, Zhou HW, Yin J (2017) Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep 7(1):1445. https://doi.org/10.1038/s41598-017-01387-y
Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL (2015) Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116(3):448–455. https://doi.org/10.1161/circresaha.116.305360
Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, Njenga J, Mwangi A, Kvalsvig J, Lacroix C, Zimmermann MB (2015) Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 64(5):731–742. https://doi.org/10.1136/gutjnl-2014-307720
Ellermann M, Gharaibeh RZ, Maharshak N, Peréz-Chanona E, Jobin C, Carroll IM, Arthur JC, Plevy SE, Fodor AA, Brouwer CR, Sartor RB (2020) Dietary iron variably modulates assembly of the intestinal microbiota in colitis-resistant and colitis-susceptible mice. Gut Microbes 11(1):32–50. https://doi.org/10.1080/19490976.2019.1599794
Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA (2018) Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol 44:34–40. https://doi.org/10.1016/j.mib.2018.07.003
Stojanov S, Berlec A, Štrukelj B (2020) The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory Bowel disease. Microorganisms. https://doi.org/10.3390/microorganisms8111715
Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R (2020) The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. https://doi.org/10.3390/nu12051474
Cuisiniere T, Calvé A, Fragoso G, Oliero M, Hajjar R, Gonzalez E, Santos MM (2021) Oral iron supplementation after antibiotic exposure induces a deleterious recovery of the gut microbiota. BMC Microbiol 21(1):259. https://doi.org/10.1186/s12866-021-02320-0
Wang C, Zhao J, Zhang H, Lee YK, Zhai Q, Chen W (2020) Roles of intestinal bacteroides in human health and diseases. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2020.1802695
Yoshida N, Emoto T, Yamashita T, Watanabe H, Hayashi T, Tabata T, Hoshi N, Hatano N, Ozawa G, Sasaki N, Mizoguchi T, Amin HZ, Hirota Y, Ogawa W, Yamada T, Hirata KI (2018) Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 138(22):2486–2498. https://doi.org/10.1161/circulationaha.118.033714
Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM, Annaert P, Delcour JA, Verbeke KA (2017) Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol 595(2):541–555. https://doi.org/10.1113/jp272613
Sun M, Wu W, Liu Z, Cong Y (2017) Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 52(1):1–8. https://doi.org/10.1007/s00535-016-1242-9
Yamada T, Hino S, Iijima H, Genda T, Aoki R, Nagata R, Han KH, Hirota M, Kinashi Y, Oguchi H, Suda W, Furusawa Y, Fujimura Y, Kunisawa J, Hattori M, Fukushima M, Morita T, Hase K (2019) Mucin O-glycans facilitate symbiosynthesis to maintain gut immune homeostasis. EBioMedicine 48:513–525. https://doi.org/10.1016/j.ebiom.2019.09.008
Tang G, Du Y, Guan H, Jia J, Zhu N, Shi Y, Rong S, Yuan W (2021) Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br J Pharmacol. https://doi.org/10.1111/bph.15693
Nogal A, Louca P, Zhang X, Wells PM, Steves CJ, Spector TD, Falchi M, Valdes AM, Menni C (2021) Circulating levels of the short-chain fatty acid acetate mediate the effect of the gut microbiome on visceral fat. Front Microbiol 12:711359. https://doi.org/10.3389/fmicb.2021.711359
Zhao J, Ning X, Liu B, Dong R, Bai M, Sun S (2021) Specific alterations in gut microbiota in patients with chronic kidney disease: an updated systematic review. Ren Fail 43(1):102–112. https://doi.org/10.1080/0886022x.2020.1864404
Felizardo RJF, de Almeida DC, Pereira RL, Watanabe IKM, Doimo NTS, Ribeiro WR, Cenedeze MA, Hiyane MI, Amano MT, Braga TT, Ferreira CM, Parmigiani RB, Andrade-Oliveira V, Volpini RA, Vinolo MAR, Mariño E, Robert R, Mackay CR, Camara NOS (2019) Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J 33(11):11894–11908. https://doi.org/10.1096/fj.201901080R
Kim DJ, Yang J, Seo H, Lee WH, Ho Lee D, Kym S, Park YS, Kim JG, Jang IJ, Kim YK, Cho JY (2020) Colorectal cancer diagnostic model utilizing metagenomic and metabolomic data of stool microbial extracellular vesicles. Sci Rep 10(1):2860. https://doi.org/10.1038/s41598-020-59529-8
Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, Zhao J, Zhang H, Chen W (2021) Blautia-a new functional genus with potential probiotic properties? Gut microbes 13(1):1–21. https://doi.org/10.1080/19490976.2021.1875796
Liu Y, Li J, Yu J, Wang Y, Lu J, Shang EX, Zhu Z, Guo J, Duan J (2018) Disorder of gut amino acids metabolism during CKD progression is related with gut microbiota dysbiosis and metagenome change. J Pharm Biomed Anal 149:425–435. https://doi.org/10.1016/j.jpba.2017.11.040
Graboski AL, Redinbo MR (2020) Gut-derived protein-bound uremic toxins. Toxins. https://doi.org/10.3390/toxins12090590
Chang JF, Hsieh CY, Liou JC, Liu SH, Hung CF, Lu KC, Lin CC, Wu CC, Ka SM, Wen LL, Wu MS, Zheng CM, Ko WC (2020) Scavenging intracellular ROS attenuates p-Cresyl sulfate-triggered osteogenesis through MAPK signaling pathway and NF-κB activation in human arterial smooth muscle cells. Toxins. https://doi.org/10.3390/toxins12080472
Li X, Lu Z, Zhou F, Jin W, Yang Y, Chen S, Xie Z, Zhao Y (2020) Indoxyl sulfate promotes the atherosclerosis through up-regulating the miR-34a expression in endothelial cells and vascular smooth muscle cells in vitro. Vasc Pharmacol 131:106763. https://doi.org/10.1016/j.vph.2020.106763
Opdebeeck B, D’Haese PC, Verhulst A (2020) Molecular and cellular mechanisms that induce arterial calcification by indoxyl sulfate and P-cresyl sulfate. Toxins. https://doi.org/10.3390/toxins12010058
Acknowledgements
The authors would like to express their gratitude to all the clinical doctors and volunteers in the Blood Purification Center of Department of Nephrology, Shanxi Provincial People's Hospital.
Funding
This research was funded by Provincial special matching funds of Shanxi Provincial People’s Hospital, Grant Number sz2019016.
Author information
Authors and Affiliations
Contributions
Conceptualization, LH and WWQ; methodology, LH; formal analysis, WWQ; investigation, LH; data curation, LH; writing—original draft preparation, LH; writing—review and editing, WWQ and LYK; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics Committee of Shanxi Provincial People's Hospital (2019 Provincial Medical Lun Shen Zi No. 149th and 2019/11/7).
Informed consent
Written informed consent has been obtained from the patients to publish this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, H., Wu, W. & Luo, Y. Oral and intravenous iron treatment alter the gut microbiome differentially in dialysis patients. Int Urol Nephrol 55, 759–767 (2023). https://doi.org/10.1007/s11255-022-03377-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03377-0