Abstract
Background
Deceased donor kidneys with acute kidney injury (AKI) are often discarded because of concerns about inferior transplant outcomes. A means of grading the quality of such kidneys is the performance of procurement biopsies.
Methods
This is a retrospective study of 221 brain death donors with marginal kidneys transplanted in 223 recipients in Germany. Marginal kidneys were defined as kidneys with procurement biopsies done exceptionally to assess suitability for transplantation in otherwise potentially discarded organs. The impact of deceased donor AKI on patient survival and death-censored graft survival at 1, 3 and 5 years and graft function at 1 and 3 years after transplantation was investigated.
Results
Recipients of kidneys with stage 3 AKI had a greater incidence of delayed graft function [DGF; ORStage 1: 1.435 (95% CI 0.438–0.702), ORStage 2: 2.463 (95% CI 0.656–9.245), ORStage 3: 4.784 (95% CI 1.421–16.101)] but a similar graft and patient survival compared to recipients of donors without AKI and with AKI stage 1 and 2 as well. The coexistence of recipient DGF and donor AKI was associated with the lowest graft survival and function rates.
Conclusion
The transplantation of deceased donor marginal kidneys with AKI confers a higher risk for DGF but is associated with acceptable graft and patient outcomes, which do not differ in comparison with marginal donor kidneys without AKI. Graft prognosis is especially poor if donor AKI and recipient DGF concur. Donor AKI was a risk factor independent of the histological lesions of procurement biopsies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplantation (KT) continues to remain the best available renal replacement therapy for most patients with end-stage renal disease. The shortage of organs and the continuously increasing number of patients on the waiting list led to the increased usage of organs from marginal donors [1]. The challenge with marginal kidneys is that delayed graft function (DGF) occurs frequently and may be associated with inferior graft and patient outcomes [2, 3].
Acute kidney injury (AKI) is very common in kidney donors and is strongly correlated with DGF [4,5,6,7,8]. Previous studies reported that the prognosis of KT from donors with AKI does not significantly differ from that of KT from donors without AKI. In contrast, other studies indicated that AKI does have an impact on long-term allograft outcome [7, 9]. Thus, the impact of donor AKI on allograft outcome is unknown and donor kidneys are discarded at a higher rate [6, 10]. Unfortunately, most studies focused on the analysis of KT performed with standard criteria donors. Therefore, it is unclear whether kidneys with AKI recovered from expanded criteria donors (ECDs) or from donors with a high kidney donor profile index (KDPI) or from donors with marginal organ quality exhibit similar results. Another important point is whether procurement biopsies are helpful in such cases [10].
Given these open questions, we aimed to investigate AKI in kidneys from marginal donors with post-explantation biopsies. We evaluated the impact of clinical donor characteristics and histological findings of their biopsies on short-term patient and graft survival and short-term graft function.
Materials and methods
Study population
We extracted data from the Deutsche Stiftung Organtransplantation (DSO) Region Nord and from the German transplant centers of kidneys allocated between January 2003 and March 2012. We included adult recipients of deceased-donor kidney-only transplants of marginal organ quality in Germany. Recipients were excluded if they were < 18 years old at the time of transplantation, if they received multiple types of organs, or if their donors were from outside of Germany. According to German regulations, only brain-dead donors were included in the study. For the same reason, normothermic ex vivo kidney perfusion systems for organ preservation were also not used.
Donor variables, procurement biopsy results, recipient variables and transplant factors included in the analysis are listed in Tables 1, 2 and 3.
Definitions
Marginal kidneys were defined according to the current clinical practice in Germany, i.e., kidneys with procurement biopsies done exceptionally to assess suitability for transplantation in otherwise potentially discarded organs. Such kidneys had, for example, proteinuria or presumed chronic kidney disease, were of poor macroscopic or perfusion quality, had heavy aortic patch and/or renal artery atherosclerosis, had multiple accessory renal arteries or were recovered from donors with long ICU stay, diabetes and multiorgan failure. Macroscopic grading of the external aspect of the donor kidney was provided by the explanting surgical team as good, medium, or poor; likewise, atherosclerosis was characterized as no, mild or severe and perfusion quality as good, medium or poor. Extended criteria donors (ECD) were classified as brain death donors 60 years of age or donors 50 to 59 years of age with at least 2 of the following features: history of hypertension, terminal serum creatinine > 1.5 mg/dl or cerebrovascular cause of death [11].
The original Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR [12]. AKI was defined as per the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [13]. DGF was defined as the need for dialysis within the first 7 days of transplantation similar to many papers focusing on this topic [14]. Duration of brain death was defined as the interval between brain death and the beginning of cold ischemia time and was calculated by subtracting the time of ICU until cross-clamp, where cold perfusion started, and the time of ICU until declaration of brain death.
Overall graft loss was defined as time from transplantation to return to dialysis, or death with a functioning graft. Death-censored graft failure was the same apart from censoring those who died with a functioning graft. Patient death was defined as the time from date of transplantation to patient death, not censored at graft failure. All survival times were censored at the end of follow-up or loss to follow-up.
Outcome measures
Four different outcomes were analyzed: (1) primary non-function (PNF), (2) DGF, (3) recipient eGFR/creatinine at 3, 12 and 36 months, and (4) graft loss, death-censored graft failure and patient survival at 1 and 3 years.
Histological assessment of procurement biopsy
All biopsies were processed in paraffin according to the routine protocol at the Institute of Pathology, Hannover Medical School, which involves multiple level sections stained with hematoxylin and eosin, periodic-acid Schiff, Jones silver, trichrome elastica. Histopathological parameters were retrospectively determined by an experienced nephropathologist and included type of biopsy, total number of glomeruli and ratio of globally sclerosed glomeruli, focal and segmental glomerulosclerosis (FSGS), number of arteries (media ≥ 2 smooth muscle cell layers), presence of FSGS, Banff Lesion Scores i, t, v, g, ptc, ci, ct, cv, cg, ah according to Banff 2011 [15,16,17,18], arteriolar fibrosis scored as absent, mild, moderate, severe, cortical tubular hypertrophy, epithelial cell flattening, brush border loss, vacuolization, luminal detritus as 0 (absent), 1 (< 25%), 2 (< 50%), 3 (≥ 50%), tubular nuclear loss 0 (absent), 1 (1 quadrant), 2 (2 quadrants), 3 (3 quadrants of the most affected tubular cross section), pyelonephritis, thrombotic microangiopathy as glomerular microthrombi.
Statistical analysis
The baseline characteristics of the study cohort were expressed as mean (SD). We created logistic regression models for the outcome of DGF, adjusting for covariates. The linearity assumption was assessed through categorization of continuous variables. We checked for interaction terms using forward elimination. Nonsignificant variables were removed from the model using backward elimination with a cutoff of P < 0.05. Variables were also considered confounders if they changed the coefficient of the explanatory variable by > 10%. The different exposure variables were inserted into the model. We compared the models using F test, adjusted R2 and the Hosmer–Lemeshow goodness of fit and the C statistic.
Three multilinear regression models for the outcomes of recipient eGFR at 3, 12 and 36 months were created, adjusting for covariates. Collinearity of different variables was assessed using the variance inflation factor. The linearity assumption was assessed using scatter plots of residual values for each continuous variable. Effect modification was assessed for using the forward elimination method. Nonsignificant variables were removed from the model using backward elimination. The different exposure variables were then assessed in the different models. The Wald test was used to assess the significance of exposure variable plus any interaction terms. We then compared the variables of interest for the different models using the F test and adjusted R2.
Three separate multivariable Cox proportional hazards models were created to assess the outcomes of death-censored graft failure, and patient death. Nonlinear continuous variables were made categorical. The nonsignificant variables were removed from the model using backward elimination. Wald statistics were used to assess the significance of exposure variables. The models were assessed using the Harrell C statistic and Akaike Information Criterion (AIC).
The variables for which the various models were adjusted for in the multivariate analyses are summarized in Supplementary File 1.
A P value below of 0.05 was considered significant in all two-sided tests. Statistical analysis was performed with SPSS software, v24 (IBM Corp, Armonk, NY) and IBM SPSS Statistics Essentials for R.
Ethical approval
The study protocol was conducted in accordance with the Declarations of Helsinki and Istanbul on organ trafficking and transplant tourism and approved by the Ethics Committee of Hannover Medical School (No. 1519-2012).
Results
Donors’ and recipients’ characteristics
From 442 kidneys of marginal quality considered for transplantation and with procurement biopsies, 149 were not transplanted. For the remaining 293 transplanted kidneys, follow-up data were available for 223 organs (Fig. 1).
Donors’ characteristics are summarized in Table 1. The mean age of non-AKI and AKI donors was 64.5 ± 15.6 and 56.9 ± 12.9 years (P = 0.001). Furthermore, the KDRI was 1.553 ± 0.506 and 1.426 ± 0.516 in the non-AKI and AKI donor groups. The mean donor BMI and the prevalence of diabetes were higher, whereas less numbers of ECDs were observed in the AKI group. No differences in donors’ gender, hypertension, smoking rate, Hepatitis B and C and CMV serology, causes of brain death and distribution of KDPI were observed between the non-AKI and AKI donor groups. Traumatic brain injury was more common in donors without AKI, but duration of brain death was comparable between groups. Donors with AKI remained for a longer time in the ICU and received less often volume expanders and steroids.
In the AKI donor group serum creatinine at admission, peak serum creatinine and creatinine and recovery were higher. Furthermore, AKI donors had more proteinuria and reduced diuresis in the last 24 h before cross-clamp.
Regarding perfusion and organ quality, there were no differences between kidneys with and without AKI (Table 2). However, post-explantation biopsies revealed more severe chronic glomerular and tubulointerstitial damage in the non-AKI group.
Table 3 presents recipients’ baseline characteristics. Of 223 recipients, 89 received kidneys from 50 AKI donors, whereas the remaining 134 received kidneys from 83 donors without AKI. A total of 153 patients (68.6%) received kidneys from ECDs. Non-immunological and immunological risk factors for renal allograft failure, such as age, history of hypertension or cardiovascular disease, prior transplantation, human leukocyte antigen (HLA) mismatches, plasma reactive antibody (PRA) titers and cold and warm ischemia time and immunosuppressive therapy did not differ between groups. Hepatitis C was more prevalent in recipients of donor kidneys with AKI.
Analysis of clinical outcomes
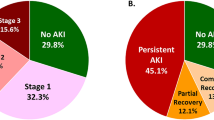
Clinical outcomes are presented in Tables 4 and 5 and illustrated in Fig. 2. There were no differences in PNF of the graft between recipients of donors with and without AKI. Although DGF was more common in recipients of donor kidneys with AKI (68.8% versus 46.2%; P = 0.002), patient and death-censored graft survival at 1, 3 and 5 years graft function at 3 months, 1 and 3 years, proteinuria and number of rejections were similar in both groups.
A Kaplan–Meier curve illustrating graft survival in AKI versus No-AKI kidney transplant recipients. B Kaplan–Meier curve illustrating graft survival according to AKI stages. C Kaplan–Meier curve illustrating graft survival in recipients with and without AKI. D Kaplan–Meier curve illustrating graft survival in recipients with and without DGF receiving kidneys from donors with and without AKI
Increasing stage of donor AKI was associated with a higher rate of DGF (stage 1 in 46.2%, stage 2 in 65.0%, stage 3 in 79.3%, Tables 6, 7), but only stage 3 remained significant after multivariable logistic regression [stage 1 odds ratio (OR) 1.435 95% CI 0.438–4.702, stage 2 OR 2.463 95% CI 0.656–9.245, stage 3 OR 4.784 95% CI 1.421–16.101, Table 8, the models had moderate discrimination and were similar across all models (C statistics: AKI = 0.772 (CI 0.703–0.842), AKIN classification = 0.784 (CI 0.714–0.853))].
Cox proportional hazard models adjusted for multiple clinical and histological variables (Supplementary File 1) showed that there was no significant association between AKI stage and patient survival, death-censored and non-death-censored graft failure (Table 8). Similar results were also observed for 5 years after transplantation (data not shown).
Interestingly, there was weak evidence of an association between 3- and 12-month recipient eGFR and increasing stage of donor AKI (Table 8). The models performed similarly, but the adjusted R2 values were worse (R < 0.30 for all models).
Results on graft survival are illustrated in Fig. 2. Between non-AKI and AKI donors, the outcome of recipients with and without DGF was calculated (Supplementary Table 1). Patient survival was unaffected in all four groups. Overall, death-censored graft survival was lower in the DGF and AKI groups, but there was a significant interaction between both. Transplant patients experiencing DGF and receiving kidneys from donors with AKI exhibited the lowest graft survival (Fig. 2D).
Discussion
The deceased donor pool is limited and living kidney donation does not suffice to close the gap in organ shortage. For donors with AKI, several aspects have to be taken into account, such as surgical issues, hemodynamic compromise, immunological issues and ischemia reperfusion injury [19]. Unfortunately, donors with AKI are either not considered for donation or kidneys from such donors are often discarded during multi-organ harvest [20]. The aim of this analysis was to assess the impact of donor AKI, as classified by the AKIN criteria, on outcomes in KT of marginal organs. Additionally, a total of 223 post-explantation biopsies of 141 donors with marginal kidneys were analyzed to find possible decisive factors that are relevant for the outcome of the transplantation.
The main findings of our study were:
-
(1)
Short-term patient and allograft survival and graft function appear acceptable after transplantation of marginal kidneys with and without AKI to expand the donor pool.
-
(2)
The incidence of DGF was significantly higher only in recipients of kidneys from marginal donor kidneys with AKIN stage 3, but not in recipients of donor kidneys with AKIN stage 1 and 2.
-
(3)
Donor AKI and recipient DGF in combination were considerably associated with graft loss.
-
(4)
Histopathological assessment of donor kidneys was not helpful in predicting outcomes;
-
(5)
The rate of cumulative rejections or the level of proteinuria at follow-up was not higher in recipients of marginal donor kidneys with AKI.
Similar to others, we confirmed classical risk factors for AKI, such as diabetes mellitus, BMI and preexisting chronic kidney disease [21,22,23]. Moreover, the longer duration of ICU stay, and the less common use of steroids and volume expanders in the donor AKI group emphasize the importance of the systemic inflammatory response and intravascular volume depletion respectively as modifiable risk factors for AKI in the ICU setting [24,25,26]. Donor age was surprisingly lower in the donor AKI group. We suppose that the presumed better organ quality associated with lower age was reassuring but the relative benefit of age was here outweighed by the risk associated with the other above-mentioned factors.
The discrepancy between macroscopic and microscopic findings in the no-AKI group (good morphology, bad histology) implicates that macroscopic assessment of a recovered organ from transplant team is a subjective and probably not accurate parameter of organ quality. Furthermore, it underpins the pitfalls of procurement biopsies in marginal kidneys and corroborates the allocation policy of the European senior program (ESP) of Euro Transplant (ET), where biopsy is not a prerequisite and indeed is not performed in the great majority of the recovered organs. Regarding histopathology, we suppose that the opposite as expected patterns in donor kidneys with AKI are due to selection bias, since only post-explantation biopsies of marginal kidneys were assessed. In that cases, histological findings are unfavorable in general and differences between marginal donor kidneys with and without AKI would not be anticipated. This was also probably the reason why, except for DGF, histological findings failed to predict clinical outcomes, in the multivariate analysis. Perhaps, the statistical analysis did not confirm the significance of these lesions, although their clinical relevance was evident, i.e., that AKI kidneys could be transplanted with satisfactory outcomes. Hence, our results do not support the routinely performance of procurement biopsies in deceased donor kidneys with AKI.
The occurrence of DGF in presence of donor AKI is plausible but the extremely poor outcomes after concurrence of both unfavorable conditions are probably due to the superimposed damage in the transplanted organ before recovery from AKI. The development and severity of AKI are known risk factors for the transition to chronic- or end-stage renal disease [27]. What’s more in the case of transplantation, is the experimental evidence linking acute epithelial tubular cell injury with an augmentation in the immunogenicity of the allograft, although this has not been confirmed in clinical studies [28]. Similar to other studies, our results reveal no disadvantage of transplanting donor kidneys with AKI stage 1 or 2, and call for caution when using AKI stage 3 donor kidneys [9, 29].
The clinical implication of our findings is that patients at high risk of developing DGF should be cautiously selected if kidneys with AKI are offered and in the case of transplantation, careful observation is required during the first three months of follow-up. Such a time frame is of relevance for the allocation policy of ET since patients can be relisted without losing previous accrued waiting-list points in case of graft failure. Insisting on rescuing a graft deemed to get lost could finally result in much longer waiting times for a second transplant. Giving-up the graft and re-initiating dialysis timely is therefore crucial for those patients.
Strengths of the study are the analysis of detailed donor items, concerning the treatment during the ICU stay and the explantation procedure, with data on kidney function from admission to the ICU until recovery, elaborate hemodynamic parameters, concomitant medications and histopathological scoring of the procurement biopsies in a center with experienced nephropathology.
Limitations of our study were the small sample size, the retrospective design, the amount of missing data and the bias toward marginal organs. Lastly, quality of life was not investigated. This is a fundamental item considering the significantly inferior outcomes in graft survival.
In conclusion, our results suggest that transplantation of marginal kidneys with AKI puts recipients in disadvantage only regarding transplanted organ survival and function but not patient survival and may be recommended, always considering an unfavorable risk-to-benefit ratio. Since functioning grafts in the long run outperform dialysis, an individualized approach in the context of a personalized medicine strategy is essential. The non-acceptance of an organ leads in the end to an increase in patients’ mortality due to their longer time on the waiting list [30].
References
Tso PL, Dar WA, Henry ML (2012) With respect to elderly patients: finding kidneys in the context of new allocation concepts. Am J Transplant 12(5):1091–1098. https://doi.org/10.1111/j.1600-6143.2011.03956.x
Sharif A, Borrows R (2013) Delayed graft function after kidney transplantation: the clinical perspective. Am J Kidney Dis 62(1):150–158. https://doi.org/10.1053/j.ajkd.2012.11.050
Melih KV, Boynuegri B, Mustafa C, Nilgun A (2019) Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation. Transplant Proc 51(4):1096–1100. https://doi.org/10.1016/j.transproceed.2019.02.013
Davenport A (2008) The brain and the kidney—organ cross talk and interactions. Blood Purif 26(6):526–536. https://doi.org/10.1159/000167800
Reese PP, Hall IE, Weng FL, Schroppel B, Doshi MD, Hasz RD, Thiessen-Philbrook H, Ficek J, Rao V, Murray P, Lin H, Parikh CR (2016) Associations between deceased-donor urine injury biomarkers and kidney transplant outcomes. J Am Soc Nephrol 27(5):1534–1543. https://doi.org/10.1681/ASN.2015040345
Hall IE, Schroppel B, Doshi MD, Ficek J, Weng FL, Hasz RD, Thiessen-Philbrook H, Reese PP, Parikh CR (2015) Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant 15(6):1623–1631. https://doi.org/10.1111/ajt.13144
Koyawala N, Parikh CR (2020) A review of donor acute kidney injury and posttransplant outcomes. Transplantation 104(8):1553–1559. https://doi.org/10.1097/TP.0000000000003144
Chan GC, Chow KM (2020) Should we use kidneys from donors with acute kidney injury for renal transplantation? Nephrology (Carlton) 25(2):105–115. https://doi.org/10.1111/nep.13679
Boffa C, van de Leemkolk F, Curnow E, Homan van der Heide J, Gilbert J, Sharples E, Ploeg RJ (2017) Transplantation of kidneys from donors with acute kidney injury: friend or foe? Am J Transplant 17(2):411–419. https://doi.org/10.1111/ajt.13966
Lentine KL, Kasiske B, Axelrod DA (2021) Procurement biopsies in kidney transplantation: more information may not lead to better decisions. J Am Soc Nephrol 32(8):1835–1837. https://doi.org/10.1681/ASN.2021030403
Rao PS, Ojo A (2009) The alphabet soup of kidney transplantation: SCD, DCD, ECD—fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol 4(11):1827–1831. https://doi.org/10.2215/CJN.02270409
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD (2013) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61(5):649–672. https://doi.org/10.1053/j.ajkd.2013.02.349
Scurt FG, Ewert L, Mertens PR, Haller H, Schmidt BMW, Chatzikyrkou C (2019) Clinical outcomes after ABO-incompatible renal transplantation: a systematic review and meta-analysis. Lancet 393(10185):2059–2072. https://doi.org/10.1016/S0140-6736(18)32091-9
Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D, Banff Meeting Report Writing Committee (2012) Banff 2011 Meeting Report: new concepts in antibody-mediated rejection. Am J Transplant 12(3):563–570. https://doi.org/10.1111/j.1600-6143.2011.03926.x
Pisarski P, Schleicher C, Hauser I, Becker JU (2016) German recommendations for pretransplantation donor kidney biopsies. Langenbecks Arch Surg 401(2):133–140. https://doi.org/10.1007/s00423-016-1384-5
Liapis H, Gaut JP, Klein C, Bagnasco S, Kraus E, Farris AB III, Honsova E, Perkowska-Ptasinska A, David D, Goldberg J, Smith M, Mengel M, Haas M, Seshan S, Pegas KL, Horwedel T, Paliwa Y, Gao X, Landsittel D, Randhawa P, Banff Working Group (2017) Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant 17(1):140–150. https://doi.org/10.1111/ajt.13929
Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M (2008) Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 8(4):753–760. https://doi.org/10.1111/j.1600-6143.2008.02159.x
Ali T, Dimassi W, Elgamal H, Alabassi A, Aleid H, Altalhi M, Shoukri M, Almeshari K (2015) Outcomes of kidneys utilized from deceased donors with severe acute kidney injury. QJM 108(10):803–811. https://doi.org/10.1093/qjmed/hcv033
Kosieradzki M, Rowinski W (2008) Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc 40(10):3279–3288. https://doi.org/10.1016/j.transproceed.2008.10.004
Carande EJ, Brown K, Jackson D, Maskell N, Kouzaris L, Greene G, Mikhail A, Obaid DR (2021) Acute kidney injury following percutaneous coronary intervention for acute coronary syndrome: incidence, aetiology, risk factors and outcomes. Angiology. https://doi.org/10.1177/00033197211040375
MacLaughlin HL, Pike M, Selby NM, Siew E, Chinchilli VM, Guide A, Stewart TG, Himmelfarb J, Go AS, Parikh CR, Ghahramani N, Kaufman J, Ikizler TA, Robinson-Cohen C, A-AS Investigators (2021) Body mass index and chronic kidney disease outcomes after acute kidney injury: a prospective matched cohort study. BMC Nephrol 22(1):200. https://doi.org/10.1186/s12882-021-02400-3
Hamroun A, Frimat L, Laville M, Metzger M, Combe C, Fouque D, Jacquelinet C, Ayav C, Liabeuf S, Lange C, Herpe YE, Zee J, Glowacki F, Massy ZA, Robinson B, Stengel B, Chronic Kidney Disease-Renal Epidemiology Information Network Study Group (2021) New insights into acute-on-chronic kidney disease in nephrology patients: the CKD-REIN Study. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfab249
Trongtrakul K, Sawawiboon C, Wang AY, Chitsomkasem A, Limphunudom P, Kurathong S, Prommool S, Trakarnvanich T, Srisawat N (2019) Acute kidney injury in critically ill surgical patients: epidemiology, risk factors and outcomes. Nephrology (Carlton) 24(1):39–46. https://doi.org/10.1111/nep.13192
Jiang YJ, Xi XM, Jia HM, Zheng X, Wang MP, Li W, Li WX (2021) Risk factors, clinical features and outcome of new-onset acute kidney injury among critically ill patients: a database analysis based on prospective cohort study. BMC Nephrol 22(1):289. https://doi.org/10.1186/s12882-021-02503-x
Cartin-Ceba R, Kashiouris M, Plataki M, Kor DJ, Gajic O, Casey ET (2012) Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract 2012:691013. https://doi.org/10.1155/2012/691013
Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK (2015) Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26(8):1765–1776. https://doi.org/10.1681/ASN.2015010006
Egelkamp J, Chichelnitskiy E, Kuhne JF, Wandrer F, Daemen K, Keil J, Brasen JH, Schmitz J, Bellmas-Sanz R, Iordanidis S, Katsirntaki K, Hake K, Akhdar A, Neudorfl C, Haller H, Blume C, Falk CS (2019) Back signaling of HLA class I molecules and T/NK cell receptor ligands in epithelial cells reflects the rejection-specific microenvironment in renal allograft biopsies. Am J Transplant 19(10):2692–2704. https://doi.org/10.1111/ajt.15417
Hall IE, Akalin E, Bromberg JS, Doshi MD, Greene T, Harhay MN, Jia Y, Mansour SG, Mohan S, Muthukumar T, Reese PP, Schroppel B, Singh P, Thiessen-Philbrook HR, Weng FL, Parikh CR (2019) Deceased-donor acute kidney injury is not associated with kidney allograft failure. Kidney Int 95(1):199–209. https://doi.org/10.1016/j.kint.2018.08.047
Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU (2009) Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol 4(7):1239–1245. https://doi.org/10.2215/CJN.01280209
Acknowledgements
We would thank the following German transplant centers for providing us with recipient data for the present analysis: Transplantationszentrum Uniklinik RWTH Aachen; Transplantation Berlin-Charité-Universität Berlin; Transplantationszentrum Bochum; Transplantationszentrum-Universitätsklinikum Bonn; Dr. Frans Zantvoort, Klinikum Bremen Mitte; Transplantationszentrum Uniklinik Düsseldorf; Transplantationszentrum-Uniklinikum Essen; Professor Dr. med. Martina Koch, Universitätsklinikum Hamburg-Eppendorf; Transplantationschirurgie-Uniklinik Innsbruck; Transplantationszentrum-Uniklinik Köln; PD Dr. med. Antje Habicht, Transplantationszentrum München der LMU-LMU Klinikum; Prof. Dr. med. Bernhard Banas, Universitäres Transplantationszentrum Regensburg; PD Dr. med. Martin Nitschke, Universitätsklinikum Schleswig-Holstein; Dr. Peter Weithofer, Transplantationszentrum Süd-Niedersachsen.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scurt, F.G., Ernst, A., Wassermann, T. et al. Short-term outcomes after transplantation of deceased donor kidneys with acute kidney injury: a retrospective analysis of a multicenter cohort of marginal donor kidneys with post-explantation biopsies. Int Urol Nephrol 55, 115–127 (2023). https://doi.org/10.1007/s11255-022-03277-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03277-3