Abstract
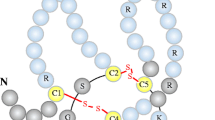
The causes of the increased cardiovascular risk associated with kidney diseases partly reside in the chronic kidney disease–mineral bone disorder (CKD–MBD) syndrome. Three cardiovascular risk factors [hyperphosphatemia, vascular calcification, and elevated fibroblast growth factor 23 (FGF23)] levels have been discovered within the CKD–MBD over the last decades. In addition, sclerostin is recently presented as a new bone and vascular disease biomarker. This 22-kDa glycoprotein, secreted mainly by osteocytes, is a soluble inhibitor of the canonical Wnt pathway that has a pivotal role in bone biology and turnover. CKD patients are reported with higher levels of sclerostin, and levels decrease during dialysis. Sclerostin is associated with vascular calcification and CV risk in CKD, although data are still controversial. The question whether serum sclerostin has protective or deleterious role in CKD–MBD pathophysiology, and therefore in cardiovascular risk and overall mortality, is still open and needs to be answered. The standardization of assays and the establishment of a clear cut-off values when sclerostin starts to switch from physiological to pathophysiological role have to be another important step. Further research is needed also to define its relationship with other CKD–MBD biomarkers for future diagnostic and therapeutic strategies.
Similar content being viewed by others
References
Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW (2013) Chronic kidney disease: global dimension and perspectives. The Lancet 382:260–272
Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX (2006) Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17:2034–2047
Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 69:1945–1953
Hruska KA, Sugatani T, Agapova O, Fang Y (2017) The chronic kidney disease-mineral bone disorder (CKD-MBD): advances in pathophysiology. Bone 100:80–86
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K et al (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Evenepoel P, D’Haese P, Brandenburg V (2015) Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 88:235–240
Viaene L, Behets GJ, Claes K, Meijers B, Blocki F, Brandenburg V, Evenepoel P, D’Haese PC (2013) Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant 28:3024–3030
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ (2011) Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependently pathway. PLoS ONE 6:25900
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33:747–783
Clarke BL, Drake MT (2013) Clinical utility of serum sclerostin measurements. Bonekey Rep. 2:361. https://doi.org/10.1038/bonekey.2013.95
Pietrzyk B, Smertka M, Chudek J (2017) Sclerostin: intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv. Clin. Exp. Med 26:1283–1291
Yavropoulou MP, Xygonakis C, Lolou M, Karaimou F, Yovos JG (2014) The sclerostin story: from human genetics to the development of novel anabolic treatment or osteoporosis. Hormones 13:476–487
Asamiya Y, Tsuchiya K, Nitta K (2016) Role of sclerostin in the pathogenesis of chronic kidney disease-mineral bone disorder. Renal Replace Ther 2:8. https://doi.org/10.1186/s41100-016-0024-4
Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D (2013) The relation between renal function and serum sclerostin in adult patients with CKD0. Clin J Am Soc Nephrol 8:819–823
Cejka D, Jager-Lansky A, Kieweg H, Weber M, Bieglmaye C, Haider DG, Diarra D, Patsch JM, Kainberger F, Bohle B et al (2011) Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant 27:226–230
Cejka D, Marculescu R, Kozakowski N et al (2014) Renal elimination of sclerostin increases with declining kidney function. J Clin Endocrinol Metab 99:248–255
Bielesz BO, Hempfing T, Kieweg H, Marculescu R, Haas M, Cejka D (2014) Sclerostin declines during hemodialysis and appears in dialysate. Blood Purif 38:30–36
Lips L, de Roij van Zuijdewijn CLM, Ter Wee PM, Bots ML, Blankestijn PJ, van den Dorpel MA, Fouque D, de Jongh R, Pelletier S, Vervloet MG et al (2017) Serum sclerostin: relation with mortality and impact of hemodiafiltration. Nephrol Dial Transplant 32:1217–1223
Yamada S, Tsuruya K, Tokumoto M, Yoshida H, Ooboshi H, Kitazono T (2015) Factors associated with serum soluble inhibitors of Wnt-β-catenin signaling (sclerostin and dickkopf-1) in patients undergoing peritoneal dialysis. Nephrology (Carlton) 20:639–645
Bonani M, Rodriguez D, Fehr T, Mohebbi N, Brockmann J, Graf N, Frey D, Wüthrich RP (2014) Sclerostin blood levels before and after kidney transplantation. Kidney Blood Press Res 39:230–239
Evenepoel P, Claes K, Viaene L, Bammens B, Meijers B, Naesens M, Sprangers B, Kuypers D (2016) Decreased circulating sclerostin levels in renal transplant recipients with persistent hyperparathyroidism. Transplantation 100:2188–2193
Sabbagh Y, Graciolli FG, O’Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C et al (2012) Repression of osteocyte Wnt/-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 27:1757–1772
Ishimura E, Okuno S, Ichii M, Norimine K, Yamakawa T, Shoji S, Nishizawa Y, Inaba M (2014) Relationship between serum sclerostin, bone metabolism markers, and bone mineral density in maintenance hemodialysis patients. J Clin Endocrinol Metab 99:4315–4320
Kanbay M, Siriopol D, Saglam M, Kurt YG, Gok M, Cetinkaya H, Karaman M, Unal HU, Oguz Y, Sari S et al (2014) Serum sclerostin and adverse outcomes in non-dialyzed chronic kidney disease patients. J Clin Endocrinol Metab 99:E1854–E1861
Drechsler C, Evenepoel P, Vervloet MG, Wanner C, Ketteler M, Marx N, Floege J, Dekker F, Brandenburg VM (2015) High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 30:288–293
Kramer I, Loots GG, Studer A, Keller H, Kneissel M (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189
Wesseling-Perry K, Harkins GC, Wang HJ, Elashoff R, Gales B, Horwitz MJ, Stewart AF, Jüppner H, Salusky IB (2010) The calcemic response to continuous parathyroid hormone (PTH) (1–34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH (7–84). J Clin Endocrinol Metab 95:2772–2780
Behets GJ, Viaene L, Meijers B, Blocki F, Brandenburg VM, Verhulst A, D’Haese PC, Evenepoel P (2017) Circulating levels of sclerostin but not DKK1 associate with laboratory parameters of CKD-MBD. PLoS One 12:e0176411
Desjardins L, Liabeuf S, Oliveira RB, Louvet L, Kamel S, Lemke HD, Vanholder R, Choukroun G, Massy ZA (2014) Uremic toxicity and sclerostin in chronic kidney disease patients. J Clin Endocrinol Metab 99:E1854–E1861
Tartaglione L, Pasquali M, Rotondi S, Muci ML, Leonangeli C, Farcomeni A, Fassino V, Mazzaferro S (2017) Interactions of sclerostin with FGF23, soluble klotho and vitamin D in renal transplantation. PLoS One 12:e0178637
de Oliveira RB, Graciolli FG, dos Reis LM, Cancela ALE, Cuppari L, Canziani ME, Carvalho AB, Jorgetti V, Moyses RMA (2013) Disturbances of Wnt/β-catenin pathway and energy metabolism in early CKD: effect of phosphate binders. Nephrol Dial Transplant 28:2510–2517
Mathew S, Lund RJ, Strebeck F, Tustison KS, Geurs T, Hruska KA (2007) Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol 18:122–130
Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc. Natl Acad Sci U S A 110:6199–6204
Kumar R, Vallon V (2014) Reduced renal calcium excretion in the absence of sclerostin expression: evidence for a novel calcium-regulating bone kidney axis. J Am Soc Nephrol 25:2159–2168
Zeng C, Guo C, Cai J, Tang C, Dong Z (2018) Serum sclerostin in vascular calcification and clinical outcome in chronic kidney disease. Diabetes Vasc Dis Res 15:99–105
He XW, Wang E, Bao YY, Wang F, Zhu M, Hu XF, Jin XP (2016) High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis 253:22–28
Teng IC, Wang JH, Lee CJ, Hou JS, Hsu BG (2018) Serum sclerostin as an independent marker of peripheral artery disease in elderly persons. Int. J. Clin. Exp. Pathol 11:2816–2821
Gaudio A, Privitera F, Pulvirenti I, Canzonieri E, Rapisar R, Fiore CE (2014) The relationship between inhibitors of the Wnt signalling pathway (sclerostin and Dickkopf-1) and carotid intima-media thickness in postmenopausal women with type 2 diabetes mellitus. Diabetes Vasc Dis Res 11:48–52
Figurek A, Spasovski G (2018) Is serum sclerostin a marker of atherosclerosis in patients with chronic kidney disease-mineral and bone disorder? Int Urol Nephrol 50:1863–1870
Morales-Santana S, Garcia-Fontana B, Garcia-Martin A, Rozas-Moreno R, Garcia-Solcedo JA, Reyes-Garcia R, Munoz-Torres M (2013) Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care 36:1667–1674
Hampson G, Edwards S, Conroy S, Blake GM, Fogelman I, Frost ML (2013) The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone 56:42–47
Chapurlat RD, Confavreux CB (2016) Novel biological markers of bone: from bone metabolism to bone physiology. Rheumatology 55:1714–1725
Lv W, Guan L, Zhang Y, Yu S, Cao B, Ji Y (2016) Sclerostin as a new key factor in vascular calcification in chronic kidney disease stages 3 and 4. Int Urol Nephrol 48:2043–2050
Krischna SM, Seto SW, Jose RJ, Li J, Morton SK, Biros E, Wang Y, Nsengiyumva V, Lindeman JHN, Loots GG et al (2017) Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II–induced aortic aneurysm and atherosclerosis. Arterioscler Thromb Vasc Biol 37:553–566
Wang XR, Yuan L, Zhang JJ, Hao L, Wang DG (2017) Serum sclerostin values are associated with abdominal aortic calcification and predict cardiovascular events in patients with chronic kidney disease stages 3–5D. Nephrology 22:286–292
Zhou H, Yang M, Li M, Cui L (2017) Radial artery sclerostin expression in chronic kidney disease stage 5 predialysis patients: a cross-sectional observational study. Int Urol Nephrol 49:1433–1437
Li M, Zhou H, Yang M, Xing C (2018) Relationship between serum sclerostin, vascular sclerostin expression and vascular calcification assessed by different methods in ESRD patients eligible for renal transplantation: a cross-sectional study. Urol. Nephrol, Int. https://doi.org/10.1007/s11255-018-2033-4
Jørgensen HS, Winther S, Dupont L, Bøttcher M, Rejnmark L, Hauge EM, Svensson M, Ivarsen P (2018) Sclerostin is not associated with cardiovascular event or fracture in kidney transplantation candidates. Clin Nephrol 90:18–26
Yang CY, Chang ZF, Chau YP, Chen A, Yang WC, Yang AH, Lee OKS (2015) Circulating Wnt/β-catenin signalling inhibitors and uraemic vascular calcifications. Nephrol Dial Transplant 30:1356–1363
Jean G, Chazot C, Bresson E, Zaoui E, Cavalier E (2016) High serum sclerostin levels are associated with a better outcome in haemodialysis patients. Nephron 132:181–190
Kirkpantur A, Balci M, Turkvatan A, Afsar B (2016) Serum sclerostin levels, arteriovenous fistula calcification and 2-years all-cause mortality in prevalent hemodialysis patients. Nefrologia 36:24–32
Ji YQ, Guan LN, Yu SX, Yin PY, Shen XQ, Sun ZW, Liu J, Lv W, Yu GP, Ren C (2018) Serum sclerostin as a potential novel biomarker for heart valve calcification in patients with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci 22:8822–8829
Chen A, Sun Y, Cui J, Zhao B, Wang H, Chen X, Mao Y (2018) Associations of sclerostin with carotid artery atherosclerosis and all-cause mortality in Chinese patients undergoing maintenance hemodialysis. BMC Nephrol 19:264
Nowak A, Artunc F, Serra AL, Pollock E, Krayenbühl PA, Müller C, Friedrich B (2015) Sclerostin quo vadis?—Is this a useful long-term mortality parameter in prevalent hemodialysis patients? Kidney Blood Press Res 40:266–276
Balci M, Kirkpantur A, Turkvatan A, Mandiroglu S, Ozturk E, Afsar B (2015) Sclerostin as a new key player in arteriovenous fistula calcification. Herz 40:289–297
Gong L, Zheng D, Yuan J, Cao L, Ni Z, Fang W (2018) Elevated levels of serum sclerostin are linked to adverse cardiovascular outcomes in peritoneal dialysis patients. Int Urol Nephrol 50:955–961
Evenepoel P, Goffin E, Meijers B, Kanaan N, Bammens B, Coche E, Claes K, Jadoul M (2015) Sclerostin serum levels and vascular calcification progression in prevalent renal transplant recipients. J Clin Endocrinol Metab 100:4669–4676
Delanaye P, Krzesinski JM, Warling X, Moonen M, Smelten N, Medart L, Bruyere O, Reginster JY, Pottel H, Cavalier E (2014) E Clinical and biological determinants of sclerostin plasma concentration in hemodialysis patients. Nephron Clin Pract 128:127–134
Schiavi SC, Moyses RM (2016) Turning over renal osteodystrophy dogma: direct actions of FGF23 on osteoblast beta-catenin pathway. Kidney Int 90:17–20
Catalano A, Pintaudi B, Morabito N, Di Vieste G, Giunta L, Bruno ML, Cucinotta D, Lasco A, Di Benedetto A (2014) Gender differences in sclerostin and clinical characteristics in type 1 diabetes mellitus. Eur J Endocrinol 171:293–300
Pietrzyk B, Wyskida K, Ficek J, Kolonko A, Ficek R, Wiecek A, Olszanecka-Glinianowicz M, Chudek J (2018) Relationship between plasma levels of sclerostin, calcium–phosphate disturbances, established markers of bone turnover, and inflammation in haemodialysis patients. Urol. Nephrol, Int. https://doi.org/10.1007/s11255-018-2050-3
Boltenstål H, Qureshi AR, Behets GJ, Lindholm B, Stenvinkel P, D’Haese PC, Haarhaus M (2018) Association of serum sclerostin with bone sclerostin in chronic kidney disease is lost in glucocorticoid treated patients. Calcif Tissue Int. https://doi.org/10.1007/s00223-018-0491-4
Brandenburg VM, Kramann R, Koos R, Krüger T, Schurgers L, Mühlenbruch G, Hüber S, Gladziwa U, Drechsler C, Ketteler M (2013) Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol 14:219
Zhu D, Mackenzie NC, Millán JL, Farquharson C, Mac Rae VE (2011) The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One 6:e19595
Carvalho Goncalves FL, Elias RM, dos Reis LM, Graciolli FG, Zampieri FG, Oliveira RB, Jorgetti V, Moyses RMA (2014) Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol 15:190
Kanbay M, Solak Y, Siriopol D, Aslan G, Afsar B, Yazici D, Covic A (2016) Sclerostin, cardiovascular disease and mortality: a systematic review and meta-analysis. Int Urol Nephrol 48:2029–2042
Piec I, Washbourne C, Tang J, Fisher E, Greeves J, Jackson S, Fraser WD (2016) How accurate is your sclerostin measurement? Comparison between three commercially available sclerostin ELISA kits. Calcif Tissue Int 98:546–555
Funding
No funding available.
Author information
Authors and Affiliations
Contributions
AF, MR and GS: Conceptualization. AF and MR: writing; original draft preparation. GS: writing; review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Figurek, A., Rroji, M. & Spasovski, G. Sclerostin: a new biomarker of CKD–MBD. Int Urol Nephrol 52, 107–113 (2020). https://doi.org/10.1007/s11255-019-02290-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02290-3