Abstract
Purpose
Acute kidney injury (AKI) frequently complicates hospitalization and is associated with in-hospital mortality (IHM). It has been reported a seasonal trend in different clinical conditions. The aim of this study was to evaluate the possible relationship between seasons of the year and IHM in elderly hospitalized patients with AKI.
Methods
We selected all admissions complicated by AKI between 2000 and 2015 recorded in the Italian National Hospital Database. ICD-9-CM code 584.xx identified subjects with age ≥ 65 years and age, sex, comorbidity burden, need of dialysis treatment and IHM were compared in hospitalizations recorded during the four seasons. Moreover, we plotted the AKI observed/expected ratio and percentage of mortality during the study period.
Results
We evaluated 759,720 AKI hospitalizations (mean age 80.5 ± 7.8 years, 52.2% males). Patients hospitalized with AKI during winter months had higher age, prevalence of dialysis-dependent AKI, and number of deceased patients. In whole population IHM was higher in winter and lower in summer, while the AKI observed/expected ratio demonstrated two peaks, one in summer and one in winter. Logistic regression analysis demonstrated that parameters such as age, autumn, winter, comorbidity burden were positively associated with IHM.
Conclusion
We conclude that a seasonality exists in AKI, however, relationship between seasons and AKI could vary depending on the aspects considered. Both autumn and winter months are independent risk factors for IHM in patients with AKI regardless of age, sex and comorbidity burden. On the contrary, summer time reduces the risk of death during hospitalizations with AKI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To establish effective public health interventions, proper evaluation of seasonally fluctuating factors is important. Environmental factors such as temperature, humidity, indoor activity, infections should be taken into account in elderly to plan prevention and educational strategies. Therefore, the identification of diseases seasonality could help health care professionals in planning preventive measures, developing effective policies and allowing for use resources more efficiently and effectively [1].
In Hippocratic writings, it is stated that diseases could be correlated with seasons or weather conditions, and mortality shows seasonal fluctuations with winter peak, suggesting that increased mortality in cooler months has been known since 400 B.C. [2].
Several epidemiological studies have reported a seasonal trend in different clinical conditions in the surgical [3], traumatological [4] and medical [5] fields, in particular cardiovascular [6,7,8,9,10,11,12], respiratory [13] and infectious illness [14] have got a winter peak in their frequency. Also, acute kidney injury (AKI) has been described to have an incidence peak during winter [15, 16].
AKI has also been reported as a frequent condition among the elderly population and recognizes several risk factors such as advanced age, sepsis, surgery and comorbidities, including systemic arterial hypertension, diabetes mellitus, heart disease, neoplasms and chronic kidney disease [17]. Furthermore, AKI is a frequent finding in hospitalized individuals complicating the clinical course of patients and AKI is associated with higher in-hospital mortality (IHM) and use of resources [18].
Despite all the recent technical and therapeutic advances, the overall mortality of AKI patients remains high, reaching 80% in ICU patients. We previously evaluated the association between AKI and IHM in a large nationwide cohort of elderly subjects in Italy and found that the increasing burden of comorbidity, dialysis-dependent AKI, and sepsis were the major risk factors for IHM [19]. Moreover, we analyzed the relationship between time of the week and IHM in elderly Italian patients admitted for AKI. Our results showed that subjects admitted during weekend with AKI are exposed to a higher risk of IHM, especially if they need dialysis treatment and have high comorbidity burden [20].
The aim of this study was to evaluate the possible relationship between seasons of the year and IHM in elderly patients discharged with AKI diagnosis recorded in the Italian National Hospital Database.
Materials and methods
Patient selection and eligibility
The design of this study is retrospective, conducted in agreement with the Declaration of Helsinki of 1975, revised in 2013. Subjects could not be identified, personal data were deleted before analysis aiming at maintaining data anonymity and confidentiality. Ethics committee approval was not required because the study was conducted in agreement with the existent Italian disposition-by-law (G.U. n.76, 31/03/2008).
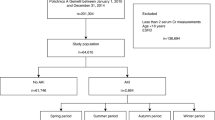
The Italian Ministry of Health (General Directorate for Health Planning) allowed to access the National Hospital Database (NHD), where all hospitalizations, both in public and private Italian hospitals, codified as discharge hospital records (DHR), are recorded. We selected all admissions complicated by AKI between December 22, 2000, and December 21, 2015. The DHR files contain information such as sex, age, date and department of admission and discharge, vital status at discharge (in-hospital death vs. discharged alive), main diagnosis, up to five comorbidities, and up to six procedures/interventions. Diagnoses and procedures are classified on the basis of the International Classification of Diseases, 9th Revision, Clinical modification (ICD-9-CM). Following the national disposition-by-law in terms of privacy, personal data and all other potential identifiers were removed from the database by the Ministry of Health. The only identifier was a consecutive number for each patient.
In administrative database codes, acute renal failure was usually the reference definition, although in clinical settings AKI replaced the term acute renal failure. Patients aged ≥ 65 years identified by the ICD-9-CM code 584.xx as a first or second discharge diagnosis were selected. The time period was divided into four 3-month intervals depending on the season, i.e., spring (21 March–20 June), summer (21 June–22 September), autumn (23 September–21 December), and winter (22 December–20 January). According to the Koppen-Geiger climate classification scheme, Italian climate could be considered as Cfb (warm temperate, fully humid, warm summer) in the north and Csa (warm temperate, summer dry, hot summer) in the south [21].
Data analysis
We evaluated IHM related to the season of hospitalization. We also evaluated the comorbidity burden, using a modified Elixhauser Index (mEI) [22, 23]. It was calculated based on the guidelines set by Quan et al. [24]. To calculate the score, the following conditions, based on administrative data, were considered: age, sex, presence of chronic kidney disease (CKD), neurological disorders, lymphoma, solid tumor with metastasis, ischemic heart disease, congestive heart disease, coagulopathy, fluid and electrolyte disorders, liver disease, weight loss, and metastatic cancer. The original score was corrected, removing the diagnosis of previous AKI; therefore, the points assigned to renal diseases were considered only if CKD was recorded. The score was calculated automatically. Finally, we also considered dialysis treatment (code ICD-9-CM 39.95).
Statistical analysis
A descriptive analysis of the whole population was performed and the data were expressed as absolute numbers, percentages, and means ± SD. Univariate analysis was carried out by using the Chi-Squared, ANOVA or Kruskal–Wallis test as appropriate, comparing age, sex, length of stay, comorbidity burden, dialysis-dependent AKI and mortality during the four seasons of the year. Moreover, to evaluate the relationship between seasonality and IHM, the latter was considered as the dependent variable in a logistic regression analysis, while demography, seasons (spring as the reference), comorbidity score, and dialysis-dependent AKI were considered as independent variables. Odds ratios (ORs) with their 95% confidence intervals (95% CI) were reported.
Moreover, we plotted the AKI observed/expected ratio and percentage of mortality during the study period. The observed numbers of AKI events per month were calculated as the monthly numbers of events over the whole study period. The expected numbers of cases per month were obtained by dividing the average total numbers of patients per year by 365.25, and by multiplying the results by the numbers of days in each month, considering 28.25 days for February. The AKI observed/expected ratio of events was, thus, calculated. All p values were two tailed, and p value < 0.05 was considered significant. Statistical product and service solution (SPSS) 23.0 for Windows (IBM Corp., Armonk, NY, USA) was used for the statistical analyses.
Results
We considered a 15-year period (2001–2015), and evaluated 759,720 AKI hospitalizations of whom 52.2% were males. Mean age of the population was 80.5 ± 7.8 years, mean length of stay was 13.7 ± 15.5 days, and dialysis-dependent AKI cases were 68,561 (9%). Mean comorbidity burden calculated by the score was 14.57 ± 6.21. AKI hospitalizations during spring were 187,128 (24.6%), during summer 197,373 (26%), during autumn 181,198 (23.9%), and winter hospitalizations 194,021 (25.5%). Deceased subjects during admissions were 210,305 (27.7%) (Table 1).
Table 2 shows the seasonal differences of the different characteristics analyzed both on the whole population and in the subgroups of patients with non-dialysis-dependent AKI and dialysis-dependent AKI. Males had higher prevalence during all seasons. Patients hospitalized with AKI during winter months had higher prevalence of dialysis-dependent AKI, higher number of deceased patients and were older. The difference in comorbidity burden, although statistically significant, could not be considered clinically impacting. The same characteristics were showed in the subgroup of patients with non-dialysis-dependent AKI, whilst in dialysis-dependent AKI population, IHM and length of stay were higher during fall. In the same way, the difference in comorbidity burden, although statistically significant, could not be considered clinically evaluable.
Logistic regression analysis (Table 3) demonstrates that age, autumn, winter, comorbidity burden are positively associated with IHM in all groups considered. Summer appears to reduce the risk of death during hospitalization, on the contrary, males have higher risk of death during hospitalization in the dialysis-dependent AKI group, whilst females have higher risk of IHM in the whole population and in the non-dialysis-dependent AKI group.
Figure 1 shows the AKI observed/expected ratio and percentage of mortality during hospitalization during the 15-year period in the whole population, considering all seasons throughout the period of the study (A) and during the four seasons including all years (b). In whole population IHM is higher in winter and lower in summer, while the AKI observed/expected ratio considering the four seasons including all years demonstrates two peaks, one in summer and one in winter. Similar patterns are calculated in non-dialysis-dependent AKI (data not shown); whilst in dialysis-dependent AKI group, a seasonal periodicity pattern is evident only for the AKI observed/expected ratio considering all seasons throughout the period of the study. Evaluation of percentage of IHM considering the four seasons including all years shows a peak in autumn (Fig. 2).
Discussion
In this study, we found that the highest number of hospitalizations with AKI is detected during summer; however, admitted subjects suffering AKI have higher winter mortality associated with higher prevalence of dialysis-dependent AKI and higher age; on the contrary, patients suffering dialysis-dependent AKI have higher mortality during Autumn. Therefore, despite a higher frequency of AKI, summer seems to reduce the risk of IHM in both non-dialysis and dialysis-dependent AKI populations, whilst autumn and winter seem to increase the risk of IHM in the two groups, as well as age and comorbidity burden. IHM has a winter peak in the whole population, whist the AKI observed/expected ratio has two peaks, in summer and winter in the whole population, but in the dialysis-dependent AKI group we see only a winter peak. In this population IHM has a peak in autumn.
Particularly interesting is the fact that male sex increases the risk of IHM in dialysis-dependent AKI population, while female sex increases the risk of IHM in non-dialysis-dependent AKI. However, it is somewhat difficult to explain this finding.
To the best of our knowledge, this is the first study considering the relationship between seasons of the year and both AKI observed/expected ration and IHM in AKI associated admissions in Italy. The relationship between seasonality and renal diseases is matter of debate and results from different studies could be interpreted as contradictory, probably depending on different populations selection, outcome, study design, and degree of renal impairment. In fact, results of different studies are different depending also on evaluation of AKI incidence or mortality.
Seasonal mortality pattern has been described in non-human primates influenced both by diet and degree of environmental seasonality [25]. Similarly to what happens in non-human primates, the relationship between seasonality and mortality in human societies should take into considerations several confounding factors, including social factors.
Indeed, in New Zealand, a temperate country, winter mortality was higher among low-income people, those living in rented accommodation and those living in cities [26]. In the same country excess of winter mortality was found to be 18% higher than expected from non-winter months [27]. Mortality from diseases of the respiratory and circulatory system was most dependent on seasonal effect. Also pathological conditions involving the blood, the endocrine system and metabolism, and diseases of the digestive system had rates of death in winter and non-winter month ratio higher than one. Similar results were also reported in less temperate areas such as Pakistan, where patients aged more than 54 years had high risk of death during the winter season as compared to the summer season, especially if they suffered from cardiovascular, respiratory and kidney diseases. On the other hand, authors underlined that the majority of people living in the analyzed region had socio-economics problem such as a very low standard of living and low quality houses residence [28]. It has also underlined that females could be more vulnerable than males to winter phenomenon [27]. In our study women had higher risk for IHM if AKI was not treated with dialysis. A review published in 2015 established that the elderly, children, and males could be considered the vulnerable populations during heat waves, demanding increased medical care especially in the presence of existing chronic diseases. Moreover, social factors, such as lower socio-economic status, could contribute to heat susceptibility. Authors suggested that relevant policies and guidelines should be developed to protect vulnerable populations, adopting morbidity indicators during heat wave early warning systems to ameliorate public health actions [29].
Climate impacts health, it has been established that global warming may influence human health [30], and increasing temperature impact health status [31]. Data from Adelaide, South Australia, established that augmentation in daily temperature of 1 °C was associated with an increased incidence for different renal disease such as urolithiasis, AKI, chronic kidney disease (CKD), urinary tract infections (UTIs), and lower urinary tract infections (LUTIs) [32]. A Korean research group reported increasing AKI admissions by 23.3% per 1 °C rise in mean temperature above the 28.8 °C flexion point in the warm season [33]. The cut-off for an increased risk of AKI has been reported to be 22.3 °C with a high correlation between ambient temperature and emergency department visit for AKI [34]. In our study, the AKI observed/expected ratio in the whole population and in the non-dialysis-dependent AKI group has a peak in summer as well as in winter time. However, this seasonal behavior appears to belong to AKI with less degree of renal impairment, in fact IHM and the AKI observed/expected ratio of hospitalization in dialysis-dependent AKI group are higher in winter. The frequency of AKI requiring only medical treatment is high especially during summer months. It was found that in patients with a median age of 80 years with high prevalence of hypertension, diabetes, cardiovascular disease and chronic liver disease, environmental heat increased the risk of AKI by 11%. Incidence of AKI during warm months was 182 cases per 100,000 person-years [35]. Similar results were reported by Bobb et al. [36] that identified causes of hospital admissions during extreme heat events evaluating 23.7 million fee-for-service beneficiaries, aged ≥ 65 years, during the period 1999–2010 with at least five summers of near-complete daily temperature data in the United States. They found that risks of hospitalization for fluid and electrolyte disorders, renal failure, UTIs, septicaemia, and heat stroke were statistically significantly higher on heat wave days relative to matched non-heat wave days [36]. Also, it has been described that heavy occupational workload in high ambient temperature is associated with acute reduction in kidney function [37]. Moreover, even in hypertensive patients, with and without chronic kidney disease, it has been demonstrated similar seasonal variations in estimated glomerular filtration rate (eGFR) with lower values during summer (June–August) compared with spring (March–May). The decrease in eGFR from spring to summer was similar for both groups, however, the percent change in eGFR was higher in hypertensive patients with renal impairment [38].
Cardiovascular disease (CVD) and renal disease are closely related [39], and a clear seasonal pattern has been reported for CVD, with the highest incidence occurring during the colder winter months [40]. CVD follows a seasonal pattern with a winter peak and clusters of all subtypes of CVD after ‘cold snaps’, and with corollary peaks linked to heat waves [41].
None of the previous studies have considered mortality, while in our study the main outcome is IHM and we take into consideration the observed/expected ratio of AKI needing hospitalization.
A retrospective study by our group, based on the database of hospital admissions of the region Emilia-Romagna of Italy (years 1998–2006), analyzed 64,191 cases of acute myocardial infarction (AMI), of whom 62.9% males, and 12.3% fatal [42]. Acute myocardial infarction was most frequent in winter and least in summer, with the highest number of cases in January and the lowest in July. Chronobiologic analysis showed winter peaks for total cases (January), females (December), and fatal cases (January) [42]. More recently, a study from the United States analyzed nearly 11 × 106 adult admissions for acute myocardial infarction using the National Inpatient Sample (2000–2017). Admissions, happened in 24.3, 22.9, 22.2, and 24.2% in spring, summer, autumn, and winter, respectively. Despite an approximately similar disease incidence, compared to spring, winter admissions had higher IHM risk, whilst summer and autumn had slightly lower IHM risk [43].
All-cause mortality and death due to CVD was higher during the cold months than during the warm ones also in patients receiving dialysis treatment [44, 45]. Dialysis treatment appears to worsen prognosis of patients admitted with AKI, and we find higher IHM in dialysis-dependent AKI group during autumn and winter, suggesting a relationship between dialysis treatment and cold months. In 2012, Usvyat et al. [44] evaluated whether mortality related with seasonal changes was present in high-risk haemodialysis patients. They evaluated more than 15,000 subjects and found that mortality, both all-cause and cardiovascular, was significantly higher during winter compared with other seasons. Seasonal variations were similar across climatologically different regions. Differences in mortality disappeared when adjusted for seasonally variable clinical parameters. Therefore, they concluded that significant seasonal variations in overall and cardiovascular mortality were consistent over different climatic regions, and that mortality differences were related to seasonality of physiologic and laboratory parameters. Higher winter mortality in dialysis patients was confirmed by the International monitoring dialysis outcomes (MONDO) consortium, but it was true only outside the tropical zones [46].
In addition, the analysis of the United States Renal Data System database 2000–2013, showed that transitioning to end-stage renal disease (ESRD) and adverse events, including all-cause, cardiovascular and infectious mortalities, were more frequent in winter and less frequent in summer [47]. In 2020, Goto et al. [48], using the Japanese database for dialysis patients, compared the fractions of all-cause and cause-specific mortality among seasons after adjustment for different variables. They concluded that all-cause mortality, and mortality from coronary heart disease, heart failure, cerebral hemorrhage, and infectious pneumonia were significantly higher in winter than in summer. Although acute aortic dissection exhibits a winter peak too [49], a further analysis on cases enrolled at various sites around the globe, belonging to the International Registry of Acute Aortic Dissection (IRAD), revealed that the winter peak was evident in both cold and temperate climate settings, suggesting that the relative change in temperature, rather than absolute temperature, and/or endogenous annual rhythms could be critical mechanistic factors [50].
Similar seasonal patterns were shown in transplant medicine, although new kidney transplantation was highest in summer, whereas outcome of transplant patients was reported to be worse during winter (January) [47] as well as graft failures due to chronic rejection [51]. Gilbertson et al. [52] evaluated data from the Center for Diseases Control and Prevention (CDC) Outpatient Influenza-like Illness Surveillance network and centers for medicare and medicaid services, and ascribed excess of mortality of patients with end-stage renal disease to serious respiratory tract infections such as influenza-like illness. Indeed, a recent study from Hong Kong revealed a relationship between admissions due to AKI and hospitalization caused by influenza, and the risk was increased significantly by low temperature [53].
Studies investigating AKI found results similar to ours. In 2017, Phillips and colleagues identified the seasonal pattern of incidence and outcome of AKI using the Welsh National Health Service. The highest frequency of both, community-acquired and hospital-acquired AKI, was detected between January and March, whilst 90-day mortality after AKI episode had two peaks, the first one was included between January and March, and the second between October and December [54]. Iwagami et al. [15] evaluated more than 80,000 patients with AKI and found that prevalence of AKI was higher in January than in June, moreover, further evaluations suggested that the seasonality of AKI was related to community-acquired AKI associated with the admission diagnosis of cardiovascular and pulmonary diseases among older patients. Also 30-day mortality was higher in winter months. Similar results were highlighted in an Italian hospital setting, underlining how winter was associated with increased risk of AKI and that low air temperature and high relative humidity increased the risk of AKI [16].
Therefore, populations with low degree of renal impairment appear to have higher risk for hospitalization during warm months, on the contrary, AKI with serious renal damage associated with comorbid burden and the need of dialysis treatment could suffer winter peak regarding hospitalization and IHM.
Limitations
Retrospective studies, based on administrative data, have major limitations without the possibility to assess the degree of renal impairment and the cause of AKI. Administrative databases do not allow to evaluate some important items, such as cause of admission and death, intensive care level or hospitals’ facilities, device use, type of treatment, and impact of clinical or biochemical parameters. However, we could differentiate the population investigated in non-dialysis-dependent AKI and dialysis-dependent AKI, a classification suggesting the degree of renal impairment. Administrative databases are used for different reasons such as reimbursement, and lack of specific clinical information and could be cause of possible misclassification [55]. Moreover, AKI was not identified on the basis of international Kidney Disease Improving Global Outcomes (KDIGO) guidelines [56], and the cause of AKI, as well as treatment setting were missing, with the exception of dialysis treatment. In the same way we could not consider socio-economic parameters. The performance of ICD-9-CM for diagnosis of AKI has been reported to have poor sensitivity, and high specificity, while positive and negative predictive values could differ [57,58,59]. However, in subjects aged ≥ 65-year sensitivity significantly increased and administrative data detected more severe AKI and higher IHM mortality [60]. For these reasons, we decided to focus on patients aged > 65 years. Data on weather conditions and environmental factors during the study period were missed, we did not analyze climatic parameters such as temperature and humidity; on the other hand, we reported how Koppen-Geiger classified Italian climate [21]. We should also underline some strengths of our study such as the sample size derived from a national database, the significant period of time considered, and the use of IHM as hard outcome.
Conclusions
Relationship between AKI, comorbidity and IHM is a very actual debate [61,62,63,64]. Our results suggest that also seasonality should be taken into account especially in Italy, a country belonging to warm temperate climates [21]. However, relationship between seasons and AKI could vary depending on the aspect considered, i.e., the AKI observed/expected ratio, IHM or degree of renal impairment. With this study we have tried to stratify an important population to better understand seasonality at a national level. We conclude that both autumn and winter months are independent risk factors for IHM in patients with AKI regardless of age, sex and comorbidity burden, while the risk of death in AKI subjects that require in-hospital treatment is lower during the summertime. Understanding disease seasonality could ameliorate clinical practice, improving hospital resource utilization and community-based preventive care.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy policy but are available from the corresponding author on reasonable request.
References
Fares A (2013) Factors influencing the seasonal patterns of infectious diseases. Int J Prev Med 4:128–132
Lloyd GER, Chadwick J, Mann WN (1978) Hippocratic writings. Penguin, London
Zangbar B, Rhee P, Pandit V, Hsu CH, Khalil M, Okeefe T, Neumayer L, Joseph B (2016) Seasonal variation in emergency general surgery. Ann Surg 263:76–81. https://doi.org/10.1097/SLA.0000000000001238
Hind J, Lahart IM, Jayakumar N, Athar S, Fazal MA, Ashwood N (2020) Seasonal variation in trauma admissions to a level III trauma unit over 10 years. Injury 51:2209–2218. https://doi.org/10.1016/j.injury.2020.07.014
Upshur RE, Moineddin R, Crighton E, Kiefer L, Mamdani M (2005) Simplicity within complexity: seasonality and predictability of hospital admissions in the province of Ontario 1988–2001, a population-based analysis. BMC Health Serv Res 5:13. https://doi.org/10.1186/1472-6963-5-13
Gallerani M, Trappella G, Manfredini R, Pasin M, Napolitano M, Migliore A (1994) Acute intracerebral haemorrhage: circadian and circannual patterns of onset. Acta Neurol Scand 89:280–286. https://doi.org/10.1111/j.1600-0404.1994.tb01681.x
Manfredini R, Gallerani M, Boari B, Salmi R, Mehta RH (2004) Seasonal variation in onset of pulmonary embolism is independent of patients’ underlying risk comorbid conditions. Clin Appl Thromb Hemost 10:39–43. https://doi.org/10.1177/107602960401000106
Manfredini R, Boari B, Smolensky MH, Salmi R, Gallerani M, Guerzoni F, Guerra V, Maria Malagoni A, Manfredini F (2005) Seasonal variation in onset of myocardial infarction–a 7-year single-center study in Italy. Chronobiol Int 22:1121–1135. https://doi.org/10.1080/07420520500398106
Manfredini R, Fabbian F, Pala M, Tiseo R, De Giorgi A, Manfredini F, Malagoni AM, Signani F, Andreati C, Boari B, Salmi R, Imberti D, Gallerani M (2011) Seasonal and weekly patterns of occurrence of acute cardiovascular diseases: does a gender difference exist? J Women Health (Larchmt) 20:1663–1668. https://doi.org/10.1089/jwh.2011.2734
Dentali F, Ageno W, Rancan E, Donati AV, Galli L, Squizzato A, Venco A, Mannucci PM, Manfredini R (2011) Seasonal and monthly variability in the incidence of venous thromboembolism. A systematic review and a meta-analysis of the literature. Thromb Haemost 106:439–447. https://doi.org/10.1160/TH11-02-0116
Gallerani M, Boari B, Manfredini F, Manfredini R (2011) Seasonal variation in heart failure hospitalization. Clin Cardiol 34:389–394. https://doi.org/10.1002/clc.20895
Vitale J, Manfredini R, Gallerani M, Mumoli N, Eagle KA, Ageno W, Dentali F (2015) Chronobiology of acute aortic rupture or dissection: a systematic review and a meta-analysis of the literature. Chronobiol Int 32:385–394. https://doi.org/10.3109/07420528.2014.983604
Mongardon N, Max A, Bouglé A, Pène F, Lemiale V, Charpentier J, Cariou A, Chiche JD, Bedos JP, Mira JP (2012) Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive care unit: a multicenter study. Crit Care 16:R155. https://doi.org/10.1186/cc11471
Fisman DN (2007) Seasonality of infectious diseases. Annu Rev Public Health 28:127–143. https://doi.org/10.1146/annurev.publhealth.28.021406
Iwagami M, Moriya H, Doi K, Yasunaga H, Isshiki R, Sato I, Mochida Y, Ishioka K, Ohtake T, Hidaka S, Noiri E, Kobayashi S (2018) Seasonality of acute kidney injury incidence and mortality among hospitalized patients. Nephrol Dial Transplant 33:1354–1362. https://doi.org/10.1093/ndt/gfy011
Lombardi G, Gambaro G, Pertica N, Naticchia A, Bargagli M, Ferraro PM (2021) Seasonality of acute kidney injury in a tertiary hospital academic center: an observational cohort study. Environ Health 20:8. https://doi.org/10.1186/s12940-021-00691-5
Yokota LG, Sampaio BM, Rocha EP, Balbi AL, Sousa Prado IR, Ponce D (2018) Acute kidney injury in elderly patients: narrative review on incidence, risk factors, and mortality. Int J Nephrol Renovasc Dis 11:217–224. https://doi.org/10.2147/IJNRD.S170203
Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS (2014) Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 9:12–20. https://doi.org/10.2215/CJN.02730313
Fabbian F, Savriè C, De Giorgi A, Cappadona R, Di Simone E, Boari B, Storari A, Gallerani M, Manfredini R (2019) Acute kidney injury and in-hospital mortality: a retrospective analysis of a nationwide administrative database of elderly subjects in Italy. J Clin Med 8(9):1371. https://doi.org/10.3390/jcm8091371
Fabbian F, De Giorgi A, Di Simone E, Cappadona R, Lamberti N, Manfredini F, Boari B, Storari A, Manfredini R (2020) Weekend effect and in-hospital mortality in elderly patients with acute kidney injury: a retrospective analysis of a National Hospital Database in Italy. J Clin Med 9:1815. https://doi.org/10.3390/jcm9061815
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Koppen-Geiger climate classification updated. Meteorol Z 15:259–263
Fabbian F, De Giorgi A, Maietti E, Gallerani M, Pala M, Cappadona R, Manfredini R, Fedeli U (2017) A modified Elixhauser score for predicting in-hospital mortality in internal medicine admissions. Eur J Intern Med 40:37–42. https://doi.org/10.1016/j.ejim.2017.02.002
De Giorgi A, Di Simone E, Cappadona R, Boari B, Savriè C, López-Soto PJ, Rodríguez-Borrego MA, Gallerani M, Manfredini R, Fabbian F (2020) Validation and comparison of a modified Elixhauser index for predicting in-hospital mortality in Italian internal medicine wards. Risk Manag Healthc Policy 13:443–451. https://doi.org/10.2147/RMHP.S247633
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1130–1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
Gogarten JF, Brown LM, Chapman CA, Cords M, Doran-Sheehy D, Fedigan LM, Grine FE, Perry S, Pusey AE, Sterck EH, Wich SA, Wright PC (2012) Seasonal mortality patterns in non-human primates: implications for variation in selection pressures across environments. Evolution 66:3252–3266. https://doi.org/10.1111/j.1558-5646.2012.01668.x
Hales S, Blakely T, Foster RH, Baker MG, Howden-Chapman P (2012) Seasonal patterns of mortality in relation to social factors. J Epidemiol Community Health 66:379–384. https://doi.org/10.1136/jech.2010.111864
Davie GS, Baker MG, Hales S, Carlin JB (2007) Trends and determinants of excess winter mortality in New Zealand: 1980 to 2000. BMC Public Health 7:263. https://doi.org/10.1186/1471-2458-7-263
Asif M, Nawaz K, Zaheer Z, Thygesen H, Abu-Shaheen A, Riaz M (2019) Seasonality of deaths with respect to age and cause in Chitral district Pakistan. PLoS ONE 14:e0225994. https://doi.org/10.1371/journal.pone.0225994
Li M, Gu S, Bi P, Yang J, Liu Q (2015) Heat waves and morbidity: current knowledge and further direction-a comprehensive literature review. Int J Environ Res Public Health 12:5256–5283. https://doi.org/10.3390/ijerph120505256
Haines A, Kovats RS, Campbell-Lendrum D, Corvalan C (2006) Climate change and human health: impacts, vulnerability and public health. Public Health 120:585–596. https://doi.org/10.1016/j.puhe.2006.01.002
Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, Tobias A, Tong S, Rocklöv J, Forsberg B, Leone M, De Sario M, Bell ML, Guo YL, Wu CF, Kan H, Yi SM, de Sousa Zanotti Stagliorio Coelho M, Saldiva PH, Honda Y, Kim H, Armstrong B (2015) Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386:369–375. https://doi.org/10.1016/S0140-6736(14)62114-0
Borg M, Bi P, Nitschke M, Williams S, McDonald S (2017) The impact of daily temperature on renal disease incidence: an ecological study. Environ Health 16:114. https://doi.org/10.1186/s12940-017-0331-4
Lim YH, So R, Lee C, Hong YC, Park M, Kim L, Yoon HJ (2018) Ambient temperature and hospital admissions for acute kidney injury: a time-series analysis. Sci Total Environ 616–617:1134–1138. https://doi.org/10.1016/j.scitotenv.2017.10.207
Kim SE, Lee H, Kim J, Lee YK, Kang M, Hijioka Y, Kim H (2019) Temperature as a risk factor of emergency department visits for acute kidney injury: a case-crossover study in Seoul. South Korea Environ Health 18:55. https://doi.org/10.1186/s12940-019-0491-5
McTavish RK, Richard L, McArthur E, Shariff SZ, Acedillo R, Parikh CR, Wald R, Wilk P, Garg AX (2018) Association between high environmental heat and risk of acute kidney injury among older adults in a Northern climate: a matched case-control study. Am J Kidney Dis 71:200–208. https://doi.org/10.1053/j.ajkd.2017.07.011
Bobb JF, Obermeyer Z, Wang Y, Dominici F (2014) Cause-specific risk of hospital admission related to extreme heat in older adults. JAMA 312:2659–2667. https://doi.org/10.1001/jama.2014.15715
Moyce S, Armitage T, Mitchell D, Schenker M (2020) Acute kidney injury and workload in a sample of California agricultural workers. Am J Ind Med 63:258–268. https://doi.org/10.1002/ajim.23076
Masugata H, Senda S, Inukai M, Himoto T, Murao K, Hosomi N, Iwado Y, Noma T, Kohno M, Goda F (2011) Seasonal variation in estimated glomerular filtration rate based on serum creatinine levels in hypertensive patients. Tohoku J Exp Med 224:137–142. https://doi.org/10.1620/tjem.224.137
Longhini C, Molino C, Fabbian F (2010) Cardiorenal syndrome: still not a defined entity. Clin Exp Nephrol 14:12–21. https://doi.org/10.1007/s10157-009-0257-4
Fares A (2013) Winter cardiovascular diseases phenomenon. N Am J Med Sci 5:266–279. https://doi.org/10.4103/1947-2714.110430
Stewart S, Keates AK, Redfern A, McMurray JJV (2017) Seasonal variations in cardiovascular disease. Nat Rev Cardiol 14:654–664. https://doi.org/10.1038/nrcardio.2017.76
Manfredini R, Manfredini F, Boari B, Bergami E, Mari E, Gamberini S, Salmi R, Gallerani M (2009) Seasonal and weekly patterns of hospital admissions for nonfatal and fatal myocardial infarction. Am J Emerg Med 27:1097–1103. https://doi.org/10.1016/j.ajem.2008.08.009
Vallabhajosyula S, Patlolla SH, Cheungpasitporn W, Holmes DR Jr, Gersh BJ (2020) Influence of seasons on the management and outcomes acute myocardial infarction: An 18-year US study. Clin Cardiol 43:1175–1185. https://doi.org/10.1002/clc.23428
Usvyat LA, Carter M, Thijssen S, Kooman JP, van der Sande FM, Zabetakis P, Balter P, Levin NW, Kotanko P (2012) Seasonal variations in mortality, clinical, and laboratory parameters in hemodialysis patients: a 5-year cohort study. Clin J Am Soc Nephrol 7:108–115. https://doi.org/10.2215/CJN.03880411
Wetmore JB, Gilbertson DT, Liu J, Collins AJ (2016) Improving outcomes in patients receiving dialysis: the peer kidney care initiative. Clin J Am Soc Nephrol 11:1297–1304. https://doi.org/10.2215/CJN.12981215
Guinsburg AM, Usvyat LA, Etter M, Xu X, Thijssen S, Marcelli D, Canaud B, Marelli C, Barth C, Wang Y, Carioni P, van der Sande FM, Kotanko P, Kooman JP, Monitoring Dialysis Outcomes (MONDO) consortium (2015) Seasonal variations in mortality and clinical indicators in international hemodialysis populations from the MONDO registry. BMC Nephrol 16:139. https://doi.org/10.1186/s12882-015-0129-y
Obi Y, Kalantar-Zadeh K, Streja E, Rhee CM, Reddy UG, Soohoo M, Wang Y, Ravel V, You AS, Jing J, Sim JJ, Nguyen DV, Gillen DL, Saran R, Robinson B, Kovesdy CP (2017) Seasonal variations in transition, mortality and kidney transplantation among patients with end-stage renal disease in the USA. Nephrol Dial Transplant 32(suppl_2):ii99–ii105. https://doi.org/10.1093/ndt/gfw379
Goto S, Hamano T, Ogata S, Masakane I (2020) Seasonal variations in cause-specific mortality and transition to renal replacement therapy among patients with end-stage renal disease. Sci Rep 10(1):2325. https://doi.org/10.1038/s41598-020-59153-6
Mehta RH, Manfredini R, Hassan F, Sechtem U, Bossone E, Oh JK, Cooper JV, Smith DE, Portaluppi F, Penn M, Hutchison S, Nienaber CA, Isselbacher EM, Eagle KA, International Registry of Acute Aortic Dissection (IRAD) Investigators (2002) Chronobiological patterns of acute aortic dissection. Circulation 106:1110–1115. https://doi.org/10.1161/01.cir.0000027568.39540.4b
Mehta RH, Manfredini R, Bossone E, Fattori R, Evagelista A, Boari B, Cooper JV, Sechtem U, Isselbacher EM, Nienaber CA, Eagle KA, International Registry of Acute Aortic Dissection (IRAD) Investigators (2005) The winter peak in the occurrence of acute aortic dissection is independent of climate. Chronobiol Int 22:723–729. https://doi.org/10.1080/07420520500179605
Astor BC, Melamed ML, Mandelbrot DA, Djamali A (2018) Seasonality of mortality and graft failure among kidney transplant recipients in the US—a retrospective study. Transpl Int 31:293–301. https://doi.org/10.1111/tri.13047
Gilbertson DT, Rothman KJ, Chertow GM, Bradbury BD, Brookhart MA, Liu J, Winkelmayer WC, Stürmer T, Monda KL, Herzog CA, Ashfaq A, Collins AJ, Wetmore JB (2019) Excess deaths attributable to influenza-like illness in the ESRD population. J Am Soc Nephrol 30:346–353. https://doi.org/10.1681/ASN.2018060581
Mohammad KN, Chan EYY, Lau SY, Lam HCY, Goggins WB, Chong KC (2021) Relationship between acute kidney injury, seasonal influenza, and environmental factors: a 14-year retrospective analysis. Environ Int 153:106521. https://doi.org/10.1016/j.envint.2021.106521
Phillips D, Young O, Holmes J, Allen LA, Roberts G, Geen J, Williams JD, Phillips AO, Welsh AKI steering group (2017) Seasonal pattern of incidence and outcome of Acute kidney injury: a national study of Welsh AKI electronic alerts. Int J Clin Pract. 71(9):e13000. https://doi.org/10.1111/ijcp.13000
Mazzali C, Duca P (2015) Use of administrative data in healthcare research. Intern Emerg Med 10:517–524. https://doi.org/10.1007/s11739-015-1213-9
Clinical practice guideline for acute kidney injury (2021) Available online: https://kdigo.org/wpcontent/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf (Accessed 4 Nov 2021)
Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL (2006) Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17:1688–1694. https://doi.org/10.1681/ASN.2006010073
Vlasschaert ME, Bejaimal SA, Hackam DG, Quinn R, Cuerden MS, Oliver MJ, Iansavichus A, Sultan N, Mills A, Garg AX (2011) Validity of administrative database coding for kidney disease: a systematic review. Am J Kidney Dis 57:29–43. https://doi.org/10.1053/j.ajkd.2010.08.031
Tomlinson LA, Riding AM, Payne RA, Abel GA, Tomson CR, Wilkinson IB, Roland MO, Chaudhry AN (2013) The accuracy of diagnostic coding for acute kidney injury in England—a single centre study. BMC Nephrol 14:58. https://doi.org/10.1186/1471-2369-14-58
Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J (2014) Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol 9:682–689. https://doi.org/10.2215/CJN.07650713
Schiffl H (2020) Gender differences in the susceptibility of hospital-acquired acute kidney injury: more questions than answers. Int Urol Nephrol 52:1911–1914. https://doi.org/10.1007/s11255-020-02526-7
Ponce D, Zamoner W, Batistoco MM, Balbi A (2020) Changing epidemiology and outcomes of acute kidney injury in Brazilian patients: a retrospective study from a teaching hospital. Int Urol Nephrol 52:1915–1922. https://doi.org/10.1007/s11255-020-02512-z
Yalin SF, Bakir A, Trabulus S, Seyahi N, Altiparmak MR (2020) The Charlson comorbidity index: can it predict the outcome in acute kidney injury? Int Urol Nephrol 52:1713–1718. https://doi.org/10.1007/s11255-020-02499-7
Duarte I, Gameiro J, Resina C, Outerelo C (2020) In-hospital mortality in elderly patients with acute kidney injury requiring dialysis: a cohort analysis. Int Urol Nephrol 52:1117–1124. https://doi.org/10.1007/s11255-020-02482-2
Acknowledgements
We thank Dr. Isabella Bagnaresi and Mr. Mauro Pasin, Clinica Medica Unit, Azienda Ospedaliero-Universitaria ‘S. Anna’, and Dr. Claudia Righini and Dr. Donato Bragatto, Biblioteca Interaziendale di Scienze della Salute, Ferrara, Italy, for their precious support.
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement. None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest directly or indirectly related to the work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Giorgi, A., Storari, A., Rodríguez-Muñoz, P.M. et al. Seasonal pattern in elderly hospitalized with acute kidney injury: a retrospective nationwide study in Italy. Int Urol Nephrol 54, 3243–3253 (2022). https://doi.org/10.1007/s11255-022-03271-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03271-9