Abstract
Background
At present, both 16G and 18G needles are used for percutaneous renal biopsy in China. This study aimed to compare the efficacy and safety of biopsy performed with the 18G needle vs. the 16G needle.
Methods
The data of patients who underwent percutaneous renal biopsy at our hospital between January 2015 and December 2019 were retrospectively analyzed. The number of glomeruli obtained by puncture and postoperative complications were compared between patients undergoing biopsy with the 16G and 18G needles. Continuous variables were compared by the t test or the Mann–Whitney U test, and categorical variables by the chi-square test. Correlation analysis was used to examine the relationship of different variables with hematoma size.
Results
Of the total 3138 kidney biopsies, 2526 were performed with the18G needle and 612 with the 16G needle. The number of glomeruli obtained was not significantly different between the two groups (P = 0.078). Large hematomas were significantly more common the 16G group than in the 18G group (9.31% vs. 5.98%, P = 0.003). Arteriovenous fistula was also more common in the 16G group (1.14% vs. 0.23%, P = 0.005). Other complications were rare, with similar incidence in the two groups.
Conclusion
The 18G needle is as effective as the 16G needle for percutaneous renal biopsy. The risk of large hematoma and arteriovenous fistula appear to be lower with the 18G needle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous renal biopsy (PRB) has been used since the early 1950s and has now become the gold standard for diagnosis of kidney pathology [1]. The gauge of the needle used for PRB varies from 14 to 22G [2,3,4,5]. In the US, the 16G needle is recommended as it is believed that this gauge is ideal for obtaining sufficient tissue, with minimal bleeding complications [6]; no similar recommendations have been made in China.
The safety and success of PRB have improved markedly with the introduction of semiautomatic biopsy needles and real-time ultrasound guidance [7,8,9]. Most centers now use semiautomatic biopsy needles, the popular choices being the 16G needles (diameter 1.65 mm, length 22 mm or 15 mm) and the 18G needles (diameter 1.27 mm, length 22 mm or 15 mm). The smaller 18G needle is usually preferred for patients with relative contraindications such as renal insufficiency, coagulation dysfunction, and small kidney size (length ≤ 9 cm) [10]. However, there is still some controversy about whether the 18G biopsy needle can obtain sufficient tissue for disease diagnosis or significantly reduce the incidence of complications.
The purpose of this retrospective study was to compare the efficacy and safety of PRB performed using the 18G needle versus the 16G needle.
Materials and methods
Patients
The data of 3138 patients who underwent PRB in our hospital between January 2015 and December 2019 were retrospectively analyzed. At our center, during 2015 and most of 2016, both 16G and 18G needles (CR Bard Inc., Tempe, AZ, USA) were used for PRB. The 18G needle was preferred for patients with relative contraindication such as renal insufficiency, coagulation disorder, and small kidney size, and the 16G needle for the other patients. However, after October 2016, only 18G needles were purchased, and so all biopsies were performed with 18G needle. Since 2017, only one type of renal puncture needle, that is, the 18G puncture needle, has been available at our center. This data analysis was conducted to analyze whether the use of 18G puncture needles causes a decrease in the number of specimens and whether there is an improvement in the risk of bleeding.
Thus, the patients could be divided into two groups: the 16G group and the 18G group. Safety and efficacy were compared between the two groups.
This study was a retrospective study that was approved by the hospital ethics committee (approval number 2020KY041). All patients provided informed consent for the renal biopsy. Informed consent from a parent and/or legal guardian was obtained for minors.
Renal biopsy procedure
Prior to the biopsy all patients underwent complete kidney ultrasound examination, blood routine examination, liver and kidney function tests, and blood coagulation function tests. Anticoagulants (including aspirin, warfarin, heparin, and direct factor Xa inhibitors) were stopped 3–7 days before the procedure.
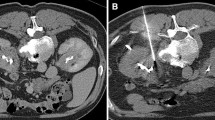
The biopsy was performed by a nephrologist, with an ultrasonologist in attendance to assist in real-time ultrasound guidance [11]. The patient was asked to empty the bladder and lie in the prone position. The lower pole of the right kidney was selected as the biopsy target. The puncture site was disinfected and infiltrated with a local anesthetic. The guide needle was inserted and advanced till the tip reached the kidney capsule to trigger the biopsy needle and initiate the kidney biopsy (Fig. 1). Since sufficient tissue was necessary for light and electron microscopy, as well as immunofluorescent examination, two biopsy specimens were generally obtained; if the tissue was insufficient, up to five specimens were obtained. The biopsy tissue was divided into three parts and sent for examination.
The patient was allowed to assume the supine position 6–8 h after the procedure, and then rested in bed for 24 h. During this period, urine color and abdominal pain were recorded, and ultrasound examination was performed to detect formation of hematoma (Fig. 2). If gross hematuria appeared, immediate ultrasound examination was carried out; otherwise, ultrasound was performed 48–72 h after the procedure to assess the size of the renal subcapsular hematoma. The length, width, and height of the renal hematoma were measured, and the hematoma volume (V) was calculated using the formula [12].
If the hematoma volume was > 40 mL, regular (once every two days) ultrasound monitoring was carried out until the hematoma had completely disappeared or the hematoma volume was < 5 mL. If an arteriovenous fistula was found, ultrasound examination was repeated regularly (once every two weeks) until the arteriovenous fistula had disappeared.
Observation indices
Safety
Safety was assessed using the indices recommended by Whittier et al. [13, 14]: i.e., incidence of lumbar or abdominal pain, gross hematuria, arteriovenous fistula formation, bleeding requiring surgical intervention or blood transfusion, decreased blood pressure, decreased hemoglobin level, severe infection, and perirenal hematoma size.
Efficacy
Efficacy of biopsy was assessed by counting the number of glomeruli obtained in the samples, with ≥ 10 glomeruli considered as an adequate sample. The adequate sample rate was compared between the two groups.
Statistical analysis
The data were analyzed using SPSS 22 (IBM Corp., Armonk, NY, USA). Quantitative data were summarized as means (± standard deviation) or as medians (and interquartile range), depending on the normality of the distribution, and compared between groups using the independent samples t test or the Mann–Whitney U test, respectively. Qualitative data were summarized as percentages and compared between groups using the chi-square test. Linear correlation was used to analyze the correlation between the indexes, and the statistic was R (Spearman rank correlation was used if the condition was not met, and the statistic was RS). P < 0.05 was regarded as statistically significant.
Results
The 3138 patients included in this study ranged in age from 10 to 79 years. The 18G group comprised 2526 patients with mean age of 41.98 ± 14.17 years, while the 16G group comprised 612 patients with mean age of 40.44 ± 14.29 years. Age was significantly higher in the 18G group (P = 0.015). Before biopsy, there were no significant differences in serum creatinine level, hemoglobin level, platelet count, and 24-h urine protein level between the two groups. The mean glomerular filtration rate (GFR) was significantly lower in the 18G group than in the 16G group: 83.44 ± 34.86 mL/min vs. 87.00 ± 37.25 mL/min, P = 0.026). The median cystatin C level was significantly higher in the 18G group than in the 16G group: 0.88 (0.70–1.22) mg/L vs. 0.82 (0.67–1.15) mg/L, P = 0.007 (Table 1).
Efficacy
The efficacy of kidney biopsy is evaluated based on whether a sufficient number of glomeruli are obtained. The mean number of glomeruli obtained by renal puncture was comparable between the groups: 23.6 ± 10.1 in the 18G group vs. 22.8 ± 9.9 in the 16G group (P = 0.078). The number of glomeruli ≤ 9 cases is considered pathological glomerulus insufficient, which had 138 cases (5.4%) in 18G group and 34 cases (5.5%) in 16G group; there is no statistical difference between the two groups (P = 0.573). A sufficient number of glomeruli is considered to be ≥ 10. The number of glomeruli was ≥ 10 in 2388 cases (94.5%) in the 18G group and 578 cases (94.4%) in the 16G group (Table 2). There was no statistical difference between the two groups (P = 0.928).
Safety
The main complication after renal puncture was perirenal hematoma. Large hematomas (i.e., maximum diameter > 5 mm) occurred in 208/3138 (6.62%) patients. They were significantly less common in the 18G group than in the 16G group: 151/2526 (5.98%) vs. 57/612 (9.31%), P = 0.003. Other complications (including arteriovenous fistula formation, abdominal pain, gross hematuria, bleeding requiring blood transfusion, hemostasis requiring intervention, and death) were rare, with incidence < 1%. Arteriovenous fistula was significantly less common in the 18G group than in the 16G group: 6/2526 (0.23%) vs. 7/612 (1.14%), P = 0.005. Serious hemorrhage, requiring renal artery embolization to stop bleeding, was necessary in 1/2526 (0.04%) patient in 18G group. The incidence of other complications was similar in the two groups (Table 3). There were no deaths in either of the two groups.
Since perirenal hematoma after renal puncture was the main complication, we compared hematoma volume between the two groups. The hematoma volume deviation was large, and the volume was calculated to analyze after log10 logarithm. As shown in Table 4, the length of the hematoma was 24.00 (18.00–33.00) mm in the 18G group vs. 26.00 (20.00–37.00) mm in the 16G group (P < 0.001). The height of the hematoma in the 18G group was 8.00 (6.00–11.00) mm, and the height of the hematoma in the 16G group was 10.00 (8.00–12.00) mm. There was a significant difference between the two groups (P < 0.001). The logarithm (log10) of the hematoma volume in the 18G group was 3.66 ± 0.54, and the logarithm (log10) of the hematoma volume in the 18G group was 3.80 ± 0.54, which had a significant difference between the two groups (P = 0.002). According to the classification of no hematoma, small hematoma (hematoma long diameter < 50 mm), large hematoma (hematoma long diameter ≥ 50 mm), there were 381 cases (15.1%) without hematoma and 151 cases (6.0%) with large hematoma in the 18G group, 68 cases (11.1%) without hematoma and 57 cases (9.3%) with large hematoma in the 16G group, with a significant difference between the two groups (P = 0.001).
We also attempted to identify the factors related to hematoma size. The scatter plot showed only a very weak correlation between age and hematoma size; the linear correlation coefficient ρ was 0.051. However, because these two indicators, especially the hematoma length, were non-normally distributed, Spearman rank correlation was used. As Table 5 shows, hematoma volume showed weak, but statistically significant, correlation with platelet count, serum creatinine, and cystatin C, all of which were weakly related.
Discussion
This retrospective study aimed to determine the efficacy and safety of the 18G needle for percutaneous renal biopsy. The ability to obtain sufficient glomeruli, with minimal bleeding, was compared between the 18G needle and the 16G needle. Although the current standards for the number of glomeruli necessary for diagnosis in autologous kidneys and transplanted kidneys are different [15, 16], it is generally believed that at least 10 glomeruli are required for light microscopic evaluation [17]. This study found that among the cases with glomerulus ≤ 9, there were 138 cases (5.4%) in the 18G group and 34 cases (5.5%) in the 16G group. There was no statistical difference between the two groups (P = 0.573). Moreover, the mean number of glomeruli obtained by renal puncture was similar in the 18G and 16G groups (23.59 ± 10.14 vs. 22.78 ± 9.93, P = 0.078). At present, the number of glomeruli is generally used to evaluate the success of renal puncture, and less than 10 glomeruli is considered as the evaluation standard. Based on this standard, the failure rates of the 18G and 16G needles were 5.5% and 5.4%, respectively, but the difference was not significant. At present, it is believed that good renal tissue samples contain more than 20 glomeruli. This criterion was met by 62.0% of the 18G needles and 59.6% of the 16G needles, respectively, but this difference was also not significant.
Previous studies have reported conflicting results regarding the efficacy of 18G needles; some studies showed that 16G needles were better than 18G needles for obtaining a sufficient number of glomeruli [10, 18], while others reported no difference between 18 and 16G needles [19, 20]. Based on the findings of the present study, we believe that there was no significant difference in efficacy between the two. However, as part of the results of the electron microscope were sent out for inspection, some of the fluorescent sections did not have any glomeruli, and then, the sections were resectioned from the wax blocks for immunohistochemical staining. Therefore, it was difficult to calculate the number of fluorescent and electron microscope spheres, so it might have an impact on the final number of the kidney. The method of cutting the light microscope and the experimental personnel are all fixed personnel, and the efficacy of renal puncture can only be evaluated from the number of small balls of the light microscope.
In this study, the main complications were perirenal hematoma and arteriovenous fistula. The risk of large hematoma and arteriovenous fistula were significantly lower with the smaller 18G needle. The overall incidence of large hematomas was 6.38%, with a significantly higher incidence in the 16G group than in the 18G group (9.31% vs. 5.98%, P = 0.003). The overall incidence of arteriovenous fistula was 0.04%, with significantly higher incidence in the 16G group than the 18G group (1.14% vs. 0.23%, P = 0.005). Large hematomas may cause abdominal pain and decrease blood hemoglobin level; some patients may require blood transfusion or even interventional hemostasis. Through our research, we believe that the thickness of the puncture needle does not affect the success rate of renal puncture, which is more likely to be related to the puncture site. Therefore, the puncture success rate of the 18G needle is not different from that of the 16G needle.
Luciano [6] describes bleeding that requires blood transfusion, gross hematuria, arteriovenous fistula formation, and perirenal hematoma as the complications that occur after renal puncture. In our study, we found that the incidence of arteriovenous fistula, abdominal pain, gross hematuria, bleeding requiring blood transfusion, surgical intervention embolization, and renal resection was low, and there was no statistically significant difference between the two groups. The main complication was the formation of large hematomas, which was significantly different between the two groups (P = 0.003): 151 patients (5.98%) in the 18G needle group and 57 patients (9.31%) in the 16G needle group developed large hematomas.
With regard to factors that affect the size of hematomas that form after needle biopsy, previous research has shown that old age, hypertension, anemia, low platelet count, and kidney dysfunction are related to the size of the hematoma [23]. In this study, hematoma volume was significantly related to platelet count, serum creatinine, and cystatin C, but it was not significantly correlated with age, gender, BMI, hemoglobin level, 24-h urine protein level, GFR, or renal size (long diameter). The 18G group was significantly older than the 16G group and had significantly poorer renal function. Based on the previous findings, this would imply that the hematoma size was significant larger in the 18G group than in the 16G group. However, this was not the case based on the present findings. Therefore, our findings imply that the 18G needle can reduce the incidence of large hematomas, irrespective of patient age or renal status.
In previous studies, the incidence of gross hematuria following kidney biopsy has ranged from 3 to 9%, with blood transfusion being required in 0.1%–3% patients [21, 22]. In our sample, the incidence rate of gross hematuria was low (0.058%), and blood transfusion was needed in only 1/3138 (0.032%) patient; there was no statistically significant difference between the two groups. The low incidence may be because we used stent-guided puncture and sampled from the lateral segment of the kidney parenchyma, avoiding the renal medulla.
This study has some limitations. First, this was a single-center retrospective study and so bias is inevitable. Second, the 18G needle was solely used in the latter part of the study; the low incidence of complications may be partly attributable to the accumulated experience of the persons performing the biopsy.
To conclude, the 18G needle appears to be as effective as the 16G for percutaneous renal biopsy, and it may be less likely to cause complication. Our findings need to be confirmed in randomized clinical trials.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PRB:
-
Percutaneous renal biopsy
- BMI:
-
Body mass index
- GFR:
-
Glomerular filtration rate
- IQR:
-
Interquartile range; SD, standard deviation
References
Alwall N. Aspiration biopsy of the kidney, including i.a. a report of a case of amyloidosis diagnosed through aspiration biopsy of the kidney in 1944 and investigated at an autopsy in 1950. Acta Med Scand. 1952;143(6):430–5.
Ocak S, Duplaquet F, Jamart J, Pirard L, Weynand B, Delos M et al (2016) Diagnostic accuracy and safety of CT-guided percutaneous transthoracic needle biopsies: 14-gauge versus 22-gauge needles. J Vasc Interv Radiol 27(5):674–681
Chunduri S, Whittier WL, Korbet SM. Adequacy and complication rates with14- vs. 16-gauge automated needles in percutaneous renal biopsy of native kidneys. Semin. Dial. 2015;28:E11–4.
Mai J, Yong J, Dixson H, Makris A, Aravindan A, Suranyi MG et al (2013) Is bigger better? A retrospective analysis of native renal biopsies with 16 Gauge versus 18 Gauge automatic needles. Nephrology 18:525–530
Clarissa A, Juarez R, Samer D, Khalili K, Avila-Casado C (2017) Effectiveness and safety of two 18-gauge needle types on native and allograft renal biopsies. Ann Diagn Pathol 28:1–6
Luciano RL, Moeckel GW (2019) Update on the native kidney biopsy: core curriculum 2019. Am J Kidney Dis 73:404–415
Birnholz JC (1985) An improved technique for ultrasound guided percutaneous renal biopsy. Kidney Int 27(1):80
Ahmed AM, Anees M, Riaz A, Mueed S (2003) Percutaneous renal biopsy by automated biopsy gun. J Coll Physicians Surg Pak 13(5):263–266
Lübbers H, Mahlke R, Haake C, Lankisch PG (1993) A new fine needle for easier, single handed, ultrasound-guided biopsies, requiring less advancing forces into solid organs. Z Gastroenterol 31(9):484
Arora K, Punia RS, D’Cruz S (2012) Comparison of diagnostic quality of kidney biopsy obtained using 16G and 18G needles in patients with diffuse renal disease. Saudi J Kidney Dis Transpl 23:88–92
Korbet SM, Volpini KC, Whittier WL (2014) Percutaneous renal biopsy of native kidneys: a single-center experience of 1,055 biopsies. Am J Nephrol 39:153–162
Mao Y, De Oliveira IS, Hedgire S, Prapruttam D, Harisinghani M (2017) Aetiology, imaging features, and evolution of spontaneous perirenal haemorrhage. Clin Radiol 72(175):e19-26
Whittier WL, Korbet SM (2004) Timing of complications in percutaneous renalbiopsy. J Am Soc Nephrol 15(1):142
Whittier WL (2012) Complications of the percutaneous kidney biopsy. Adv Chronic Kidney Dis 19(3):179
Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T et al (1999) The Banff 97 working classifification of renal allograft pathology. Kidney Int 55:713–722
Wang HJ, Kjellstrand CM, Cockfifield SM, Solez K (1998) On the inflfluence of sample size on the prognostic accuracy and reproducibility of renal transplant biopsy. Nephrol Dial Transpl 13:165–172
Dhaun N, Bellamy CO, Cattran DC, Kluth DC. Utility of renal biopsy in the clinical management of renal disease. Kidney Int 2014;85:1039–48. https://doi.org/10.1038/ki.2013.512.
Peters B, Mölne J, Hadimeri H, Hadimeri U, Stegmayr B (2017) Sixteen gauge biopsy needles are better and safer than 18 gauge in native and transplant kidney biopsies. Acta Radiol 58(2):240–248
Mai J, Yong J, Dixson H, Makris A, Aravindan A, Suranyi MG et al (2013) Is bigger better? A retrospective analysis of native renal biopsies with 16 Gauge versus 18 Gauge automatic needles. Nephrology (Carlton) 18:525–530. https://doi.org/10.1111/nep.12093
Weiji Xie , Jing Xu , Yi Xie,et al.Adequacy and complication rates of percutaneous renal biopsy with 18- vs. 16-gauge needles in native kidneys in Chinese individuals. BMC Nephrology (2020) 21:337–43.
Anuj M, Rajb T, Basma E, Tamer A, Ahmad E, Ehtuish FE (2011) Percutaneous ultrasound-guided renal biopsy. Saudi J Kidney Dis Transpl 22:746–750
Corapi KM, Chen JL, Balk EM, Gordon CE (2012) Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis 60:62–73
Da-min Xu, Chen M, Zhou F-d et al (2017) Risk factors for severe bleeding complications in percutaneous renal biopsy. Am J Kidney Sci 3:230–235
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Basic Public Welfare Research Program of Zhejiang Province (Grant No. GF18H180031) and Zhejiang Traditional Chinese Medical Scientific Technology (Grant No. 2020ZA080).
Author information
Authors and Affiliations
Contributions
JZY and SYX collected the data, interpreted the statistical results, and wrote the first draft. LLM and JZL contributed to the design of the protocol and revised the manuscript. WXY and ZXZ performed percutaneous renal biopsy with ultrasonography together. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University (approval number 2020KY041). All the patients (or their guardians in the case of minors) provided their written informed consent before data collection.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, S., Ma, L., Lin, J. et al. Efficacy and safety of percutaneous renal biopsy performed using 18G needle versus 16G needle: a single-center retrospective study. Int Urol Nephrol 54, 3255–3261 (2022). https://doi.org/10.1007/s11255-022-03276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03276-4