Abstract
Purpose
Considering its prognostic usefulness and the relationship with chronic kidney disease, we analyzed the clinical utility of soluble urokinase plasminogen activator receptor (suPAR) in end-stage renal disease patients undergoing hemodialysis treatment. We focused on the association between suPAR levels and clinical outcomes, especially those related to cardiovascular events and mortality as well as the effect of hemodialysis on the protein levels.
Methods
We enrolled 64 patients. Blood samples for laboratory tests were collected before and after the midweek hemodialysis. The concentration of suPAR was assessed using suPARNostic ELISA, ViroGates.
Results
Spearman rank analyses showed a positive association between suPAR and creatinine, cystatin C, galectin 3, N-terminal prohormone of brain natriuretic peptide and troponin T (p < 0.05). In ROC analysis, the suPAR concentration equal to 11.5 ng/mL was established to be the cutoff value for the prediction of mortality in the analyzed patients. Simultaneous analysis of creatinine and suPAR increased the predictive value of the latter—the area under curve increased to 0.84 (95% CI 0.70–0.94, p < 0.0001). Logistic regression analysis revealed that increase in the suPAR level was associated with the increase in odds ratio for death by 1.3 (95% CI 1.1–1.6, χ2 = 8.2, p = 0.004). In multivariable analysis, the prediction power of suPAR appeared to be stronger after including creatinine (p = 0.0005).
Conclusions
Elevated suPAR levels provide independent information on mortality risk in patients undergoing hemodialysis. The protein appears not to cross the dialysis membrane; thus, blood collection before the second hemodialysis session seems to give reliable information on the suPAR level for clinical interpretation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soluble urokinase plasminogen activator receptor (suPAR) circulates in the human bloodstream and bodily fluids as a consequence of proteolytic cleavage of the glycosyl-phosphatidylinositol anchor of the urokinase plasminogen activator receptor that is expressed on membranes of various cells, including immunologically active cells and endothelial cells [1]. The protein is present in healthy individuals in a low concentration, while higher levels have been observed in persons with infectious diseases and other inflammatory disorders. Therefore, suPAR was considered to be a marker of immune system activation [1, 2]. The protein is involved in numerous signaling pathways, including those involved in cellular proliferation, migration, adhesion, and differentiation.
The level of soluble urokinase plasminogen activator receptor has been demonstrated to provide prognostic data concerning the risk of cardiovascular events and all-cause mortality in general population and critically ill patients [3, 4]. In the kidneys, the presence of the protein leads to proteinuria following podocyte migration and injury and is known as an important factor in the development of focal segmental glomerulosclerosis (FSGS) [1, 5]. Nevertheless, it was reported that in a population of patients with all-cause chronic kidney disease (CKD), suPAR correlated with reduced glomerular filtration rate (GFR) [5,6,7]. Considering its prognostic usefulness and the relationship with CKD, we decided to analyze the clinical utility of suPAR in end-stage renal disease patients. Due to the fact that heart failure (HF) is a leading cause of mortality in CKD [8, 9], we focused on the association between suPAR levels and clinical outcomes, especially those related to cardiovascular events and all-cause mortality. Additionally, we compared suPAR with other biomarkers with predictive value, such as N-terminal prohormone of brain natriuretic peptide (NT-proBNP), galectin-3 (Gal-3), high-sensitive troponin T (hsTnT), and high-sensitive C-reactive protein (hsCRP). The aim of this study was to analyze the prognostic potential of suPAR for predicting morbidity and mortality of HD patients as well as the effect of dialysis on the protein levels.
Materials and methods
We enrolled 64 out of the 74 patients of the Department of Nephrology, Hypertension, and Family Medicine—Central Dialysis Unit of the Medical University of Lodz, Poland, undergoing hemodialysis treatment 3 times per week, excluding those with biopsy-proven FSGS. The additional excluding criteria were: malignancy, hepatic diseases, rheumatic and autoimmune diseases, and prior transplants. We enrolled stable HD patients only, already on dialysis for 4–73 months, median: 20 (6–56) months. All the enrolled patients had no symptoms of infection nor any other acute disease. The causes of CKD which progressed to end-stage renal disease in the group of the studied patients included: diabetic nephropathy (n = 27, 42%); hypertensive nephropathy (n = 13, 20%); interstitial nephritis (n = 9, 14%); obstructive nephropathy (n = 8, 12%); polycystic kidney disease (n = 5, 9%); and ischemic nephropathy (n = 2, 3%). Twenty (31%) of the patients had a history of coronary artery disease (CAD), while HF was diagnosed in 41 (64%) patients.
The patients were treated with HD in four-hour session using dialyzers based on polynephron membrane (Elisio-190M, Nipro, Osaka, Japan). All the patients had an arteriovenous fistula as vascular access. A single pool Kt/V was calculated according to the Daugirdas formula based on serum urea concentration before and after the midweek dialysis and before the next dialysis of the week to assess a dialysis adequacy. Blood flow and ultrafiltration rates were adjusted to the individual needs of the patients and kept constant.
Blood samples for laboratory tests were collected before and after the midweek session to determine the levels of laboratory markers, including NT-proBNP, Gal-3, hsTnT, hsCRP, cystatin C, urea, creatinine, albumin, total cholesterol, LDL, calcium, phosphate, parathormone (PTH), hemoglobin, ferritin, and total iron binding capacity (TIBC). Blood analysis before dialysis was performed to describe patients’ baseline characteristics, while the measurement of urea, suPAR, and albumin, also after dialysis, gave insight into how the dialysis session influenced the levels of those parameters. The concentration of suPAR was analyzed using suPARNostic ELISA, ViroGates, Denmark; Gal3 using enzyme-linked fluorescence assay (ELFA), bioMerieux, France; NT-proBNP and hsTnT using electro-chemiluminescence immunoassay (ECLIA), Roche, Switzerland; and cystatin C, hsCRP, immunoturbidimetric assay (ITA), Beckman Coulter, USA.
Heart failure (HF) was diagnosed according to HF criteria [10]. Transthoracic echocardiography was performed on inclusion to the study. Left ventricular ejection fraction (EF) was assessed by the modified Simpson’s formula. During a 3-year follow-up (2014-2016), we focused on clinical outcomes, including all-cause mortality, non-fatal cardiovascular events (myocardial infarction, stroke, hospitalization for heart failure), infections, and hospitalization for other reasons.
Statistical analysis
All results, including baseline characteristics, are presented as mean ± standard deviation (SD) or median values with 5–95th percentiles, if necessary, for continuous variables and as percentages for categorical variables. Differences in analyzed parameters between studied groups of patients were analyzed using the Mann–Whitney test for independent samples and the Wilcoxon test for paired samples. The relation between the suPAR level and baseline characteristics was assessed using the Spearman or Pearson correlation analysis. A comparison of appropriate parameter levels between groups, with and without the endpoints, was performed with the Kruskal–Wallis test. Logistic regression models were used for the analysis of the relations between determined laboratory biomarkers and patients’ outcomes during the follow-up period. Forward selection was used. Additionally, a receiver operating characteristic (ROC) curve analysis was performed to determine the predictive value of biomarkers as well as to define their optimal cutoff values. The statistical analysis was performed using MedCalc Statistical Software version 17.5.5 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2017). The significance level of p < 0.05 was used in all tests.
Results
The study group consisted of 64 persons (42 men and 22 women) with a mean age of 66 ± 13 years. The mean serum concentration of suPAR measured before hemodialysis was 14.6 ± 6.0 ng/mL. When performing the Spearman rank and Pearson analyses, we found a positive association between suPAR concentrations and creatinine, cystatin C, Gal-3, NT-proBNP, and hsTnT, as well as the dialysis vintage (p < 0.05). We also observed a negative association with serum albumin and cholesterol; however, these associations were rather weak (− 0.37; p = 0.022 and − 0.30; p = 0.058, respectively). There was no correlation between suPAR level and others among known associations (Table 1).
Significantly higher suPAR concentrations were observed in all patients with a history of cardiovascular disease (17.5 vs. 12.6 ng/L; p = 0.004). Additionally, patients with a history of CAD had considerably higher suPAR levels (14.9 vs. 9.6 ng/L; p = 0.012). Despite the observed differences between baseline suPAR level in patients with and without diagnosed HF (15.1 vs. 9.1 ng/mL; p = 0.0004), there were no differences in the suPAR level between HF patients with preserved and reduced EF. There were no differences between suPAR levels in patients with and without atrial fibrillation (14.6 vs. 13.2 ng/L; p = 0.7).
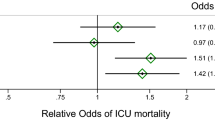
During the follow-up period, we observed 15 non-fatal cerebrocardiovascular events (2 myocardial infarctions, 4 strokes, 4 hospitalizations for decompensation of heart failure, 5 peripheral arteries angioplasty procedures) and 37 hospitalizations for other reasons. We observed statistically relevant differences in baseline suPAR level in patients who finally reached endpoint (14.7 vs. 11.2 ng/mL, p = 0.043), and the ROC analysis showed the predictive power of the protein in the prediction of cerebrocardiovascular endpoint (AUC = 0.7, p = 0.029). Nevertheless, these data were not statistically relevant in uni- or multivariate regression analysis (p > 0.05).
During the follow-up period, 33 (51%) patients died. Due to the low number of deaths of cardiovascular cause (n = 12), we analyzed the relationship between suPAR and all-cause mortality. The logistic regression analysis performed in a forward stepwise manner revealed that a 1 ng/mL increase in suPAR level was associated with an increase in OR for death by 1.3 (95% CI 1.1–1.6, χ2 = 8.2, p = 0.004). A statistically relevant relationship was also found in the case of albumin concentration. An increase in albumin concentration by 1 g/L correlated with a decrease in OR for death by 0.6 (95% CI 0.5–0.9, χ2 = 13.5, p = 0.0002). In multivariable analysis, the prediction power of suPAR appeared to be stronger after including creatinine concentrations (χ2 = 15.3, p = 0.0005). Known risk factors, like the patients’ age, dialysis vintage, and diabetes, as well as other biomarkers in question: NT-proBNP, Gal-3, hsCRP, and hsTnT, were also included in the analysis, but they did not retain in the regression model (p > 0.1).
ROC analysis enabled the assessment of suPAR usefulness as the predictor of mortality in the analyzed patients. A suPAR concentration equal to 11.5 ng/mL was established to be the best-fit cutoff value with 90% sensitivity but only 53% specificity (p = 0.006). Nevertheless, the positive (PPV) and negative (NPV) predictive values, assuming that the ratio of cases in the positive and negative groups reflects the prevalence of outcomes, were 72 and 82%, respectively. Therefore, we used this concentration level as an optimal criterion to determine a division into patient groups for the further analysis. The clinical and laboratory characteristics of patients with or without elevated suPAR levels are presented in Table 2.
Although the predictive value of creatinine levels was shown to be of borderline relevance in the ROC analysis (AUC = 0.66, p = 0.079), simultaneous analysis of creatinine and suPAR increased the predictive value of the latter—the AUC of suPAR increased to 0.84 (95% CI 0.70–0.94, p < 0.0001). The comparative ROC analysis is presented in Fig. 1. These results are in accordance with the correlation analysis and the logistic regression analysis presented above. Additionally, Kaplan–Meier survival analysis showed a 5.6 (95% CI 2.3–13.8) times higher hazard ratio of mortality in the group of patients with suPAR level higher than 11.5 ng/mL; p = 0.007 (Fig. 2).
We observed differences in the numerical values of suPAR serum concentrations before and after hemodialysis session in individual patients (means 14.6 vs. 14.8 ng/mL, respectively) and the differences in the protein level ranged between 1.7 and 26.8%. Nevertheless, the paired-samples t test showed no statistical relevance of the differences (p = 0.1), while there was a high correlation between the concentrations (r = 0.92, p < 0.0001). Due to the fact that there are no data concerning suPAR concentration following dialysis, we tried to assess whether its concentration changes as a result of going through dialysis membranes. We compared the suPAR (60 kDa) level changes with albumin (66 kDa) level changes (the easily detectable protein that does not cross the barrier, with a similar molecular mass) and the urea reduction rate (URR). The median concentrations of albumin before and after hemodialysis session were 38.6 and 43.5 g/L, respectively, and the differences in the protein levels ranged between 1.7 and 22.5%. The URR ranged from 0.52 to 0.88 with the mean 0.65 ± 0.06. We found a correlation between changes in suPAR levels and changes in albumin levels (p = 0.001) but no correlation with URR (p = 0.4).
Discussion
The mortality risk of patients undergoing hemodialysis is undisputedly high and frequently remains in association with cardiovascular complications [11]. In this study, we observed a significantly higher baseline suPAR level in patients with diagnosed HF and in patients with a history of cardiovascular disease. ROC analysis showed the predictive power of suPAR protein in the prediction of cerebrocardiovascular endpoint (AUC = 0.7, p = 0.03). Meijers et al. [12] in their study also showed that suPAR level was directly and significantly associated with cardiovascular events in a group of 476 patients with mild-to-moderate kidney disease. However, in contrast to this study, we failed to find statistically relevant association with CV events in our patients, while, what is worth to point, the mean suPAR concentration in this group of the patients was already significantly higher. Additionally, in the population without CKD, elevated plasma suPAR levels were associated with the presence and severity of angiographic CAD. In Eapen et al.’s [13] study, suPAR levels were higher in patients with significant CAD compared to those with normal coronary arteries or insignificant CAD, and a greater severity of CAD was associated with higher suPAR levels.
In our multivariate analysis, only suPAR, creatinine, and hypoalbuminemia were associated with mortality. We found that a 1 ng/mL increase in suPAR level was associated with an increase in OR for death by 1.3. Moreover, only suPAR appeared to give independent information for risk stratification in the studied patients against other analyzed parameters: NT-proBNP, Gal-3, hsCRP, and hsTnT. Eapen et al. [13] confirmed that suPAR was a significant predictor of incident mortality and morbidity in patients with suspected or established CAD. Moreover, in their study, suPAR significantly improved discrimination of future death and MI risk over a standard clinical model.
Our study confirmed the known relationship between albumin concentration and mortality. The increase in albumin concentration by 1 g/L correlated with a decrease in OR for death by 0.6 (95% CI 0.5–0.9, χ2 = 13.5, p = 0.0002). In other studies, similar associations were observed [14,15,16,17].
It was previously proved that suPAR levels are elevated in association with cardiovascular disease and they hold potential information concerning short- and long-term cardiovascular prediction [2, 3, 18]. Many other markers have also recently been studied in the context of cardiovascular prediction, including galectin-3, NT-proBNP, and troponin T, among others [19,20,21]. Additionally, in the context of cardiovascular risk in dialysis patients, markers of cardiac dysfunction were recently studied [22, 23]. The results of our studies show a correlation between suPAR and the markers of heart failure and heart injury. A suPAR concentration equal to 11.5 ng/mL was established to be the cutoff value with 90% sensitivity, but only 53% specificity (p = 0.006). This relatively low specificity may be due to the fact that the calculated optimal level is much above the concentration recognized as associated with a moderate risk (6 ng/mL) and a high risk (9 ng/mL) in a particular patient and associated with critical illness anyway [3, 4]. Though high concentration of creatinine in the studied group of patients is a complex consequence of nutrition, HD adequacy and residual kidney function, simultaneous analysis of creatinine and suPAR increased the predictive value of the latter—the AUC of suPAR increased to 0.84 (95% CI 0.70–0.94, p < 0.0001). Additionally, Kaplan–Meier survival analysis showed a 5.6 times higher hazard ratio of mortality in the group of patients with suPAR level higher than 11.5 ng/mL (p = 0.007). Only suPAR fit the final model of the risk estimation, which made the biomarker an independent predictor of overall mortality in the studied group. Although a past history of cardiovascular disease was associated with mortality in our patients, we could not study associations between the markers in question and mortality due to cardiovascular events only because of the relatively small number of events.
Due to the fact that there are no data concerning suPAR concentration following dialysis, we also tried to assess whether its concentration changes as a result of going through dialysis membranes. A strong correlation between suPAR level changes and albumin level changes measured before and after the hemodialysis session and no association with URR proves that the molecular form of the protein in question appears not to cross the dialysis membrane: thus, blood collection before the second hemodialysis session seems to give reliable information on the suPAR level for clinical interpretation. We concluded that the observed serum suPAR level changes during hemodialysis session are not due to crossing the hemodialysis membrane, but are the results of changes in the volume of bodily fluids (dehydration after the session) and/or other influences of the preanalytical phase.
The limitations of this study include the relatively small study group and the fact that it enrolled HD patient only from one dialysis unit. Nevertheless, the mean concentration of suPAR in our studied group (14.6 ng/mL) was in accordance with the data obtained by Griveas et al. [24], who described the same mean concentration of the marker in question (14.7 ng/mL) in a similar group, but which was twice as big (n = 127) of HD patients.
Conclusions
Elevated suPAR levels provide independent information on all-cause mortality risk in patients undergoing hemodialysis. The protein appears not to cross the dialysis membrane; thus, blood collection before the midweek hemodialysis session seems to give reliable information on the suPAR level for clinical interpretation.
References
Thuno M, Macho B, Eugen-Olsen J (2009) suPAR: the molecular crystal ball. Dis Markers 27:157–172
Sehestedt T, Lyngbaek S, Eugen-Olsen J et al (2011) Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis 216:237–243
Eugen-Olsen J, Anderson O, Linneberg A et al (2010) Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med 268:296–308
Koch A, Voigt S, Kruschinski C et al (2011) Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 15(1):R63
Wei C, El Hindi S, Li J et al (2011) Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17(8):952–960
Wei C, Moller CC, Altintas MM et al (2008) Modification of kidney barrier function by the urokinase receptor. Nat Med 14(1):55–63
Hayek SS, Sever S, Ko YA et al (2015) Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373(20):1916–1925
Genovesi S, Valsecchi MG, Rossi E et al (2009) Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transpl 24(8):2529–2536
Herzog CA, Mangrum JM, Passman R (2008) Sudden cardiac death and dialysis patients. Semin Dial 21:300–307
McMurray JJ, Adamopoulos S, Anker SD et al (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33(14):1787–1847
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR et al (2013) Chronic kidney disease and cardiovascular risk: epidemiology, mechanism and prevention. Lancet 382:339–352
Meijers B, Poesen R, Claes K et al (2015) Soluble urokinase receptor is a biomarker of cardiovascular disease in chronic kidney disease. Kidney Int 87:210–217
Eapen DJ, Manocha P, Ghasemzedah N et al (2014) Soluble urokinase plasminogen activator receptor level is an independent predictor of the presence and severity of coronary artery disease and of future adverse events. J Am Heart Assoc. https://doi.org/10.1161/JAHA.114.001118
Friedman AN, Fadem SZ (2010) Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 21(2):223–230
Honda H, Qureshi AR, Heimbürger O et al (2006) Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47(1):139–148
Kalantar-Zadeh K, Kilpatrick RD, Kuwae N et al (2005) Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transpl 20(9):1880–1888
Koot BG, Houwen R, Pot DJ et al (2004) Congenital analbuminaemia: biochemical and clinical implications—a case report and literature review. Eur J Pediatr 163:664–670
Wlazel R, Migala M, Zielinska M et al (2016) Soluble urokinase plasminogen activator receptor in one-year prediction of major adverse cardiac events in patients after first myocardial infarction treated with primary percutaneous intervention. Arch Med Sci. https://doi.org/10.5114/aoms.2016.63596
Szadkowska I, Wlazeł RN, Migała M et al (2013) The association between galectin-3 and clinical parameters in patients with first acute myocardial infarction treated with primary percutaneous coronary angioplasty. Cardiol J 20(6):577–582
Gleissner CA, Erbel C, Linden F et al (2017) Galectin-3 binding protein, coronary artery disease and cardiovascular mortality: insights from the LURIC study. Atherosclerosis 260:121–129
Szadkowska I, Pawlicki L, Kowalski J et al (2009) Left ventricular dysfunction and NT-proBNP levels in patients with one-vessel disease after first ST-elevation myocardial infarction treated with primary coronary angioplasty. Kardiol Pol 67(11):1201–1206
Obokata M, Sunaga H, Ishida H et al (2016) Independent and incremental prognostic value of novel cardiac biomarkers in chronic hemodialysis patients. Am Heart J 179:29–41
Franczyk B, Gluba-Brzózka A, Bartnicki P et al (2017) Are markers o cardiac dysfunction useful in the assessment of cardiovascular risk in dialysis patients? Curr Pharm Des. https://doi.org/10.2174/1381612823666170508152501
Griveas I, Andpriopoulos C, Sitaras P et al (2015) Soluble urokinase plasminogen receptor: a future risk marker for hemodialysis patients. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfv200.66
Acknowledgements
The study was supported by the scientific grant for the statutory activity of the Medical University of Lodz, Poland, No 503/5-020-01/503-51-001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Informed consent
All patients gave written informed consent, and the study was approved by the local bioethics committee.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wlazeł, R.N., Szadkowska, I., Bartnicki, P. et al. Clinical and prognostic usefulness of soluble urokinase plasminogen activator receptor in hemodialysis patients. Int Urol Nephrol 50, 339–345 (2018). https://doi.org/10.1007/s11255-017-1778-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1778-5