Abstract
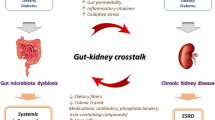
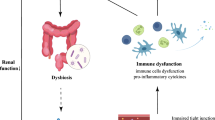
The complicated communities of microbiota colonizing the human gastrointestinal tract exert a strong function in health maintenance and disease prevention. Indeed, accumulating evidence has indicated that the intestinal microbiota plays a key role in the pathogenesis and development of chronic kidney disease (CKD). Modulation of the gut microbiome composition in CKD may contribute to the accumulation of gut-derived uremic toxins, high circulating level of lipopolysaccharides and immune deregulation, all of which play a critical role in the pathogenesis of CKD and CKD-associated complications. In this review, we discuss the recent findings on the potential impact of gut microbiota in CKD and the underlying mechanisms by which microbiota can influence kidney diseases and vice versa. Additionally, the potential efficacy of pre-, pro- and synbiotics in the restoration of healthy gut microbia is described in detail to provide future directions for research.

Similar content being viewed by others
Abbreviations
- BUN:
-
Blood urea nitrogen
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular disease
- DMA:
-
Dimethylamine
- ESKD:
-
End-stage kidney disease
- GFR:
-
Glomerular filtration rate
- GIT:
-
Gastrointestinal tract
- GLP-1:
-
Glucagon-like peptide 1
- GOSs:
-
Galacto-oligosaccharides
- IL-6:
-
Interleukin-6
- IS:
-
Indoxyl sulfate
- LPS:
-
Lipopolysaccharides
- NKT:
-
Natural killer T
- PCS:
-
P-cresyl sulfate
- PYY:
-
Peptide YY
- RS:
-
Resistant starch
- SCFAs:
-
Short-chain fatty acids
References
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR et al (2013) Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382(9889):339–352
Evenepoel P, Meijers BK, Bammens BR et al (2009) Uremic toxins originating from colonic microbial metabolism. Kidney Int 76:S12–S19
Anders H-J, Andersen K, Stecher B (2013) The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 83(6):1010–1016
Szeto C-C, Kwan BC-H, Chow K-M et al (2008) Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 3(2):431–436
Housman A, Shropshire Lad A Incidence and prevalence. United States Renal Data System. In: Proceedings of the 2010 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States, vol 2. Atlas of ESRD
Cerf-Bensussan N, Eberl G (2012) The dialog between microbiota and the immune system: shaping the partners through development and evolution. Semin Immunol 24(1):1–2
Gonçalves S, Pecoits-Filho R, Perreto S et al (2006) Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant 21(10):2788–2794
Le Chatelier E, Nielsen T, Qin J et al (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500(7464):541–546
Cani PD, Delzenne NM (2009) The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 15(13):1546–1558
Vaziri ND (2012) CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens 21(6):587
Bäckhed F, Ley RE, Sonnenburg JL et al (2005) Host-bacterial mutualism in the human intestine. Science 307(5717):1915–1920
Hooper LV, Midtvedt T, Gordon JI (2002) How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22(1):283–307
Ley RE, Turnbaugh PJ, Klein S et al (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022
Lam V, Su J, Koprowski S et al (2012) Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 26(4):1727–1735
Wang Z, Klipfell E, Bennett BJ et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472(7341):57–63
Hida M, Aiba Y, Sawamura S et al (1996) Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin®, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74(2):349–355
Simenhoff M, Dunn S, Zollner G et al (1995) Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab 22(1–3):92–96
Vaziri ND, Wong J, Pahl M et al (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83(2):308–315
Aron-Wisnewsky J, Clément K (2016) The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol 12(3):169–181
Mafra D, Lobo JC, Barros AF et al (2014) Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol 9(3):399–410
Sabatino A, Regolisti G, Brusasco I et al (2015) Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant 30(6):924–933
Carrero JJ, Stenvinkel P (2009) Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol 4(Supplement 1):S49–S55
Neirynck N, Vanholder R, Schepers E et al (2013) An update on uremic toxins. Int Urol Nephrol 45(1):139–150
Sirich TL, Funk BA, Plummer NS et al (2014) Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 25(3):615–622
Soulage CO, Koppe L, Fouque D (2013) Protein-bound uremic toxins… new targets to prevent insulin resistance and dysmetabolism in patients with chronic kidney disease. J Ren Nutr 23(6):464–466
Hughes R, Magee E, Bingham S (2000) Protein degradation in the large intestine: relevance to colorectal cancer. Curr Issues Intest Microbiol 1(2):51–58
Meijers BK, Verbeke K, Dehaen W et al (2009) The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54(5):891–901
Meijers BK, Claes K, Bammens B et al (2010) p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5(7):1182–1189
Koppe L, Pillon NJ, Vella RE et al (2013) p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 24(1):88–99
Mutsaers HA, Stribos EG, Glorieux G et al (2015) Chronic kidney disease and fibrosis: the role of uremic retention solutes. Front Med 2:60
Meijers BK, Evenepoel P (2011) The gut–kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant 26(3):759–761
Barreto FC, Barreto DV, Liabeuf S et al (2009) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4(10):1551–1558
Raff AC, Meyer TW, Hostetter TH (2008) New insights into uremic toxicity. Curr Opin Nephrol Hypertens 17(6):560–565
Faure V, Dou L, Sabatier F et al (2006) Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost 4(3):566–573
Tumur Z, Shimizu H, Enomoto A et al (2010) Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-ĸB activation. Am J Nephrol 31(5):435–441
Lekawanvijit S, Adrahtas A, Kelly DJ et al (2010) Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J 31(14):1771–1779
Aoki K, Teshima Y, Kondo H et al (2015) Role of indoxyl sulfate as a predisposing factor for atrial fibrillation in renal dysfunction. J Am Heart Assoc 4(10):e002023
Gross P, Massy ZA, Henaut L et al (2015) Para-cresyl sulfate acutely impairs vascular reactivity and induces vascular remodeling. J Cell Physiol 230(12):2927–2935
Lin C-J, Pan C-F, Liu H-L et al (2012) The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis 225(1):173–179
Lin C-J, Wu V, Wu P-C et al (2015) Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 10(7):e0132589
Nii-Kono T, Iwasaki Y, Uchida M et al (2007) Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int 71(8):738–743
Lin C-J, Pan C-F, Chuang C-K et al (2014) Association of indoxyl sulfate with fibroblast growth factor 23 in patients with advanced chronic kidney disease. Am J Med Sci 347(5):370–376
Hirata J, Hirai K, Asai H et al (2015) Indoxyl sulfate exacerbates low bone turnover induced by parathyroidectomy in young adult rats. Bone 79:252–258
Howitt MR, Garrett WS (2012) A complex microworld in the gut: gut microbiota and cardiovascular disease connectivity. Nat Med 18(8):1188–1189
Koeth RA, Wang Z, Levison BS et al (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19(5):576–585
Rak K, Rader DJ (2011) Cardiovascular disease: the diet-microbe morbid union. Nature 472(7341):40–41
Stubbs JR, House JA, Ocque AJ et al (2016) Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 27(1):305–313
Missailidis C, Hällqvist J, Qureshi AR et al (2016) Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE 11(1):e0141738
Kaysen GA, Johansen KL, Chertow GM et al (2015) Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr 25(4):351–356
Saito A, Takagi T, Chung T et al (1983) Serum levels of polyamines in patients with chronic renal failure. Kidney Int 16:S234–S237
Lutz W (1979) A uremic peptide containing polyamine: formation and possible role in uremic hypertriglyceridemia. Physiol Chem Phys 12(5):451–456
Chiang C-K, Tanaka T, Inagi R et al (2011) Indoxyl sulfate, a representative uremic toxin, suppresses erythropoietin production in a HIF-dependent manner. Lab Investig 91(11):1564–1571
Nangaku M, Mimura I, Yamaguchi J et al (2015) Role of uremic toxins in erythropoiesis-stimulating agent resistance in chronic kidney disease and dialysis patients. J Ren Nutr 25(2):160–163
Ahmed MSE, Abed M, Voelkl J et al (2013) Triggering of suicidal erythrocyte death by uremic toxin indoxyl sulfate. BMC Nephrol 14(1):244
Yoshida K, Yoneda T, Kimura S et al (2006) Polyamines as an inhibitor on erythropoiesis of hemodialysis patients by in vitro bioassay using the fetal mouse liver assay. Ther Apheresis Dial 10(3):267–272
Cario E, Gerken G, Podolsky D (2007) Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132(4):1359–1374
Schlee M, Harder J, Köten B et al (2008) Probiotic lactobacilli and VSL# 3 induce enterocyte β-defensin 2. Clin Exp Immunol 151(3):528–535
Kiechl S, Lorenz E, Reindl M et al (2002) Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med 347(3):185–192
Muccioli GG, Naslain D, Bäckhed F et al (2010) The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 6(1):392
Sivapalaratnam S, Farrugia R, Nieuwdorp M et al (2011) Identification of candidate genes linking systemic inflammation to atherosclerosis; results of a human in vivo LPS infusion study. BMC Med Genomics 4(1):64
Ryu M, Kulkarni OP, Radomska E et al (2011) Bacterial CpG-DNA accelerates Alport glomerulosclerosis by inducing an M1 macrophage phenotype and tumor necrosis factor-α-mediated podocyte loss. Kidney Int 79(2):189–198
Patole PS, Pawar RD, Lichtnekert J et al (2007) Coactivation of Toll-like receptor-3 and -7 in immune complex glomerulonephritis. J Autoimmun 29(1):52–59
Farhadi A, Banan A, Fields J et al (2003) Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol 18(5):479–497
Magnusson M, Magnusson K-E, Sundqvist T et al (1990) Increased intestinal permeability to differently sized polyethylene glycols in uremic rats: effects of low-and high-protein diets. Nephron 56(3):306–311
Magnusson M, Magnusson K, Sundqvist T et al (1991) Impaired intestinal barrier function measured by differently sized polyethylene glycols in patients with chronic renal failure. Gut 32(7):754–759
Kang J (1993) The gastrointestinal tract in uremia. Dig Dis Sci 38(2):257–268
de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M et al (2004) Bacterial translocation in experimental uremia. Urol Res 32(4):266–270
Vaziri ND, Yuan J, Nazertehrani S et al (2013) Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol 38(2):99–103
Vaziri N, Dure-Smith B, Miller R et al (1985) Pathology of gastrointestinal tract in chronic hemodialysis patients: an autopsy study of 78 cases. Am J Gastroenterol 80(8):608–611
Ding L-A, Li J-S (2003) Gut in diseases: physiological elements and their clinical significance. World J Gastroenterol 9(11):2385–2389
Wang F, Zhang P, Jiang H et al (2012) Gut bacterial translocation contributes to microinflammation in experimental uremia. Dig Dis Sci 57(11):2856–2862
Wang F, Jiang H, Shi K et al (2012) Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology 17(8):733–738
Alegre ML, Mannon RB, Mannon PJ (2014) The microbiota, the immune system and the allograft. Am J Transplant 14(6):1236–1248
Bromberg JS, Fricke WF, Brinkman CC et al (2015) Microbiota [mdash] implications for immunity and transplantation. Nat Rev Nephrol 11(6):342–353
Stenvinkel P (2005) Inflammation in end-stage renal disease–a fire that burns within. Contrib Nephrol 149:185–199
Kato S, Chmielewski M, Honda H et al (2008) Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3(5):1526–1533
Stearns-Kurosawa DJ, Osuchowski MF, Valentine C et al (2011) The pathogenesis of sepsis. Annu Rev Pathol Mech Dis 6:19–48
Carrero JJ, Stenvinkel P (2010) Inflammation in end-stage renal disease—What have we learned in 10 years? Semin Dial 23(5):498–509
Harris K, Kassis A, Major G et al (2012) Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J Obes 2012:879151
Chow J, Tang H, Mazmanian SK (2011) Pathobionts of the gastrointestinal microbiota and inflammatory disease. Curr Opin Immunol 23(4):473–480
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12(1):5–9
Wen L, Ley RE, Volchkov PY et al (2008) Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455(7216):1109–1113
Macpherson AJ, Harris NL (2004) Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 4(6):478–485
Kranich J, Maslowski KM, Mackay CR (2011) Commensal flora and the regulation of inflammatory and autoimmune responses. Semin Immunol 23(2):139–145
Niebauer J, Volk H-D, Kemp M et al (1999) Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 353(9167):1838–1842
McIntyre CW, Harrison LE, Eldehni MT et al (2011) Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6(1):133–141
Lee YK, Menezes JS, Umesaki Y et al (2011) Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 108(Supplement 1):4615–4622
Vaziri ND, Pahl MV, Crum A et al (2012) Effect of uremia on structure and function of immune system. J Ren Nutr 22(1):149–156
Janus N, Vacher L-V, Karie S et al (2008) Vaccination and chronic kidney disease. Nephrol Dial Transplant 23(3):800–807
Hotchkiss RS, Coopersmith CM, McDunn JE et al (2009) The sepsis seesaw: tilting toward immunosuppression. Nat Med 15(5):496–497
Mutsaers HA, Engelke UF, Wilmer MJ et al (2013) Optimized metabolomic approach to identify uremic solutes in plasma of stage 3–4 chronic kidney disease patients. PLoS ONE 8(8):e71199
Konstantinov SR, Smidt H, de Vos WM et al (2008) S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci 105(49):19474–19479
Van Baarlen P, Troost FJ, van Hemert S et al (2009) Differential NF-κB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci 106(7):2371–2376
Murthy M, Venkitanarayan K, Rangavajhyala N et al (2000) Delineation of beneficial characteristics of effective probiotics. JAMA 3(2):38–43
Chen L, Liu W, Li Y et al (2013) Lactobacillus acidophilus ATCC 4356 attenuates the atherosclerotic progression through modulation of oxidative stress and inflammatory process. Int Immunopharmacol 17(1):108–115
Ranganathan N, Patel B, Ranganathan P et al (2005) Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Sci World J 5:652–660
Ranganathan N, Patel BG, Ranganathan P et al (2006) In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J 52(1):70–79
Niwa T (2011) Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: experimental and clinical effects of oral sorbent AST-120. Ther Apheresis Dial 15(2):120–124
Ueda H, Shibahara N, Takagi S et al (2008) AST-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysis. Ren Fail 30(9):856–860
Takayama F, Taki K, Niwa T (2003) Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis 41(3):S142–S145
Taki K, Takayama F, Niwa T (2005) Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J Ren Nutr 15(1):77–80
Piñero-Lambea C, Ruano-Gallego D, Fernández LÁ (2015) Engineered bacteria as therapeutic agents. Curr Opin Biotechnol 35:94–102
Mandell DJ, Lajoie MJ, Mee MT et al (2015) Biocontainment of genetically modified organisms by synthetic protein design. Nature 518(7537):55–60
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125(6):1401
Gibson GR, Probert HM, Van Loo J et al (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17(02):259–275
Silk D, Davis A, Vulevic J et al (2009) Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 29(5):508–518
Meijers BK, De Preter V, Verbeke K et al (2010) p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 25(1):219–224
Salmean YA, Segal MS, Langkamp-Henken B et al (2013) Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J Ren Nutr 23(2):e29–e32
Cani PD, Neyrinck A, Fava F et al (2007) Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50(11):2374–2383
Broekaert WF, Courtin CM, Verbeke K et al (2011) Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr 51(2):178–194
Gibson GR, Beatty ER, Wang X et al (1995) Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108(4):975–982
Pylkas AM, Juneja LR, Slavin JL (2005) Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J Med Food 8(1):113–116
Reimer R, McBURNEY MI (1996) Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology 137(9):3948–3956
Dumoulin V, Moro F, Barcelo A et al (1998) Peptide YY, glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology 139(9):3780–3786
Drucker DJ (2006) The biology of incretin hormones. Cell Metab 3(3):153–165
Ranganath L, Beety J, Morgan L et al (1996) Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38(6):916–919
Batterham RL, Cowley MA, Small CJ et al (2002) Gut hormone PYY3-36 physiologically inhibits food intake. Nature 418(6898):650
Delzenne NM, Cani PD, Daubioul C et al (2005) Impact of inulin and oligofructose on gastrointestinal peptides. Br J Nutr 93(S1):S157–S161
Alimentarius C (2010) Guidelines on nutrition labelling CAC/GL 2-1985 as last amended 2010. Joint FAO/WHO Food Standards Programme, Secretariat of the Codex Alimentarius Commission, FAO, Rome
Chiavaroli L, Mirrahimi A, Sievenpiper J et al (2015) Dietary fiber effects in chronic kidney disease: a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 69(7):761
Vaziri ND, Liu S-M, Lau WL et al (2014) High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 9(12):e114881
Sirich TL, Plummer NS, Gardner CD et al (2014) Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 9(9):1603–1610
Evenepoel P, Bammens B, Verbeke K et al (2006) Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: a pilot study. Kidney Int 70(1):192–198
Rossi M, Johnson DW, Morrison M et al (2016) Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 11(2):223–231
Nakabayashi I, Nakamura M, Kawakami K et al (2011) Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant 26(3):1094–1098
Acknowledgements
We thank Yue Cai for critical review and important intellectual contributions to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interests exists.
Rights and permissions
About this article
Cite this article
Pan, W., Kang, Y. Gut microbiota and chronic kidney disease: implications for novel mechanistic insights and therapeutic strategies. Int Urol Nephrol 50, 289–299 (2018). https://doi.org/10.1007/s11255-017-1689-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1689-5