Abstract
Purpose
To investigate whether cystine crystal-induced production of reactive oxygen species (ROS) and activation of NLRP3 inflammasome contribute to cystine calculi formation.
Methods
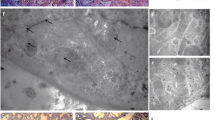
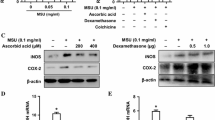
Slc7a9-knockout rats were created as cystine calculi animal models. Kidney histological examination using TEM and immunohistochemistry were performed. The protein expression of NLRP3 and IL-1β and the concentrations of oxidative stress markers such as ROS, MDA and H2O2 in kidney tissues were estimated. In parallel, HK-2 human renal proximal tubule cells were exposed to cystine crystals and NAC treatment. The protein and mRNA expression levels of NLRP3 were evaluated. Finally, cell apoptosis and cystine crystal adherence were also assessed.
Results
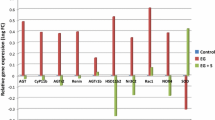
Activation of the NLRP3 inflammasome and marked elevations in MDA, H2O2 and ROS levels were observed both in vivo and in vitro. In particular, the protein and mRNA expression of NLRP3 was significantly increased by cystine crystals, but could be restored by an inhibitor of ROS. In addition, cell apoptosis and cystine crystal adherence were promoted by the NLRP3 inflammasome. The expression of CD44, OPN and HA in HK-2 cells was markedly increased by cystine crystals, but could be decreased by NLRP3 siRNA treatment.
Conclusion
Notably, we found that the activation of NLRP3 by cystine crystal-induced ROS production was of major importance in the pathogenesis of cystine calculi formation.






Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Claes DJ, Jackson E (2012) Cystinuria: mechanisms and management. Pediatr Nephrol 27(11):2031–2038
Andreassen KH, Pedersen KV, Osther SS, Jung HU, Lildal SK, Osther PJ (2016) How should patients with cystine stone disease be evaluated and treated in the twenty-first century? Urolithiasis 44(1):65–76
Prencipe G, Caiello I, Cherqui S, Whisenant T, Petrini S, Emma F, De Benedetti F (2014) Inflammasome activation by cystine crystals: implications for the pathogenesis of cystinosis. J Am Soc Nephrol 25(6):1163–1169
Erickson SB, Vrtiska TJ, Canzanello VJ, Lieske JC (2011) Cystone® for 1 year did not change urine chemistry or decrease stone burden in cystine stone formers. Urol Res 39(3):197–203
Mulay SR, Evan A, Anders HJ (2014) Molecular mechanisms of crystal-related kidney inflammation and injury Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial Transplant 29(3):507–514
Knauf F, Asplin JR, Granja I, Schmidt IM, Moeckel GW, David RJ, Flavell RA, Aronson PS (2013) NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int 84(5):895–901
Darisipudi MN, Knauf F (2016) An update on the role of the inflammasomes in the pathogenesis of kidney diseases. Pediatr Nephrol 31(4):535–544
Komada T, Muruve DA (2019) The role of inflammasomes in kidney disease. Nat Rev Nephrol 15(8):501–520
Elliott EI, Sutterwala FS (2015) Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265(1):35–52
Gross O, Thomas CJ, Guarda G, Tschopp J (2011) The inflammasome: an integrated view. Immunol Rev 243(1):136–151
Tsai PY, Ka SM, Chang JM, Lai JH, Dai MS, Jheng HL, Kuo MT, Chen P, Chen A (2012) Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum 64(1):232–242
Yifan Z, Luwei X, Kai L, Liuhua Z, Yuzheng G, Ruipeng J (2019) Protective effect of salvianolic acid B against oxidative injury associated with cystine stone formation. Urolithiasis 47(6):503–510
Liu Y, Duan C, Chen H, Wang C, Liu X, Qiu M, Tang H, Zhang F, Zhou X, Yang J (2018) Inhibition of COX-2/mPGES-1 and 5-LOX in macrophages by leonurine ameliorates monosodium urate crystal-induced inflammation. Toxicol Appl Pharmacol 15(351):1–11
Fernandes MJ, Naccache PH (2018) The role of inhibitory receptors in monosodium urate crystal-induced inflammation. Front Immunol 20(9):1883
Anders HJ, Muruve DA (2011) The inflammasomes in kidney disease. J Am Soc Nephrol 22(6):1007–1018
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440(7081):237–241
Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ (2013) Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123(1):236–246
Asselman M, Verhulst A, De Broe ME, Verkoelen CF (2003) Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol 14(12):3155–3166
Verkoelen CF, Van Der Boom BG, Romijn JC (2000) Identification of hyaluronan as a crystal-binding molecule at the surface of migrating and proliferating MDCK cells. Kidney Int 58(3):1045–1054
Vervaet BA, D’Haese PC, De Broe ME, Verhulst A (2009) Crystalluric and tubular epithelial parameters during the onset of intratubular nephrocalcinosis: illustration of the “fixed particle” theory in vivo. Nephrol Dial Transplant 24(12):3659–3668
Verkoelen CF (2006) Crystal retention in renal stone disease: a crucial role for the glycosaminoglycan hyaluronan? J Am Soc Nephrol 17(6):1673–1687
Alelign T, Petros B (2018) Kidney stone disease: an update on current concepts. Adv Urol 2018:3068365
Aggarwal KP, Narula S, Kakkar M, Tandon C (2013) Nephrolithiasis: molecular mechanism of renal stone formation and the critical role played by modulators. Biomed Res Int 2013:292953
Chi T, Taylor E, Stoller ML (2014) Treating the cystine stone former presents a singular clinical challenge. Transl Androl Urol 3(3):234
Funding
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (Grant Numbers 81570613 and 81370853), Medical research project of Jiangsu Commission of Health (Grant Numbers Z2021074) and Xinghuo Talent Program of Nanjing First Hospital.
Author information
Authors and Affiliations
Contributions
ZYF, SLM and CW were responsible for the conception and design, collection and/or assembly of data, manuscript writing, and final approval of the manuscript. XLW was responsible for the data analysis, manuscript writing and final approval of the manuscript. XZ performed the experiments and data collection. JRP was responsible for the conception and design, manuscript correction, financial and administrative support, and final approval of the manuscript. ZY, SL and CW are co-first authors.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Ethical approval
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Consent for publication
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yifan, Z., Luming, S., Wei, C. et al. Cystine crystal-induced reactive oxygen species associated with NLRP3 inflammasome activation: implications for the pathogenesis of cystine calculi. Int Urol Nephrol 54, 3097–3106 (2022). https://doi.org/10.1007/s11255-022-03347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03347-6