Abstract
Background
Enoxaparin, a low molecular weight heparin, has been shown to be a safe and effective anticoagulant to prevent clotting in the extracorporeal circulation during hemodialysis. Enoxaparin also possesses antiproliferative properties, and reduces neointimal proliferation following vascular injury in animals. Use of enoxaparin during hemodialysis may be associated with decreased myointimal proliferation and diminished vascular access stenosis or failure.
Aim
The aim of our study was to test the efficacy of enoxaparin to reduce the incidence of recurrent vascular access stenosis in chronic hemodialysis patients.
Methods
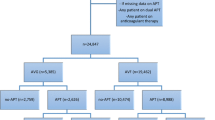
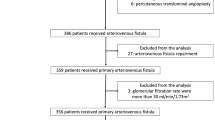
Twenty-nine hemodialysis patients who suffered from recurrent arteriovenous (A-V) access stenosis were studied retrospectively to compare the incidence of vascular access procedures before and during enoxaparin administration. Enoxaparin was administered intravenously as a single bolus at the start of hemodialysis.
Results
Twenty-eight patients (14 male) were analyzed. Ten required a new fistula during the study period. Observed treatment times (years/patient) were 1.20 ± 0.87 for unfractionated heparin (UFH) and 3.04 ± 2.19 for enoxaparin (P = 0.0001). Angiographic procedure rates (procedures/year) were 1.76 ± 0.92 in the UFH group and 1.30 ± 1.01 in the enoxoparin group (P = 0.0786). There were no significant differences in time to first stenosis between the two groups (P = 0.5315). One patient receiving enoxaparin developed upper gastrointestinal bleeding and a second patient sustained a subdural hematoma after a fall.
Conclusion
Our study demonstrated a trend toward a decreased number of angiographic procedures for maintaining A-V access patency in selected chronic hemodialysis patients treated with enoxaparin in comparison with UFH as anticoagulant during dialysis.

Similar content being viewed by others
References
Swedberg SH, Brown BG, Sigley R et al (1989) Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation 80:1726–1731
Rekhter M, Nicholls S, Ferguson M et al (1993) Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb 13:609–617
Stracke S, Konner K, Kostlin I et al (2002) Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int 61:1011–1019
Sterpetti AV, Cucina A, Santoro L et al (1992) Modulation of arterial smooth muscle cell growth by hemodynamic forces. Eur J Vasc Surg 6:16–20
Hsieh HJ, Li NQ, Frangos JA et al (1991) Shear stress increases endothelial platelet derived growth factor mRNA levels. Am J Physiol 260:H642–H646
Hofstra L, Bergmans DC, Hoeks AP et al (1994) Mismatch in elastic properties around anastomoses of interposition grafts for hemodialysis access. J Am Soc Nephrol 5:1243–1247
Welt FG, Woods TC, Edelman ER (2001) Oral heparin prevents neointimal hyperplasia after arterial injury: inhibitory potential depends on type of vascular injury. Circulation 104(25):3121–3124
Fletcher JP, Ao PY, Hawthorne WJ (2004) Antiproliferative effects of low molecular weight UFH. ANZ J Surg 74:793–796
Pecly IM, Gonzalves RG, Rangel EP et al (2006) Effects of low molecular weight UFH in obstructed kidneys: decrease of collagen, fibronectin and TGF-beta, and increase of chondroitin/dermatan sulfate proteoglycans and macrophage infiltration. Nephrol Dial Transplant 21:1212–1222
Klingel R, Schwarting A, Lotz J et al (2004) Safety and efficacy of single bolus anticoagulation with enoxaparin for chronic hemodialysis. Results of an openlabel post-certification study. Kidney Blood Press Res 27:211–217
Saltissi D, Morgan C, Westhuyzen J et al (1999) Comparison of low-molecular weight UFH (enoxaparin sodium) and standard unfractionated UFH for haemodialysis anticoagulation. Nephrol Dial Transplant 14:2698–2703
Reach I, Thebaud HE, Dupuy CA et al (1994) Optimization of enoxaparin dose in the prevention of coagulation in the circuits of hemodialysis: results of a multicenter study. Nephrologie 15:395–401
Pouzol P, Dechelette E, Jurkovitz C et al (1988) Enoxaparin in the prevention of thrombosis of extracorporeal circulation during dialysis of patients with chronic renal failure. Rev Med Interne 9:321–326
Schrader J, Stibbe W, Kandt M et al (1990) Low molecular weight UFH versus standard UFH. A long-term study in hemodialysis and hemofiltration patients. ASAIO Trans 36:28–32
Akdeniz S, Harman M, Atmaca S et al (2005) The management of lichen planus with low-molecular-weight UFH (enoxaparin). Int J Clin Pract 59:1268–1271
Balzarotti M, Fontana F, Marras C et al (2006) In vitro study of low molecular weight UFH effect on cell growth and cell invasion in primary cell cultures of high-grade gliomas. Oncol Res 16:245–250
Sagedal S, Hartmann A, Sundstrom K et al (2001) Anticoagulation intensity sufficient for haemodialysis does not prevent activation of coagulation and platelets. Nephrol Dial Transplant 16:987–993
Farooq V, Hegarty J, Chandrasekar T et al (2004) Serious adverse incidents with the usage of low molecular weight UFHs in patients with chronic kidney disease. Am J Kidney Dis 43:531–537
Gerlach AT, Pickworth KK, Seth SK et al (2000) Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy 20:771–775
Lim W, Dentali F, Eikelboom JW et al (2006) Meta-analysis: low-molecular-weight UFH and bleeding in patients with severe renal insufficiency. Ann Intern Med 144:673–684
Leonard A, Shapiro FL (1975) Subdural hematoma in regularly hemodialyzed patients. Ann Intern Med 82:650–658
Raju S (1987) PTFE grafts for hemodialysis access: techniques for insertion and management of complications. Ann Surg 206:666–673
Schuman E, Quinn S, Standage B et al (1994) Thrombolysis versus thrombectomy for occluded hemodialysis grafts. Am J Surg 167:473–476
Sugarman JR, Frederick PR, Frankenfield DL et al (2003) Developing clinical performance measures based on the Dialysis Outcomes Quality Initiative Clinical Practice Guidelines: process, outcomes, and implications. Am J Kidney Dis 42:806–812
Bessias N, Paraskevas KI, Tziviskou E et al (2009) Vascular access in elderly patients with end-stage renal disease. Int Urol Nephrol 40:1133–1142
Windus DW (1994) The effect of comorbid conditions on hemodialysis access patency. Adv Ren Replace Ther 1:148–154
Kojima F, Uchida K, Ogawa T et al (2008) Plasma levels of fibroblast growth factors-23 and mineral metabolism in diabetic and non-diabetic patients on chronic hemodialysis. Int Urol Nephrol 40:1067–1074
Gelev S, Spasovski G, Dzikova S et al (2008) Vascular calcification and atherosclerosis in hemodialysis patients: what can we learn from the routine clinical practice? Int Urol Nephrol 40:763–770
Lockhart ME, Robbin ML, McNamara MM et al (2004) Association of pelvic arterial calcification with arteriovenous thigh graft failure in haemodialysis patients. Nephrol Dial Transplant 19:2564–2569
Stolic RV, Trajkovic GZ, Peric VM et al (2008) The influence of atherosclerosis and d-dimer concentration in patients with a functioning arteriovenous fistula for maintenance hemodialysis. Int Urol Nephrol 40:503–508
Disclaimers
No financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is a retrospective study which by Israeli law does not require informed consent or Institutional Review Board (IRB)/Ethics Committee approval.
Rights and permissions
About this article
Cite this article
Shavit, L., Lifschitz, M., Lee, S. et al. Use of enoxaparin to diminish the incidence of vascular access stenosis/thrombosis in chronic hemodialysis patients. Int Urol Nephrol 43, 499–505 (2011). https://doi.org/10.1007/s11255-009-9703-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-009-9703-1