Abstract
The coronavirus disease 2019 (COVID-19) is associated with increased mortality in patients with chronic kidney disease (CKD), dialysis patients and kidney transplant recipients (KTR). Cardiovascular complications, such as sudden arrhythmias, thromboembolic events, coronary events, cardiomyopathies and heart failure, may present in about 10–20% of patients with COVID-19. Patients with CKD, dialysis patients and KTR are all at increased cardiovascular risk and present with more cardiovascular complications after COVID-19 compared to the general population. During the pandemic, health care giving has rapidly changed by reducing elective outpatient reviews, which may refrain these high-risk patients from the appropriate management of their medical conditions, further increasing cardiovascular risk. Importantly, acute kidney injury (AKI) is another common complication of severe COVID-19 and associates with increased mortality. A large proportion of the AKI patients need renal replacement treatment, while 30% of them may not present renal function recovery and remain dialysis-dependent after discharge, thereby having potentially increased future cardiovascular risk. This review summarizes current knowledge regarding the cardiovascular events and mortality in patients with CKD or undergoing hemodialysis and in KTR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus disease 2019 (COVID-19), caused by a coronavirus strain known as acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is a novel disease primarily affecting the respiratory system and causing from influenza-like symptoms to viral pneumonia, which may escalate to acute respiratory distress syndrome (ARDS) in some individuals [1, 2]. According to the World Health Organization, COVID-19 has evolved to a global pandemic, impacting > 250 million individuals and resulting in > 5.1 million deaths globally to date [3]. Mortality is higher in the elderly and in patients with existing comorbidities, such as hypertension, diabetes mellitus and cardiovascular disease [4]. In the general population, COVID-19 may also present a spectrum of atypical manifestations, such as gastrointestinal symptoms, acute kidney injury (AKI), neurological and upper respiratory symptoms, such as anosmia or taste loss [5]. However, COVID-19 can also lead to both onset of new, or the exacerbation of underlying, cardiovascular disease [6]. Importantly, COVID-19 has been tightly associated with severe cardiovascular complications including hypercoagulability, cardiac arrhythmias, acute coronary syndrome and decompensation of heart failure [7]. Whether potential long-term cardiovascular consequences, associated with COVID-19, exist is not yet known [8]. Moreover, the pandemic has introduced major problems in healthcare systems ranging from constraints on health care delivery to difficulties in accessing health services for patients with chronic diseases, which may further increase cardiovascular risk in the future [9].
The global burden of chronic kidney disease (CKD) is high, with prevalence being estimated at 9.1% and has increased by 29.3% during the past three decades [10]. Cardiovascular events are considered the main cause of mortality in these patients with infections being second [11]. Mortality is even higher in patients with end-stage renal disease (ESRD) undergoing maintenance dialysis with cardiac and cerebrovascular events accounting for > 50%, while infections account for 15% of deaths from known causes [12]. CKD generates a high-risk phenotype with a clinical profile encompassing inflammation, protein-energy wasting, altered function of the autonomic and central nervous systems, cardiopulmonary, vascular and bone diseases [13]. Moreover, CKD is tightly associated with immune system dysfunction that contributes to the high prevalence of infections and more frequent infection-related complications among these individuals [14]. Observational data suggest that patients with CKD are at higher risk for severe COVID-19 disease and mortality during admission [15, 16]. Thus, a recent consensus document from the Working Group of the European Renal Association COVID-19 Database (ERACODA) delineated the greater vulnerability for severe COVID-19 infection and associated mortality in patients with CKD [17]. Possible cardiovascular involvement, related to the COVID-19, may further increase the cardiovascular risk in this compromised population both in the short and in the long term. Herein, we discuss all existing evidence on cardiovascular risk, complications and mortality associated with COVID-19 in patients with CKD or undergoing hemodialysis and in KTR.
Pathophysiology of cardiovascular involvement in COVID-19
COVID-19 is strongly associated with the occurrence of cardiovascular complications, such as sudden arrhythmias, thromboembolic events, coronary events, cardiomyopathies and a heart failure [18]. The mechanistic background of the cardiovascular involvement in patients with COVID-19 is complex (Fig. 1). The SARS-CoV-2 uses a structural protein, known as spike, to gain intracellular entry to the host cells, through binding to the cell-associated and soluble angiotensin-converting enzyme-2 (ACE2) receptor in synergy with the transmembrane serine protease 2 (TMPRSS2) [19,20,21]. ACE2 receptors are expressed in a variety of cells, such as the lung alveolar, nasal and oral mucosa epithelial cells, enterocytes, proximal tubular cells, endothelial cells, and the vascular smooth muscle cells [22]. After entry, the expression of ACE2 is downregulated, which minimizes the protective organ effects of the receptor [23]. This decrease in ACE2 receptors has a direct negative impact on the cardiovascular system by inducing myocardial inflammation, remodeling and injury, as well as contractility disorders [24]. It is also known that the downregulation of ACE2 in the heart, induced by coronavirus strains, also promotes macrophage infiltration in the myocardium leading to myocardial inflammation [25]. These changes also induce an imbalance between ACE2 and angiotensin-2 levels and a decrease in the cardioprotective effects of angiotensin-2 [26]. Animal studies have shown that loss of ACE2 causes severely impaired cardiac contractility and increases susceptibility to experimentally induced heart failure [27]. Importantly, patients with CKD have increased ACE2 activity compared to healthy individuals [28, 29].
Pathophysiologic mechanisms leading to cardiovascular complications in patients with COVID-19. ACE2 angiotensin-converting enzyme-2 receptor; CCL chemokines ligands; IL interleukin; SARS-CoV-2 acute respiratory syndrome coronavirus-2; TMPRSS2 transmembrane serine protease 2; TNF tumor necrosis factor
In some patients, SARS-CoV-2 may provoke an acute systemic inflammatory response and cytokine storm, characterized by excessive increase in proinflammatory cytokines: interleukin (IL)-1b, IL-6, IL-8 and IL-10, tumor necrosis factor (TNF), and chemokines ligands (CCL)-2, CCL-3, and CCL-5 [30]. This reaction can result in endothelial cells damage and apoptosis leading to vascular leakage mainly in the lung, but also in several organs causing shock, acute cardiac injury and failure, as well as renal and hepatic failure [31]. In the heart, systemic inflammatory response and hypoxia increases cardiometabolic demands and causes an altered myocardial demand–supply ratio predisposing patients to cardiovascular events [24]. Systemic inflammation and increased shear stress, due to the hemodynamic changes associated with severe disease, can also cause atheromatous plaque rupture and destabilization, which may cause acute coronary syndrome and the formation of microthrombi from the release of foamy macrophages in the bloodstream [32]. Patients with COVID-19 present vascular inflammation and endothelial dysfunction which per se constitutes a hypercoagulable state [33]. In fact, evidence suggests the development of antiphospholipid antibodies, complement activation and deposition (C5-b9, C4d and mannose-binding lectin-associated serin protease) on the endothelial cells and microthrombi formation in some patients [34, 35]. These phenomena may be more pronounced in patients on dialysis, in whom endothelial cells have increased adhesiveness to leukocytes and platelets, as well as present increased synthesis of vasoactive molecules, cytokines and procoagulant factors [36].
Cardiovascular complications in CKD patients with COVID-19
So far, several studies showed that CKD is among the most common underlying diseases in patients with COVID-19, with prevalence ranging between 2% and 7% in unselected populations needing hospital admission [37, 38]. Importantly, the most common causal conditions of CKD, such as hypertension, diabetes mellitus and cardiovascular disease, have also been strongly associated with increased mortality risk after COVID-19 [39]. In one of the earlier studies in the field, patients with pre-existing CKD presented more severe COVID-19 infection (septic shock requiring vasoactive medications, or respiratory failure requiring mechanical ventilation) compared to those without CKD (38.1% vs. 15.7%; P < 0.001) [40]. Later observational data suggest that patients with CKD are at higher risk for severe COVID-19 disease and mortality during admission, ranging from 2.1-fold [95% confidence interval (CI): 1.36–3.26] higher for elevated serum creatinine, to 3.9-fold higher (95%CI: 2.57–6.14) for elevated blood urea nitrogen levels [15]. These observations have been confirmed in several subsequent studies [41,42,43]. In the largest study, including > 17 million primary care records from the United Kingdom (UK) and 10,926 COVID-19-related deaths, patients with estimated glomerular filtration rate (eGFR) 30–60 ml/min/1.73 m2 [Hazard Ratio (HR): 1.33, 95%CI: 1.28–1.40] and < 30 ml/min/1.73 m2 (HR: 2.52, 95%CI: 2.33–2.72) had higher mortality risk compared to those with eGFR > 60 ml/min/1.73 m2, corresponding to mortality rates of 0.4% and 1.11%, respectively [16]. Results from another retrospective, observational study, including 289 CKD patients, 390 hemodialysis patients, 81 KTR and 450 controls, who were hospitalized due to COVID-19, showed increased mortality risk only in the CKD and hemodialysis groups and not for KTR (CKD, HR: 2.88, 95% CI: 1.52–5.44; hemodialysis: HR: 2.32, 95% CI: 1.21–4.46; KTR: HR: 1.87, 95%CI: 0.81–4.28, respectively) compared to controls [44].
Studies directly comparing cardiovascular events and mortality in patients with and without CKD are scarce. The results of the main studies directly comparing outcomes in patients with and without CKD are presented in Table 1. So far, most studies have included unselected populations, in whom CKD patients represent only a fraction of the patients included. Earlier case-series provided the first evidence on a possible association between CKD and increased cardiovascular complications due to SARS-CoV-2 infection, but results were contradictory [45,46,47]. Results from a later retrospective cohort study, including 4264 patients (12.2% with pre-existing CKD), who were admitted to intensive care units across the United States (US) with COVID-19, indicated that CKD patients had higher risk for 28-day in-hospital death (HR: 1.25, 95%CI: 1.08–1.44) compared to those with normal kidney function [48]. Among the secondary outcomes of this study, authors also evaluated the risk for ventricular arrhythmia or cardiac arrest (HR: 1.23, 95%CI: 0.99–1.53) and for thromboembolic events (HR: 0.90, 95%CI: 0.62–1.29), which was similar in patients with compared to those without CKD [48]. In another study, including 777 consecutively admitted patients with COVID-19 (29.6% with and 71.4% without CKD), results showed significantly higher all-cause mortality (59% vs. 26%; P < 0.001) and cardiovascular events rates (11% vs. 18%; P = 0.010) in patients with CKD compared to those with normal renal function, over a mean follow-up of 35 ± 22 days [49]. An observational study including a large population of 7341 patients with COVID-19, only a small fraction of whom had CKD (3.2%) or were undergoing maintenance dialysis (< 0.2%), showed a significant difference in the occurrence of cardiac arrest (0.5% vs 2.1% vs. 7.1%; P < 0.001), myocardial infraction (3.4% vs. 7.1% vs. 7.1%; P = 0.006) and acute heart failure (5.1 vs. 8.0 vs. 14.3%; P = 0.046) among patients without and those with CKD or on dialysis, respectively [50]. Despite the fact that hemodialysis patients represented a very small fraction of the population studied, these results further support a higher incidence of all cardiovascular end-points in CKD patients compared to those without CKD [50]. In the same context, results from an observational multi-ethnic multi-centre study in a UK cohort of 434 patients admitted across 6 hospitals and diagnosed COVID-19 positive indicated an independent association between CKD and myocardial injury (OR: 9.12, 95%CI: 4.24–19.64), defined as positive troponin during admission [51]. An observational study, including 4906 patients, showed that CKD was associated with increased risk (OR: 2.10, 95%CI: 1.47–3.0) for the primary outcome of adjudicated venous or arterial thromboembolism or all-cause mortality [52]. In another analysis of outcomes in 8308 women from the US VA COVID-19 shared data repository, CKD was independently associated with increased risk for cardiovascular events within 60 days of testing positive for COVID-19 (OR: 2.24, 95%CI: 1.49–3.38) [53]. Similarly, results from another retrospective study including 8574 patients with COVID‐19 from 88 US hospitals indicated that CKD and ESRD were associated with mortality or major adverse cardiac events compared to patients without CKD, but the association was rendered insignificant after multivariate adjustment for existing risk factors [54]. Results from a smaller retrospective study including 700 patients, 11% of whom had history of CKD, indicated no significant association between CKD and cardiac arrhythmias, such as atrial fibrillation, bradyarrhythmia and non-sustained ventricular tachycardia [55]. In the only study evaluating factors possibly associated with pulmonary embolism in 689 consecutive COVID-19 patients admitted to cardiology departments across Italy, CKD was not associated with increased risk for pulmonary embolism (HR: 0.45, 95%CI: 0.16–1.25) over 15 (9–24) days of follow-up [56].
Importantly, COVID-19 may itself result in glomerular injury, evidenced by de novo proteinuria associated with acute tubular injury, collapsing focal segmental glomerulosclerosis or post-AKI proteinuria in some cases [57, 58]. The severity of proteinuria is strongly associated with increasing COVID-19-related mortality risk for higher levels [59]. Evidence on cardiovascular mortality after COVID-19 in patients with proteinuric CKD is also scarce (Table 1). In one of the earlier studies in this field, including 168 Chinese patients admitted with mild-to-moderate COVID-19 symptoms, results showed that 18.4% of the patients had proteinuria and only 1 patient had AKI on admission, while proteinuria was associated with increased risk for development of severe COVID-19 infection [relative risk (RR): 7.37, 95%CI: 2.45–22.18] [60]. A case–control study included 40 patients with known glomerulonephritis and COVID-19 [median baseline proteinuria 1.0 (0.33–3.20) g/d], as well as 80 COVID-19 controls from the general population matched for the time of infections and results suggested significantly higher mortality rate (15% vs. 5%; P = 0.026) and numerically higher myocardial infarctions (5.6% vs. 0.0%; P = 0.12) for patients with glomerulonephritis, whereas controls presented more episodes of cardiac arrhythmias (2.8% vs. 7.9%; P = 0.034) over a median follow-up of 17.0 (9.0–22.0) days [61]. In the study by Cheng et al., a total 43.9% of the patients had albuminuria on admission and results showed that mortality rates increased proportionally to the severity of albuminuria (measured semi-quantitatively by dipstick: 1+, HR: 4.12, 95%CI: 1.97–8.62; 2–3+, HR: 10.92, 95%CI: 5.00–23.86) compared to those without albuminuria [15].
Cardiovascular complications in COVID-19 hemodialysis patients
During the pandemic of COVID-19, patients receiving hemodialysis are among the few populations with long-term conditions that continued their usual treatment by attending to their dialysis units, usually on a thrice weekly schedule, encountering other patients and medical staff in a limited space for several hours. Despite the implementation of preventative measures, such as social distancing and isolation of infected individuals, in-center transmission was not uncommon [62, 63]. Patients undergoing in-center hemodialysis present a high infection rate from SARS-CoV-2, ranging between 19.6% and 22.2% by real-time polymerase chain reaction (RT-PCR) and 36.2% by serologic testing, according to studies from the UK [64, 65]. The first case-series studies published since the COVID-19 outbreak, including patients undergoing maintenance hemodialysis in European countries and the United States, reported mortality rates between 31% and 41% [66,67,68]. Two studies, including COVID-19 hemodialysis patients across Europe, showed that mortality rate was about 20% at 28 days after diagnosis [69, 70]. In another large study from the US, being on dialysis was associated with higher mortality (31.7% vs. 25.4%, HR: 1.38, 95%CI: 1.12–1.70) compared to those without ESRD [71]. Results from a secondary analysis of the previously mentioned study by Williamson et al. showed a much lower mortality rate of 0.8% and a significantly higher mortality risk (HR 3.69, 95%CI:3.09–4.39) in patients with compared to those without history of hemodialysis or ESRD, but the former patient group represented only 2.3% of the population studied [16].
Limited data exist on cardiovascular events and mortality in patients undergoing dialysis. The studies evaluating the association of hemodialysis with cardiovascular events and mortality are presented in Table 2. In one of the first studies published in this field, including 49 hemodialysis and 52 patients without CKD who were hospitalized due to COVID-19 in Wuhan China, mortality rate (14% vs. 4%; P < 0.001), incidence of arrhythmia (18% vs. 2%; P < 0.001) and myocardial injury (29% vs. 8%; P < 0.001) were higher in dialysis patients [72]. Similar were the results in other observational studies including Chinese hemodialysis patients, with results indicating cardiovascular events as the most common cause of death after COVID-19 [31, 73, 74]. A retrospective cohort study from the US in 7948 hemodialysis patients, among 5.5% of the population who developed COVID-19, 24.9% died in total, while pulmonary (51.9%), cardiac (9.3%) a combination of cardiac and pulmonary causes (7.4%) were the three most common causes of death during the 90-day follow-up period [75]. Results from a smaller study in a US population also showed a large number of in-hospital cardiac arrests in hemodialysis patients hospitalized due to COVID-19 [76]. In a multi-centre French cohort study, cardiovascular events (sudden death, heart failure, arterial or venous thrombosis, myocarditis) was again the second more common cause of death in 2336 hemodialysis patients enrolled, 5.5% of whom were COVID-19 cases [77]. A smaller single-center study evaluating clinical characteristics and outcomes in 37 hemodialysis patients in Romania hospitalized with COVID-19, 19% of whom died during follow-up, found that the main cause of death was cardiovascular events (13.5%) followed by respiratory distress syndrome (5.4%), while 20% of the patients with severe infection presented cardiac injury, defines as serum troponin-I levels above the 99th percentile upper reference levels [78].
In contrast, results from some studies, mainly in European populations, have not shown increased cardiovascular mortality during or after COVID-19. In a prospective, multicenter study from Belgium, including a total 4297 hemodialysis patients who were followed-up for 12 weeks (March 2–May 25, 2020), COVID-19 incidence was 2.54% (2.23–2.89%), mortality rate was similar to the mean rate during the same period of 2015–2019 (standardized mortality ratio: 51.02, 95%CI, 0.88 to 1.16; P = 0.82) and the rates of cardiovascular events (4.7% vs. 13.2%) and sudden death (3.1 vs. 16.7%; P = 0.006) were significantly lower in patients with compared to those without COVID-19 [79]. Importantly, the cause of death was identified as “infection” in 76.5% of the hemodialysis patients with COVID-19, which may limit the study’s results regarding the true rates of death causes [79]. In the study by Flythe et al. analyzed above, 143 patients undergoing hemodialysis who represented 3.4% of the total population, presented a 28-day in-hospital mortality rate of 50% and had significantly higher mortality risk (HR: 1.41; 95%CI: 1.09–1.81), and insignificantly higher risk for cardiovascular and thromboembolic events compared to those with normal kidney function [48]. In another small study evaluating outcomes in 15 in-center dialysis patients with SARS-CoV-2 infection from Italy, 40% of whom died during a median follow-up of 5.5 days after diagnosis, but cardiac ischemia was identified as the cause of death only in 1 patient [80].
Cardiovascular complications in kidney transplant patients with COVID-19
Transplantation is the renal replacement modality of choice for patients with ESRD, as it confers improved survival, lower healthcare cost and higher quality of life compared to dialysis [81,82,83]. However, immunosuppression treatment results in increased susceptibility to infections and the expression of diminished signs and symptoms of the invasive infection [84]. With regards to COVID-19, organ transplantation was among the comorbidities associated with the highest mortality risk (HR: 3.53, 95%CI: 2.77–4.49) in the largest available study [16]. These results were also supported from the analyses performed in COVID-19 patients from the ERA-EDTA Registry, which showed that 28-day mortality was 19.9% (17.5%–22.5%) in KTR, while mortality risk was higher compared with age- and sex-matched dialysis patients (HR: 1.28, 95%CI 1.02–1.60) [69, 70]. Results from studies in the US were in same direction, further confirming that kidney transplantation was associated with increased mortality due to COVID-19 [85,86,87]. In KTR, the relevant rate of cardiovascular mortality is 20 times that of age- and sex-matched members of the general population [88]. In the study by De Meester et al. analyzed above, none of the six KTR with COVID-19 who died during follow-up experienced cardiovascular events [79]. Despite significant improvements over the past decades, cardiovascular disease still remains the leading cause of death among KTR during the first 10 years post-transplant period [89, 90]. So far, no study has evaluated whether KTR have higher risk for cardiovascular mortality after COVID-19 compared to the general population. In a case-report presenting the post-mortem findings of a 71-year old KTR who died due to COVID-19 showed the presence of viral elements within endothelial cells, diffuse endothelial inflammation and inflammatory cell death in several organs, including the kidney allograft [91].
Indirect cardiovascular consequences of the COVID-19 in patients with renal diseases
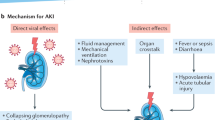
The COVID-19 pandemic has evolved in a serious challenge for all healthcare systems around the world. To cope with the pressure and to decrease the possibility of in-hospital transmission during COVID-19 waves, most of the healthcare systems worldwide postponed non-essential outpatient activities or elective operations and took measures to limit the number of inpatients for several months [92]. However, these infection control policies may have resulted in poor management of pre-existing CKD or many concomitant diseases such as diabetes, hypertension and cardiovascular disease, which in turn may further increase ESRD incidence and future cardiovascular risk in these patients [93].
Moreover, AKI is very common in severe COVID-19 cases (e.g., critically ill patients in the intensive care unit needing mechanical ventilation), a complication associated with increased mortality. Several studies showed that AKI occurs in 20–46% of patients with COVID-19, while 11–19% of these patients require renal replacement treatment during admission [94,95,96]. Importantly, 30–35% of the patients with COVID-19 and AKI remain dependent to renal replacement treatment at discharge [94,95,96]. Results from a previous metanalysis of 11 studies suggested that patients experiencing AKI present a significant risk for developing CKD (HR: 8.8, 95%CI 3.1–25.5) and ESRD (HR 3.1, 95%CI 1.9–5.0) [97] in the long term [97]. Occurrence of CKD after COVID-19 infection may indirectly increase cardiovascular events and further increase cardiovascular mortality in the future [98].
Unfortunately, patients with advanced CKD or ESRD have been excluded from many studies evaluating treatment options for COVID-19 [99, 100]. In contrast, most of the vaccination studies have not excluded patients with renal diseases from the populations studied, which may help in the prevention of COVID-19 in these patients [101,102,103]. Thus, patients with CKD and their treating physicians may have been left without an adequate evidence base from which to offer tested treatments to a group of patients known to be at increased risk of the worst outcomes from COVID-19 [104].
Conclusion
Patients with renal diseases present a high-risk phenotype that may result in increased susceptibility to cardiovascular complications after COVID-19. So far, several studies showed that these patients present higher all-cause mortality risk after SARS-CoV-2 infection compared to the general population. However, data on the possible association with cardiovascular mortality are scarce. Observational studies, including unselected populations, indicated a significant association between CKD and hemodialysis with cardiovascular events and mortality after COVID-19. Evidence associating cardiovascular events after COVID-19 in KTR is scarce. Importantly, most of the available studies in the field have not recorded a specific death cause, while in others, the cause of death has been identified as infection, possibly providing a false impression of the actual cardiovascular mortality rates in CKD and dialysis patients. Whether the association between renal dysfunction and cardiovascular events after SARS-CoV-2 infection can be attributed to the compromised cardiovascular background of these patients or to the cardiovascular manifestations of the COVID-19 is unsure.
COVID-19 is usually complicated by AKI in the general population, while a large proportion of these patients remain dialysis dependent after discharge, which in turn may further increase future cardiovascular risk. Moreover, management of CKD requires frequent outpatient reviews, particularly in those with advanced renal dysfunction, and health care measures implemented to reduce in-hospital transmissions of COVID-19 deprived proper follow-up during several months in the past few years. The same may be also applicable in patients with other diseases, such as hypertension, diabetes mellitus and cardiovascular disease, which all are among the most common causal conditions of CKD. Whether COVID-19 will potentially increase the demand for maintenance dialysis in the future or may escalate the long-term cardiovascular risk in patients with renal diseases is a matter requiring further investigation.
Data availability
Not applicable.
Code availability
Not applicable.
References
Sun P, Lu X, Xu C, Sun W, Pan B (2020) Understanding of COVID-19 based on current evidence. J Med Virol 92(6):548–551. https://doi.org/10.1002/jmv.25722
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273. https://doi.org/10.1038/s41586-020-2012-7
WHO (2021) Coronavirus disease (COVID-19) Dashboard. https://covid19.who.int/. (Accessed 22 Feb 2021).
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan China. Intensive Care Med 46(5):846–848. https://doi.org/10.1007/s00134-020-05991-x
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW (2020) Extrapulmonary manifestations of COVID-19. Nat Med 26(7):1017–1032. https://doi.org/10.1038/s41591-020-0968-3
Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O (2020) Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 5(7):831–840. https://doi.org/10.1001/jamacardio.2020.1286
Krittanawong C, Kumar A, Hahn J, Wang Z, Zhang HJ, Sun T, Bozkurt B, Ballantyne CM, Virani SS, Halperin JL, Jneid H (2020) Cardiovascular risk and complications associated with COVID-19. Am J Cardiovasc Dis 10(4):479–489
Lambert H, Gupte J, Fletcher H, Hammond L, Lowe N, Pelling M, Raina N, Shahid T, Shanks K (2020) COVID-19 as a global challenge: towards an inclusive and sustainable future. Lancet Planetary Health 4(8):e312–e314. https://doi.org/10.1016/s2542-5196(20)30168-6
Blumenthal D, Fowler EJ, Abrams M, Collins SR (2020) Covid-19 - implications for the health care system. N Engl J Med 383(15):1483–1488. https://doi.org/10.1056/NEJMsb2021088
(2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395(10225):709–733. https://doi.org/10.1016/s0140-6736(20)30045-3
Wen CP, Matsushita K, Coresh J, Iseki K, Islam M, Katz R, McClellan W, Peralta CA, Wang H, de Zeeuw D, Astor BC, Gansevoort RT, Levey AS, Levin A (2014) Relative risks of chronic kidney disease for mortality and end-stage renal disease across races are similar. Kidney Int 86(4):819–827. https://doi.org/10.1038/ki.2013.553
Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V (2019) US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 73(3 Suppl 1):A7–A8. https://doi.org/10.1053/j.ajkd.2019.01.001
Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Parati G, Rossignol P, Wiecek A, London G (2017) The systemic nature of CKD. Nat Rev Nephrol 13(6):344–358. https://doi.org/10.1038/nrneph.2017.52
Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, Tranaeus A, Stenvinkel P, Lindholm B (2008) Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3(5):1526–1533. https://doi.org/10.2215/cjn.00950208
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97(5):829–838. https://doi.org/10.1016/j.kint.2020.03.005
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821):430–436. https://doi.org/10.1038/s41586-020-2521-4
(2021) Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 36(1):87–94. https://doi.org/10.1093/ndt/gfaa314
Walsh MN, Sorgente A, Fischman DL, Bates ER, Grapsa J (2020) The COVID-19 pandemic and cardiovascular complications: what have we learned so far? JACC Case Rep 2(9):1235–1239. https://doi.org/10.1016/j.jaccas.2020.06.017
Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L (2020) Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 17(6):613–620. https://doi.org/10.1038/s41423-020-0400-4
South AM, Diz DI, Chappell MC (2020) COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318(5):H1084-h1090. https://doi.org/10.1152/ajpheart.00217.2020
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e278. https://doi.org/10.1016/j.cell.2020.02.052
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637. https://doi.org/10.1002/path.1570
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4):586–590. https://doi.org/10.1007/s00134-020-05985-9
Bansal M (2020) Cardiovascular disease and COVID-19. Diabetes Metab Syndr 14(3):247–250. https://doi.org/10.1016/j.dsx.2020.03.013
Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J (2009) SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 39(7):618–625. https://doi.org/10.1111/j.1365-2362.2009.02153.x
Patel VB, Bodiga S, Basu R, Das SK, Wang W, Wang Z, Lo J, Grant MB, Zhong J, Kassiri Z, Oudit GY (2012) Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ Res 110(10):1322–1335. https://doi.org/10.1161/circresaha.112.268029
Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417(6891):822–828. https://doi.org/10.1038/nature00786
Roberts MA, Velkoska E, Ierino FL, Burrell LM (2013) Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol Dial Transplant 28(9):2287–2294. https://doi.org/10.1093/ndt/gft038
Soler MJ, Wysocki J, Batlle D (2013) ACE2 alterations in kidney disease. Nephrol Dial Transplant 28(11):2687–2697. https://doi.org/10.1093/ndt/gft320
Ye Q, Wang B, Mao J (2020) The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect 80(6):607–613. https://doi.org/10.1016/j.jinf.2020.03.037
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Petrovic V, Radenkovic D, Radenkovic G, Djordjevic V, Banach M (2020) Pathophysiology of cardiovascular complications in COVID-19. Front Physiol 11:575600. https://doi.org/10.3389/fphys.2020.575600
Xie Y, Wang X, Yang P, Zhang S (2020) COVID-19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging 2(2):e200067. https://doi.org/10.1148/ryct.2020200067
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382(17):e38. https://doi.org/10.1056/NEJMc2007575
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J (2020) Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 220:1–13. https://doi.org/10.1016/j.trsl.2020.04.007
Zoccali C, Mallamaci F, Tripepi G (2003) Inflammation and atherosclerosis in end-stage renal disease. Blood Purif 21(1):29–36. https://doi.org/10.1159/000067852
Emami A, Javanmardi F, Pirbonyeh N, Akbari A (2020) Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 8(1):e35
Fathi M, Vakili K, Sayehmiri F, Mohamadkhani A, Hajiesmaeili M, Rezaei-Tavirani M, Eilami O (2021) The prognostic value of comorbidity for the severity of COVID-19: a systematic review and meta-analysis study. PLoS ONE 16(2):e0246190. https://doi.org/10.1371/journal.pone.0246190
D’Marco L, Puchades MJ, Romero-Parra M, Gimenez-Civera E, Soler MJ, Ortiz A, Gorriz JL (2020) Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J 13(3):297–306. https://doi.org/10.1093/ckj/sfaa104
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX, China Medical Treatment Expert Group for C (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55(5):2000547. https://doi.org/10.1183/13993003.00547-2020
Portolés J, Marques M, López-Sánchez P, de Valdenebro M, Muñez E, Serrano ML, Malo R, García E, Cuervas V (2020) Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Trans 35(8):1353–1361. https://doi.org/10.1093/ndt/gfaa189
Gok M, Cetinkaya H, Kandemir T, Karahan E, Tuncer İB, Bukrek C, Sahin G (2021) Chronic kidney disease predicts poor outcomes of COVID-19 patients. Int Urol Nephrol. https://doi.org/10.1007/s11255-020-02758-7
Dirim AB, Demir E, Yadigar S, Garayeva N, Parmaksiz E, Safak S, Bahat KA, Ucar AR, Oruc M, Oto OA, Medetalibeyoglu A, Basaran S, Orhun G, Yazici H, Turkmen A (2021) COVID-19 in chronic kidney disease: a retrospective, propensity score-matched cohort study. Int Urol Nephrol. https://doi.org/10.1007/s11255-021-02783-0
Ozturk S, Turgutalp K, Arici M, Odabas AR, Altiparmak MR, Aydin Z, Cebeci E, Basturk T, Soypacaci Z, Sahin G, Elif Ozler T, Kara E, Dheir H, Eren N, Suleymanlar G, Islam M, Ogutmen MB, Sengul E, Ayar Y, Dolarslan ME, Bakirdogen S, Safak S, Gungor O, Sahin I, Mentese IB, Merhametsiz O, Oguz EG, Genek DG, Alpay N, Aktas N, Duranay M, Alagoz S, Colak H, Adibelli Z, Pembegul I, Hur E, Azak A, Taymez DG, Tatar E, Kazancioglu R, Oruc A, Yuksel E, Onan E, Turkmen K, Hasbal NB, Gurel A, Yelken B, Sahutoglu T, Gok M, Seyahi N, Sevinc M, Ozkurt S, Sipahi S, Bek SG, Bora F, Demirelli B, Oto OA, Altunoren O, Tuglular SZ, Demir ME, Ayli MD, Huddam B, Tanrisev M, Bozaci I, Gursu M, Bakar B, Tokgoz B, Tonbul HZ, Yildiz A, Sezer S, Ates K (2020) Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant 35(12):2083–2095. https://doi.org/10.1093/ndt/gfaa271
Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA (2020) Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 396(10247):320–332. https://doi.org/10.1016/s0140-6736(20)31305-2
Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M (2020) Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA 323(16):1612–1614. https://doi.org/10.1001/jama.2020.4326
Al-Wahaibi K, Al-Wahshi Y, Mohamed Elfadil O (2020) Myocardial injury is associated with higher morbidity and mortality in patients with 2019 novel coronavirus disease (COVID-19). SN Compr Clin Med. https://doi.org/10.1007/s42399-020-00569-6
Flythe JE, Assimon MM, Tugman MJ, Chang EH, Gupta S, Shah J, Sosa MA, Renaghan AD, Melamed ML, Wilson FP, Neyra JA, Rashidi A, Boyle SM, Anand S, Christov M, Thomas LF, Edmonston D, Leaf DE (2021) Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis 77(2):190-203.e191. https://doi.org/10.1053/j.ajkd.2020.09.003
Russo E, Esposito P, Taramasso L, Magnasco L, Saio M, Briano F, Russo C, Dettori S, Vena A, Di Biagio A, Garibotto G, Bassetti M, Viazzi F (2021) Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa. Northern Italy J Nephrol 34(1):173–183. https://doi.org/10.1007/s40620-020-00875-1
Kang SH, Kim SW, Kim AY, Cho KH, Park JW, Do JY (2020) Association between chronic kidney disease or acute kidney injury and clinical outcomes in COVID-19 patients. J Korean Med Sci 35(50):e434. https://doi.org/10.3346/jkms.2020.35.e434
Papageorgiou N, Sohrabi C, Prieto Merino D, Tyrlis A, Atieh AE, Saberwal B, Lim WY, Creta A, Khanji M, Rusinova R, Chooneea B, Khiani R, Wijesuriya N, Chow A, Butt H, Browne S, Joshi N, Kay J, Ahsan S, Providencia R (2021) High sensitivity troponin and COVID-19 outcomes. Acta Cardiol. https://doi.org/10.1080/00015385.2021.1887586
Giannis D, Allen S, Tsang J, Flint S, Pinhasov T, Williams S, Tan G, Thakur R, Leung C, Snyder MS, Bhatia C, Garrett D, Cotte C, Isaacs S, Gugerty E, Davidson A, Marder GS, Schnitzer A, Goldberg B, McGinn T, Davidson K, Barish MA, Qiu M, Zhang M, Goldin M, Matsagkas M, Arnaoutoglou EM, Spyropoulos AC, Northwell Health CC (2021) Post-discharge thromboembolic outcomes and mortality of hospitalized COVID-19 patients: the CORE-19 registry. Blood. https://doi.org/10.1182/blood.2020010529
Tsai S, Nguyen H, Ebrahimi R, Barbosa MR, Ramanan B, Heitjan DF, Hastings JL, Modrall JG, Jeon-Slaughter H (2021) COVID-19 associated mortality and cardiovascular disease outcomes among US women veterans. Sci Rep 11(1):8497. https://doi.org/10.1038/s41598-021-88111-z
Rao A, Ranka S, Ayers C, Hendren N, Rosenblatt A, Alger HM, Rutan C, Omar W, Khera R, Gupta K, Mody P, DeFilippi C, Das SR, Hedayati SS, de Lemos JA (2021) Association of kidney disease with outcomes in COVID-19: results from the american heart association COVID-19 cardiovascular disease registry. J Am Heart Assoc 10(12):e020910. https://doi.org/10.1161/jaha.121.020910
Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Moss J, Chahal AA, Anesi G, Denduluri S, Domenico CM, Arkles J, Abella BS, Bullinga JR, Callans DJ, Dixit S, Epstein AE, Frankel DS, Garcia FC, Kumareswaram R, Nazarian S, Riley MP, Santangeli P, Schaller RD, Supple GE, Lin D, Marchlinski F, Deo R (2020) COVID-19 and cardiac arrhythmias. Heart Rhythm 17(9):1439–1444. https://doi.org/10.1016/j.hrthm.2020.06.016
Ameri P, Inciardi RM, Di Pasquale M, Agostoni P, Bellasi A, Camporotondo R, Canale C, Carubelli V, Carugo S, Catagnano F, Danzi G, Dalla Vecchia L, Giovinazzo S, Gnecchi M, Guazzi M, Iorio A, La Rovere MT, Leonardi S, Maccagni G, Mapelli M, Margonato D, Merlo M, Monzo L, Mortara A, Nuzzi V, Piepoli M, Porto I, Pozzi A, Provenzale G, Sarullo F, Sinagra G, Tedino C, Tomasoni D, Volterrani M, Zaccone G, Lombardi CM, Senni M, Metra M (2020) Pulmonary embolism in patients with COVID-19: characteristics and outcomes in the Cardio-COVID Italy multicenter study. Clin Res Cardiol. https://doi.org/10.1007/s00392-020-01766-y
Carriazo S, Kanbay M, Ortiz A (2020) Kidney disease and electrolytes in COVID-19: more than meets the eye. Clin Kidney J 13(3):274–280. https://doi.org/10.1093/ckj/sfaa112
Ferlicot S, Jamme M, Gaillard F, Oniszczuk J, Couturier A, May O, Grünenwald A, Sannier A, Moktefi A, Le Monnier O, Petit-Hoang C, Maroun N, Brodin-Sartorius A, Michon A, Dobosziewicz H, Andreelli F, Guillet M, Izzedine H, Richard C, Dekeyser M, Arrestier R, Sthelé T, Lefèvre E, Mathian A, Legendre C, Mussini C, Verpont MC, Pallet N, Amoura Z, Essig M, Snanoudj R, Brocheriou-Spelle I, François H, Belenfant X, Geri G, Daugas E, Audard V, Buob D, Massy ZA, Zaidan M (2021) The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfab042
Huart J, Bouquegneau A, Lutteri L, Erpicum P, Grosch S, Resimont G, Wiesen P, Bovy C, Krzesinski JM, Thys M, Lambermont B, Misset B, Pottel H, Mariat C, Cavalier E, Burtey S, Jouret F, Delanaye P (2021) Proteinuria in COVID-19: prevalence, characterization and prognostic role. J Nephrol. https://doi.org/10.1007/s40620-020-00931-w
Hong D, Long L, Wang AY, Lei Y, Tang Y, Zhao JW, Song X, He Y, Wen E, Zheng L, Li G, Wang L (2020) Kidney manifestations of mild, moderate and severe coronavirus disease 2019: a retrospective cohort study. Clin Kidney J 13(3):340–346. https://doi.org/10.1093/ckj/sfaa083
Waldman M, Soler MJ, García-Carro C, Lightstone L, Turner-Stokes T, Griffith M, Torras J, Valenzuela LM, Bestard O, Geddes C, Flossmann O, Budge KL, Cantarelli C, Fiaccadori E, Delsante M, Morales E, Gutierrez E, Niño-Cruz JA, Martinez-Rueda AJ, Comai G, Bini C, La Manna G, Slon MF, Manrique J, Agraz I, Sinaii N, Cravedi P (2021) Results from the IRoc-GN international registry of patients with COVID-19 and glomerular disease suggest close monitoring. Kidney Int 99(1):227–237. https://doi.org/10.1016/j.kint.2020.10.032
Rombolà G, Heidempergher M, Pedrini L, Farina M, Aucella F, Messa P, Brunori G (2020) Practical indications for the prevention and management of SARS-CoV-2 in ambulatory dialysis patients: lessons from the first phase of the epidemics in Lombardy. J Nephrol 33(2):193–196. https://doi.org/10.1007/s40620-020-00727-y
La Milia V, Bacchini G, Bigi MC, Casartelli D, Cavalli A, Corti M, Crepaldi M, Limardo M, Longhi S, Manzoni C, Ravasi C, Stucchi V, Viganò S (2020) COVID-19 outbreak in a large hemodialysis center in Lombardy Italy. Kidney Int Rep 5(7):1095–1099. https://doi.org/10.1016/j.ekir.2020.05.019
Corbett RW, Blakey S, Nitsch D, Loucaidou M, McLean A, Duncan N, Ashby DR (2020) Epidemiology of COVID-19 in an Urban dialysis center. J Am Soc Nephrol 31(8):1815–1823. https://doi.org/10.1681/asn.2020040534
Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, Lightstone L, Kelleher P, Pickering MC, Thomas D, Charif R, Griffith M, McAdoo SP, Willicombe M (2020) High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 31(9):1969–1975. https://doi.org/10.1681/asn.2020060827
Goicoechea M, Sanchez Camara LA, Macias N, Munoz de Morales A, Rojas AG, Bascunana A, Arroyo D, Vega A, Abad S, Verde E, Garcia Prieto AM, Verdalles U, Barbieri D, Delgado AF, Carbayo J, Mijaylova A, Acosta A, Melero R, Tejedor A, Benitez PR, Perez de Jose A, Rodriguez Ferrero ML, Anaya F, Rengel M, Barraca D, Luno J, Aragoncillo I (2020) COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int 98(1):27–34. https://doi.org/10.1016/j.kint.2020.04.031
Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Husain SA (2020) Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 31(7):1409–1415. https://doi.org/10.1681/asn.2020040470
Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Lucca B, Cortinovis R, Terlizzi V, Zappa M, Sacca C, Pezzini E, Calcaterra E, Piarulli P, Guerini A, Boni F, Gallico A, Mucchetti A, Affatato S, Bove S, Bracchi M, Costantino EM, Zubani R, Camerini C, Gaggia P, Movilli E, Bossini N, Gaggiotti M, Scolari F (2020) A report from the brescia renal COVID task force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 98(1):20–26. https://doi.org/10.1016/j.kint.2020.04.030
Hilbrands LB, Duivenvoorden R, Vart P, Franssen CFM, Hemmelder MH, Jager KJ, Kieneker LM, Noordzij M, Pena MJ, Vries H, Arroyo D, Covic A, Crespo M, Goffin E, Islam M, Massy ZA, Montero N, Oliveira JP, Roca Muñoz A, Sanchez JE, Sridharan S, Winzeler R, Gansevoort RT (2020) COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 35(11):1973–1983. https://doi.org/10.1093/ndt/gfaa261
Jager KJ, Kramer A, Chesnaye NC, Couchoud C, Sánchez-Álvarez JE, Garneata L, Collart F, Hemmelder MH, Ambühl P, Kerschbaum J, Legeai C, Del Pino YPMD, Mircescu G, Mazzoleni L, Hoekstra T, Winzeler R, Mayer G, Stel VS, Wanner C, Zoccali C, Massy ZA (2020) Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 98(6):1540–1548. https://doi.org/10.1016/j.kint.2020.09.006
Ng JH, Hirsch JS, Wanchoo R, Sachdeva M, Sakhiya V, Hong S, Jhaveri KD, Fishbane S (2020) Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int 98(6):1530–1539. https://doi.org/10.1016/j.kint.2020.07.030
Wu J, Li J, Zhu G, Zhang Y, Bi Z, Yu Y, Huang B, Fu S, Tan Y, Sun J, Li X (2020) Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China Clin J Am Soc Nephrol 15(8):1139–1145. https://doi.org/10.2215/CJN.04160320
Min Y, Cheng L, Tu C, Li H, He D, Huang D, Chen D, Huang X, Chen F, Xiong F (2021) Clinical characteristics of deceased hemodialysis patients affected by COVID-19. Int Urol Nephrol. https://doi.org/10.1007/s11255-020-02700-x
Zou R, Chen F, Chen D, Xu CL, Xiong F (2020) Clinical characteristics and outcome of hemodialysis patients with COVID-19: a large cohort study in a single Chinese center. Ren Fail 42(1):950–957. https://doi.org/10.1080/0886022x.2020.1816179
Hsu CM, Weiner DE, Aweh G, Miskulin DC, Manley HJ, Stewart C, Ladik V, Hosford J, Lacson EC, Johnson DS, Lacson E Jr (2021) COVID-19 infection among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. https://doi.org/10.1053/j.ajkd.2021.01.003
Fisher M, Yunes M, Mokrzycki MH, Golestaneh L, Alahiri E, Coco M (2020) Chronic hemodialysis patients hospitalized with COVID-19: short-term outcomes in the bronx New York. Kidney 360(8):755–762. https://doi.org/10.34067/kid.0003672020
Lano G, Braconnier A, Bataille S, Cavaille G, Moussi-Frances J, Gondouin B, Bindi P, Nakhla M, Mansour J, Halin P, Levy B, Canivet E, Gaha K, Kazes I, Noel N, Wynckel A, Debrumetz A, Jourde-Chiche N, Moal V, Vial R, Scarfoglière V, Bobot M, Gully M, Legris T, Pelletier M, Sallee M, Burtey S, Brunet P, Robert T, Rieu P (2020) Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin Kidney J 13(5):878–888. https://doi.org/10.1093/ckj/sfaa199
Stefan G, Mehedinti AM, Andreiana I, Zugravu AD, Cinca S, Busuioc R, Miler I, Stancu S, Petrescu L, Dimitriu I, Moldovanu E, Crasnaru DE, Gugonea G, Georgescu V, Strambu VD, Capusa C (2021) Clinical features and outcome of maintenance hemodialysis patients with COVID-19 from a tertiary nephrology care center in Romania. Ren Fail 43(1):49–57. https://doi.org/10.1080/0886022x.2020.1853571
De Meester J, De Bacquer D, Naesens M, Meijers B, Couttenye MM, De Vriese AS (2021) Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol 32(2):385–396. https://doi.org/10.1681/asn.2020060875
Fontana F, Giaroni F, Frisina M, Alfano G, Mori G, Lucchi L, Magistroni R, Cappelli G (2020) SARS-CoV-2 infection in dialysis patients in northern Italy: a single-centre experience. Clin Kidney J 13(3):334–339. https://doi.org/10.1093/ckj/sfaa084
Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J (2011) Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11(10):2093–2109. https://doi.org/10.1111/j.1600-6143.2011.03686.x
Rose C, Gill J, Gill JS (2017) Association of kidney transplantation with survival in patients with long dialysis exposure. Clin J Am Soc Nephrol 12(12):2024–2031. https://doi.org/10.2215/cjn.06100617
Ogutmen B, Yildirim A, Sever MS, Bozfakioglu S, Ataman R, Erek E, Cetin O, Emel A (2006) Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc 38(2):419–421. https://doi.org/10.1016/j.transproceed.2006.01.016
Fishman JA, Davis JA (2008) Infection in renal transplant recipients. Kidney Transplant. https://doi.org/10.1016/B978-1-4160-3343-1.50033-5
Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, Redondo-Pachón MD, Murphy B, Florman S, Cyrino LG, Grafals M, Venkataraman S, Cheng XS, Wang AX, Zaza G, Ranghino A, Furian L, Manrique J, Maggiore U, Gandolfini I, Agrawal N, Patel H, Akalin E, Riella LV (2020) COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transplant 20(11):3140–3148. https://doi.org/10.1111/ajt.16185
Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS Jr, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC (2020) COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant 20(7):1800–1808. https://doi.org/10.1111/ajt.15941
Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, Kinkhabwala M (2020) Covid-19 and kidney transplantation. N Engl J Med 382(25):2475–2477. https://doi.org/10.1056/NEJMc2011117
Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ (2010) Reduction in cardiovascular death after kidney transplantation. Transplantation 89(7):851–857. https://doi.org/10.1097/TP.0b013e3181caeead
Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, Navaneethan SD, Ramanathan V (2018) Trends in the causes of death among kidney transplant recipients in the United States (1996–2014). Am J Nephrol 48(6):472–481. https://doi.org/10.1159/000495081
Ying T, Shi B, Kelly PJ, Pilmore H, Clayton PA, Chadban SJ (2020) Death after kidney transplantation: an analysis by era and time post-transplant. J Am Soc Nephrol 31(12):2887–2899. https://doi.org/10.1681/ASN.2020050566
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234):1417–1418. https://doi.org/10.1016/s0140-6736(20)30937-5
Sinsky C, Linzer M (2020) Practice and policy reset post-COVID-19: reversion, transition, or transformation? Health Aff (Millwood) 39(8):1405–1411. https://doi.org/10.1377/hlthaff.2020.00612
Becker RC (2020) Anticipating the long-term cardiovascular effects of COVID-19. J Thromb Thrombolysis 50(3):512–524. https://doi.org/10.1007/s11239-020-02266-6
Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Bottinger EP, Glicksberg BS, Coca SG, Nadkarni GN (2021) AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 32(1):151–160. https://doi.org/10.1681/ASN.2020050615
Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, Sutherland A, Puri S, Srivastava A, Leonberg-Yoo A, Shehata AM, Flythe JE, Rashidi A, Schenck EJ, Goyal N, Hedayati SS, Dy R, Bansal A, Athavale A, Nguyen HB, Vijayan A, Charytan DM, Schulze CE, Joo MJ, Friedman AN, Zhang J, Sosa MA, Judd E, Velez JCQ, Mallappallil M, Redfern RE, Bansal AD, Neyra JA, Liu KD, Renaghan AD, Christov M, Molnar MZ, Sharma S, Kamal O, Boateng JO, Short SAP, Admon AJ, Sise ME, Wang W, Parikh CR, Leaf DE (2021) AKI treated with renal replacement therapy in critically Ill patients with COVID-19. J Am Soc Nephrol 32(1):161–176. https://doi.org/10.1681/ASN.2020060897
Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, Ross DW, Sharma P, Sakhiya V, Fishbane S, Jhaveri KD (2021) Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 77(2):204-215e201. https://doi.org/10.1053/j.ajkd.2020.09.002
Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81(5):442–448. https://doi.org/10.1038/ki.2011.379
Bruchfeld A (2021) The COVID-19 pandemic: consequences for nephrology. Nat Rev Nephrol 17(2):81–82. https://doi.org/10.1038/s41581-020-00381-4
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fatkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC (2020) Remdesivir for the treatment of Covid-19 - final report. N Engl J Med 383(19):1813–1826. https://doi.org/10.1056/NEJMoa2007764
Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh M-d, Kim E-S, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie M-C, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH (2020) Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2031994
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383(27):2603–2615. https://doi.org/10.1056/NEJMoa2034577
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384(5):403–416. https://doi.org/10.1056/NEJMoa2035389
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O’Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397(10269):99–111. https://doi.org/10.1016/s0140-6736(20)32661-1
Major R, Selvaskandan H, Makkeyah YM, Hull K, Kuverji A, Graham-Brown M (2020) The exclusion of patients with CKD in prospectively registered interventional trials for COVID-19-a rapid review of international registry data. J Am Soc Nephrol 31(10):2250–2252. https://doi.org/10.1681/asn.2020060877
Funding
This paper was not supported by any source and represents an original effort of the authors.
Author information
Authors and Affiliations
Contributions
CL drafted the paper and prepared the graphical illustrations, AGP updated the literature, EP performed a critical analysis of the manuscript, GAP provided supervision and mentorship.
Corresponding author
Ethics declarations
Conflict of interest
All authors disclose that they do not have any financial or other relationships, which might lead to a conflict of interest regarding this paper.
Humans and animals
Not applicable.
Ethics approval
Not applicable.
Consent to participate
All authors reviewed the final version of the manuscript and approved the submission to the Journal.
Consent for publication
All author consented for the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loutradis, C., Pitoulias, A.G., Pagkopoulou, E. et al. Cardiovascular complications after COVID-19 in chronic kidney disease, dialysis and kidney transplant patients. Int Urol Nephrol 54, 1551–1563 (2022). https://doi.org/10.1007/s11255-021-03059-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-021-03059-3