Abstract
Municipal authorities around the world have come to recognize the importance of making conservation and restoration a priority. Multiple urban restoration programs now foster insects and other pollinators through planting and sowing flowering plants, many of them within residential areas. But residents are not only walking next to pollinators visiting flowering sidewalk grass verges, they are also walking on top of them, nesting in the cracks and interstices of urban pavements.
Combining morphological and molecular monitoring schemes, we conducted a survey of urban pavements at twelve locations across Berlin and found that pavements can foster a surprising number and quantity of soil dwelling insects—in particular wild bees and wasps. Pavements located within 200 m to an insect-friendly flower garden were covered with significantly more nests of wild bees and solitary wasps, and showed higher species richness of these groups, while the degree of sealed surfaces in the surrounding had no effect per se. This underlines the positive impact that insect-friendly gardens can have for pollinators and other insects, even in highly sealed areas. Also, it shows the potential of cobbled pavements as valuable nesting sites in highly sealed urban areas. We provide a list of 55 species of ground-nesting Hymenoptera found in Berlin pavements, including 28 species of wild bees and 22 apoid wasps. In our study, the molecular approach only detected three Hymenoptera species and did not yield comparable results to classical monitoring. Nonetheless, using eDNA methods might be a promising tool for further studying soil nesting insects in the future, and to gain insights into the web of life in urban pavements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

For once, walk with your head down. Look beneath your steps in this bustling city. Can you see it?
Look between the concrete slabs that define urban areas. Look at the hidden life of this pavement crack. For in the fringes of this human-made world, nature has formed new bonds, emerging in places that had never been intended to harbour the more-than-human. Finding refuge in human-made cracks to escape human-made destruction.
– Susanne Wieland, The Hidden Life of a Pavement Crack, 2022
Introduction
Each year, world cities are expanding and growing in human population (UN-DESA 2019). This results in the landscape conversion of natural habitats into more impervious urban environments, with profound impacts on local biodiversity (McKinney 2002 and 2006; Seto et al. 2012; Li et al. 2022). With more than 50% of the human population globally already living in urban areas, cities are also often the main places where people encounter biodiversity. Experiencing nature can be a source of fascination (Sonti et al. 2020), as well as health and wellbeing (Marselle et al. 2021). Contact with everyday biodiversity experienced in daily routine activities or in nearby parks may be especially beneficial (Hunter et al. 2019), such as encountering street trees (Marselle et al. 2020), garden birds (Cameron 2020), roadside vegetation (Säumel et al. 2016) and possibly also pollinating insects (Klein 2018; Garibaldi et al. 2022).
Although urbanisation poses a major threat to biodiversity (Svenningsen et al. 2022), cities have been described as ‘sanctuaries’ (Lepczyk et al. 2023) and ‘hotspots” (Ives et al. 2016) for specific taxonomic groups and can entail locally very species rich habitats (Turrini and Knop 2015). For example, the city of Berlin (Germany) is home to 213 endangered plant species which are mainly distributed in remnant natural habitats (Planchuelo et al. 2020). In Berlin, urban ecology research began to flourish in the second half of the twentieth century, when West Berlin was surrounded by a wall (Kowarik 2023). Research in Berlin and other cities has shown that urban nature can thrive in unexpected places—on wastelands, graveyards, playgrounds, along streets, and other novel, anthropogenically altered habitats (Sukopp and Weiler 1988). And sometimes, it can thrive directly beneath our feet: in the cracks and interstices of the urban pavement.
Within research on sustainable cities, pavements—apart from its function as a walkway—have mainly been assessed with respect to their sealing and heating properties (Fini et al. 2017). Organisms that inhabit urban pavements must withstand harsh conditions: Pavements can be subject to periodic flooding followed by periodic dry stress (Frazer 2005; Chithra et al. 2015), large temperature fluctuations (Yu and Lu 2014) and periodic or continuous disturbance from trampling, vehicles, and maintenance work (Cervelli et al. 2013; Wheather 2020), and can be characterized by either a lack (Wheather 2020) or excess of nutrients (Del Tredici 2014)—depending on the location of the pavement. Nevertheless, the diversity of plants inhabiting pavements has repeatedly surprised ecologists. Pescott (2016) recorded 183 plant taxa in the pavements of Sheffield (UK), Jasprica and Milovic (2020) described 57 plant species between the cobblestones of Dubrovnik Old City (Croatia), and Bonthoux et al. (2019) found more than 300 plant species in, and next to, the urban pavement of Blois (France), speaking of a “neglected element of urban biodiversity”. There are a number of studies focusing on urban sidewalk vegetation (Scheuermann and Wein 1938; Pescott 2016; Bonthoux et al. 2019; Jasprica and Milovic 2020; Heikkinen et al. 2023) and ‘guerrilla botanists’ have sparked a growing awareness of sidewalk vegetation using the hashtags #morethanweeds (#krautschau in german) as a collective campaign (https://theurbanactivist.com/idea/more-than-weeds-rebel-plants-and-our-obsession-to-control-nature/). Meanwhile, there is little research and no public awareness of urban pavements as potential habitat for wild bees and other insects. In a German standard textbook on urban ecology, Die Ökologie der Großstadtfauna (Klausnitzer 1987), urban pavements are described as a potentially preferred habitat (Vorzugshabitat) of aculeate insects. Yet, almost 40 years later, there is only little research about pavement nesting insects. To our knowledge, apart from the work of Haeseler (1982) who described 22 species of pavement nesting bees and wasps in the city of Oldenburg (Germany), only Noël et al. (2023) have systematically assessed pavements as habitats for ground nesting bees and wasps in Brussels (Belgium). Both studies suggest that urban microclimates beneath pavement tiles create favourable conditions for aculeate insects (i.e. bees and wasps, including ants) in cities, when open, sunlit soil is rare. The stones paving the sidewalks shield the underground, and may accumulate heat, leading to elevated temperatures of the soil below.
Since wild bees and other pollinators depend on the availability of floral resources, adjacent greenspaces and gardens play an important role in providing a wide range of foraging resources and potential nesting sites for urban animals. Such insect-friendly greenspaces have been shown to increase the abundance and species diversity of wild bees and other insects (Delahay et al. 2023). One recent study focusing on urban grassland sites along an urbanisation gradient showed that the presence of endangered bee species was associated with flower abundance – not urbanisation per se (Buchholz et al. 2020). Similarly, Lanner et al. (2020) identified flower abundance in communal gardens as main driver of species richness in wild bees. This implies that enhancing even highly sealed urban areas with insect-friendly flower patches could significantly improve conditions for wild bees. In Berlin, initiatives such as “Treffpunkt Vielfalt” (Stiftung Mensch und Umwelt) or “Pilotprojekt Vielfalt Leben” (Stiftung Naturschutz Berlin) are cooperating with housing associations across Berlin to create high quality urban green spaces with wildlife friendly gardening concepts in residential areas. Areas and structures within the gardens aim to support the habitat needs of local wildlife, such as hedgehogs, lizards, butterflies and bees, for example through piles of dead wood, sand or stones, and a diversity of wildflower plants. These gardens are freely accessible and are used to educate residents about urban biodiversity.
To assess the potential of urban pavements as nesting habitat for ground-nesting insects, we investigated their diversity and abundance at twelve comparable pavements in residential areas distributed among three different location types based on the degree of surface sealing as well as the presence or absence of high-quality foraging resources in the vicinity (‘insect-friendly flower gardens’). Nest counting and classical monitoring was combined with a molecular analysis of nest entrance substrate, as identifying ground-nesting insects that nest beneath the pavement typically involves hours of careful observation and catching them at the right moment ( Haeseler 1982; Dijon et al. 2023; Noël et al. 2023).
The primary goals of our study were (a) to investigate the species composition of pavement-inhabiting arthropods in Berlin, Germany, and (b) to assess the impact of soil sealing and the presence of insect-friendly flower patches near the pavement on the diversity and abundance of ground dwelling insects. In addition (c), we tested the potential use of environmental DNA (eDNA) extracted from soil samples of nest entrances located between pavement tiles to assess the biodiversity of pavement inhabiting insects and discuss whether eDNA could potentially complement or replace classical monitoring techniques.
Materials and Methods
A. Study sites and sampling period
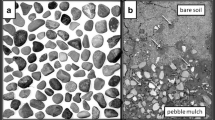
This study was conducted on 12 sampling sites, each of which was a 200 m length of pavement, within the borders of Berlin, Germany (Fig. 1). Each sampling site was composed of larger granite tiles, flanked with smaller stones laid in a mosaic pattern (‘Bernburger Muster’, Fig. 2a), a pattern typical for sidewalks in Berlin. The gaps between the slabs and stones are mostly filled with sand, providing potential nesting habitats for ground dwelling insects (Fig. 2a). Our sites were all situated in residential areas, with frequent, but not excessive pedestrian circulation.
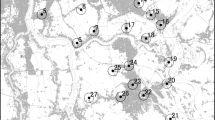
Locations of the sampling sites in Berlin. The three different categories are indicated by circles for locations adjacent to an insect-friendly flower garden, squares for medium sealed locations with no flower garden nearby and triangles for highly sealed locations. Detailed views show the locations of each sampling site including the 200 m perimeter. The colours represent the percentage of soil surface sealing. For additional information on each site, see Online Resource 1, Table S1. Source of map material: Senatsverwaltung für Stadtentwicklung und Wohnen (2016)
We classified each sampling site according to the degree of soil surface sealing and the presence or absence of high-quality, insect-friendly flower garden within a radius of 200 m. We defined two degrees of soil surface sealing: medium sealed (soil surface coverage between 30 and 70%) and highly sealed (soil surface coverage of < 80%). Data of soil surface sealing was retrieved from ’Umweltatlas Berlin’ released by the Berlin Senate (https://www.berlin.de/umweltatlas/boden/versiegelung/2016/karten). Insect-friendly flower gardens were chosen from four different neighbourhood projects and located in comparable residential areas across Berlin. All flower gardens had a wide variety of native wild plants and an ecological mowing concept. Three of the four gardens were freely accessible. One garden was only accessible to residents of the surrounding building. In this case the janitor granted us access so we could inspect the garden. A list with these sites and references to the respective projects is provided as supplementary information in Online Resource 1 (Table S1).
a Aggregation of nest entrances from ground-nesting insects (here: wild bees and wasps) in the sand-filled interstices between stones of a typical urban sidewalk composed of larger slabs and smaller cobble stones (‘Bernburger Muster’, upper part of the picture). Nest entrances can be found in interstices regardless of stone size, but the diggable surface area is much greater between the smaller cobble stones. All transects were chosen along pavements consistently containing both patterns. b Wildflower patch that forms part of a wildlife friendly garden located near one of the transects (Felixstraße). Piles of dead logs and branches, stacks of stones and endemic wildflowers provide potential nesting sites, shelter, and foraging resources for urban wildlife. Pictures: SL (a) and CW (b)
This resulted in three categories of sampling sites: 1) locations of medium soil surface sealing and the presence of an insect-friendly flower garden within 200 m of the transect (medium sealed + flower garden, n = 4); 2) locations of medium sealed soil surface coverage and absence of insect-friendly flower garden (medium sealed locations, n = 4); 3) locations of high soil surface coverage and absence of insect-friendly flower garden (highly sealed locations, n = 4).
Sampling occurred from July 19 to September 14, 2021, and from April 13 to June 14, 2022, during the hours of 9 am to 4 pm on days with a minimum temperature of 18 °C at 10 am, and either clear or partly cloudy skies. All sampling sites were visited once per month during the sampling period, with approximately four weeks between each visit.
B. Monitoring of nest abundance
Nests on each 200 m pavement transect were mapped on a detailed printed map of the location. For this, the pavement was walked slowly in a zigzag path. Ant nests were not included in the analysis, because ant colonies often have several entrances and cannot be attributed to one nest.
C. Classical monitoring techniques for insect identification and classification
Insects were collected along a transect during a period of 45 min using an insect collecting net (30 cm diameter). They were killed on site with ethyl acetate in a killing jar. If target insects were seen at the opening of the nest entrance, the insect net, or a drosophila vessel (50 × 100 mm) was used to cover the entrance in order to catch the animal when it crawled out of the nest. In the first round (July 2021) of sampling, only insects caught at a nest entrance were collected. The method was subsequently adapted to include insects crawling on the pavement or flying within a one metre range (“waist-high”) above the pavement stones, as this space was considered to be part of the pavement habitat. All killed insects were mounted on insect pins and dried before being transferred into an insect collection box. For determination, a Zeiss Stemi DV4 (magnification power 8X to 32X, zoom range 4:1) stereo microscope was used. Insect identification keys are listed in the Supplemental information (see Online Resource 1, Table S2). A sampling permit was granted by the Berlin Senate for Urban Mobility, Transport, Climate and the Environment (Online Resources 3 and 4).
D. Molecular identification of insects
Collection of soil samples for eDNA analysis
At each sampling site, the soil of two transects of 20 m were sampled in 2021. The sampling method was slightly adapted during the season: In the first month of the sampling period, i.e. July 2021 the first nest encountered served as the starting point for the 20 m sampling transect and a soil sample was collected from every visible nest or colony entrance. After completing the first 20 m, the next nest encountered served as the starting point for the second 20 m transect with the same sampling approach. In August and September 2021, only every tenth or twentieth nest (depending on the quantity of nests counted during the mapping conducted prior to sampling), with a maximum of five, were sampled within the sampling site. Soil was collected from nests between pavement tiles with a small spatula (sterilised with ethanol and flame before each sample collection) from the centre of the nests. If possible, the upper part of the inner lining of the nest entrance was also scraped out. The soil was transferred to a clean 1.5 ml or 2 ml Eppendorf tube, labelled, and stored in a cryo-box at ambient temperature. At the end of the sampling day, all samples were stored dry at 4 °C and DNA extraction was done within 14 days of collection. If extraction was not possible within this time frame, samples were stored at -20 °C and defrosted before extraction. All samples were processed within six weeks after collection.
eDNA Extraction
The extraction of DNA from the soil samples was done using the DNeasy Power Soil Pro (Qiagenbiodi, Hilden, Germany) following the manufacturer’s protocol. Samples were homogenised using a Tissuelyzer Retsch MM 400. DNA was eluted from the silica membrane using 50 µl of elution buffer to maximise the DNA concentration. DNA was extracted from soil samples in batches of no more than ten samples at a time. In the first three extraction batches, autoclaved soil samples were used as controls. After extraction, DNA concentration and quality was measured with a NanoDrop 2000 spectrophotometer. Genomic DNA was diluted to 30 ng/µl for further processing. The remaining undiluted samples were stored at -20 °C. For DNA metabarcoding, a 313-bp fragment of the CO1 region was amplified using the primers mICOIintF and jgHCO2198 (Leray et al. 2013) together with Illumina adapter sequences. PCR products were run on a 2% agarose gel to verify the size of the amplicons and exclude contamination. The autoclaved control samples showed no bands. Paired end sequencing (2 × 300 bp, 1 Mio reads) was performed on a MiSeq Illumina system with a MiSeq reagent kit v3 at the Berlin Center for Genomics in Biodiversity Research (BeGenDiv).
E. Data analysis
DNA data processing and analysis
Raw Illumina sequencing files were processed using VSEARCH v. 2.17.1 (Rognes et al. 2016). The pipeline is described in more detail in Sickel et al. (2023) and is available from GitHub (https://github.com/monagrland/MB_Pipeline; v.1.0 of the pipeline was used). Briefly, paired reads were merged, quality filtered, and primer sequences were trimmed. Remaining reads were clustered to obtain amplicon sequence variants (ASVs), which were dereplicated and taxonomically classified as described in Sickel et al. (2023), imported into R v. 4.2.2 (R Core Team 2022) and analysed using phyloseq v. 1.42.0 (McMurdie and Holmes 2013). The data set was subset to include only Eukaryota and Metazoa and the number of taxonomically classified ASVs was assessed for all taxonomic levels, with a focus on insects and other arthropods. We assessed the number of arthropod and hymenopteran ASVs per location type and checked for differences in community composition between the location types.
Statistical analysis
We used R version 4.3.1. to analyse our dataset from the classical monitoring. To test the taxa richness (i.e., variable to explain), regarding the location type category (i.e., biodiversity, medium sealed and highly sealed) as predictor, a Generalized Linear Mixed-Model (GLMM) was performed by selecting Poisson’s error distribution as model family (count data) using the lme4 R package (Bates et al. 2015). The sampling site per collection round and year was set-up as a random effect. The comparison between the location type category in our mixed model was performed using glht function of multcomp R package (Hothorn et al. 2008) with Bonferonni’s correction. Sample-size-based rarefaction and extrapolation sampling curves were calculated to show the taxa richness among the location type category using iNEXT R package (Hsieh et al. 2022). Taxonomic groups that were not assessed systematically (Diptera and Formicidae) were excluded. As the normality of the data is not met, a Kruskal–Wallis test followed by a non-parametric post-hoc pairwise comparison using Dunn’s test (Bonferroni method) was performed on nest counting data.
Figures were generated using ggplot2 R package (Wickham 2016).
Results
A. Diversity and abundance of pavement dwelling bees and wasps: Results from classical vs. molecular assessment
Classical monitoring
Sixty-six species belonging to Apoidae, Vespidae, Ichneumonidae, Diptera and Formicidae were identified over the course of the sampling period and over all sites using classical monitoring techniques (see Online Resource 2). All soil-nesting bee and wasp species are listed in Table 1. Ants and parasitic flies were observed at all sites, but not identified systematically. See Fig. 3 for examples of species observed at the sites.
Bees and wasps inhabiting pavement cracks in Berlin. a Pantaloon bee, Dasypoda hirtipes; b parasitoid bee Sphecodes sp., c bee wolf, Philanthus triangulum digging its characteristic, cone shaped nest entrance; d fly hunting wasp Oxybelus bipunctatus transporting its prey pierced on its abdominal sting; e parasitoid emerald wasp Hedychrum sp. resting on a cobble stone after inspecting several nest entrances of Cerceris arenaria; f nest aggregation of Anthophora plumipes between cobble stones. Pictures: SL (a, b, d, e, f) and CW (c)
Species richness ranged from 5 – 25 at any transect and was highest at site 7 (Arnulfstraße) with 25 species, followed by site 6 (Kniephofstraße) with 16 species, both adjacent to an insect-friendly flower garden. The smallest number of species were found at site 10 (Borussiastraße) and site 5 (Malmöer Straße) with five species each (see Online Resource 1 and 2).
Diversity analysis
Sampling sites adjacent to a flower garden significantly exhibited greater species richness compared to locations in medium sealed (z-value = -2.713; p-value = 0.018) and highly sealed sites (z-value = -2.573; p-value = 0.027). This is illustrated by a curve depicting higher taxa richness in flower garden locations (Fig. 4). Sampling sites considered as medium sealed did not differ significantly from highly sealed sites (z-value = 0.028; p-value = 1.00).
 = flower garden,
= flower garden,
 = medium sealed,
= medium sealed,
 = highly sealed). Lasioglossum sextrigatum and Cerceris rybyensis were also identified in the eDNA analysis
= highly sealed). Lasioglossum sextrigatum and Cerceris rybyensis were also identified in the eDNA analysiseDNA analysis
Based on 1,021,264 reads (15,473.7 ± 6,776.05 reads per sample), 2,536 ASVs were detected with the VSEARCH pipeline. Of these, 1,681 ASVs could be taxonomically classified. The remaining ASVs were of taxa not considered as target in our classical monitoring approach, including 107 insect taxa (i.e. Thysanoptera, n = 9; Orthoptera, n = 1; Coleoptera, n = 8; Ephemeroptera, n = 25; Lepidoptera, n = 6; Diptera, n = 2, Psocoptera, n = 2; Phthiraptera, n = 2; Unclassified Insecta, n = 52), 82 other Arthropod taxa (unclassified, n = 79; Collembola, n = 1, Arachnida, n = 2), and 1,489 other Eukaryota, including fungi, molluscs and amoebae. We detected 17 of these non-target arthropod taxa at sites adjacent to an insect-friendly flower garden, and 18 non-target arthropod taxa at medium and highly sealed sites with no flower garden nearby. The remaining 855 ASVs remained unclassified. The detected species composition was very similar across the location types, regardless of soil sealing intensity. L. sexstrigatum and C. rybyensis were the only bee and wasp species identified by eDNA analysis and detected across all location types. Lasius sp. could be identified to genus level.
B. Abundance of nest entrances at the different location sites
A total of 6,301 nests entrances (see Fig. 5 for examples) were recorded during the sampling period on all sites, 3,049 on insect-friendly sites, 1,798 on medium sealed sites and 1,454 on highly sealed sites. A Kruskal–Wallis Test showed a statistically significant difference (chi-squared = 16.229, df = 2, p-value < 0.001) among the location types. A post-hoc pairwise comparison using Dunn’s test indicates significantly more nests at locations adjacent to an insect-friendly flower garden compared to both medium sealed locations (p = 0.013) and highly sealed locations (p-value < 0.001), while the number of nests between lower-quality medium and highly sealed locations did not differ significantly (p = 0.88) (Fig. 6, left).
Most nest entrances were recorded in June 2022 on insect-friendly flower garden locations (n = 687). The smallest number of nests was recorded in July 2021 on highly sealed locations (n = 126). Over the whole sampling period most nests were found at sites adjacent to an insect-friendly flower garden (n = 3,049), followed by medium sealed (n = 1,798) and highly sealed locations (n = 1,454). Overall, there was a wide variance on the number of nest entrances recorded among the location types and sampling months. The mean number of nests entrances recorded on insect-friendly flower garden locations was 127 (SD = 74.2), 74.9 (SD = 60.4) for medium sealed locations and 60.6 (SD = 56.8) for highly sealed locations (Fig. 6). Pictures: CW (a–f)
Left: Boxplot of number of nest entrances counted on all locations during all sampling rounds sorted by location type: insect-friendly flower garden (green), medium sealed (orange) and highly sealed locations (purple). Asterisks shows significant differences: ** for p-values <0.05, *** for p-values <0.001. Right: Number of nests counted for all location times and sampling rounds. The number of nests counted ranged from n=687 in June 2022 over all insect-friendly flower garden locations to a total of n=126 nests at highly sealed locations in August 2021. Sampling began in July 2021 and continued in spring 2022
Discussion
Our study showed a diverse insect fauna of urban pavements with a total of 66 species from different groups of wild bees, solitary and parasitoid wasps, ants and flies, collected at 12 sites in Berlin (Online Resource 2). We found the highest number and density of pavement nesting insects, as well as the highest diversity of species, on the plots next to insect-friendly flower gardens. Surprisingly, the amount of green cover had no impact on the number of nest entrances. Floral resources serve as a source for nectar and pollen, in addition to hosting insect prey communities, that are susceptible to predation by insect predators found in this study such as Cerceris spp. and other hunting wasp species. These findings underline the importance of greenspace quality over quantity for sustaining insect populations regardless of surface sealing (Turo and Gardiner 2021), while at the meantime also showing the potential of pavements as nesting habitat, if floral resources are abundant. A list of 55 ground-nesting wild bees and wasp (apoid, pompilid and chrysid species), that also includes three additional observations, is provided, and intended as a first repertoire of pavement nesting hymenopteran insects in Berlin. Ants were not systematically assessed throughout the entire sampling period, but were present at all sites, with Lasius niger, Formica cinerea and Tetramorium caespitum being the most common species. Ants could be assessed in a future project, similar to the assessment of pavement-dwelling ants by Dijon et al. (2023) in Brussels. In addition, studying the interactions between parasitoid flies and ground nesting wild bees and wasps could lead to a more detailed understanding of the urban pavement as a miniature ecosystem, with its own trophic network.
Berlin is, as are many other cities, undergoing a rapidly increasing urbanisation process. While many of the pavements are still retained in their original, patchy style, the formerly sand-filled interstices are being replaced by concrete, or larger slabs are installed, reducing the potentially inhabitable area. Urban pavements can be suited to harbour a variety of ground nesting insects, including species of conservation concern (Noël et al. 2023 and this study) and great charismatic appeal, such as the pantaloon bee, Dasypoda hirtipes, that could be well suited for environmental education projects. While there is big support in the population to help wild bees and other insects with “wild bee hotels” and upright nesting structures (MacIvor 2017), finding a nesting site in the bare soil can be difficult due to the urban expansion of impervious surface which closes the access to the soil substrate: Urban areas have repeatedly been shown to disproportionally favour cavity-nesting wild bees over soil nesting bees (Neame et al. 2013; Geslin et al. 2016; Buchholz and Egerer 2020, Banaszak-Cibicka and Dylewski 2021 and references therein).
Around the nesting sites, the landscape matrix can influence floral and prey resources as well as foraging distance (Pardee and Philpott 2014). The integration of evidence-based urban planning and conservation strategies can enhance local and landscape-level pollination ecosystem services. Under the right conditions, cities can sustain numerous floral resources for wild pollinators such as bees and wasps. Urban private and community gardens (e.g. Kaluza et al. 2016), street trees (Somme et al. 2016) and ornamental patches (Daniels 2020), green roofs (Kratschmer et al. 2018), as well as remnant or novel vegetation patches (Hülsmann et al. 2015; Lowenstein et al. 2019) provide pollen and nectar to bees, wasps, and other pollinating insects. Creating insect-friendly plant arrangements, understanding additional habitat needs, and prioritising habitat creation within cities (Schueller et al. 2023) can support insect communities (Pfeiffer et al. 2023)—with urban pavement potentially providing valuable nesting sites for ground-nesting species.
There has recently been a shift from perceiving flora in pavement cracks as pest (e.g. Melander 2009; Fagot et al. 2011) towards appreciation for the diversity (Bonthoux et al. 2019) and ecosystem services (Coombes et al. 2021; Sikorska et al. 2021) provided by spontaneous vegetation in pavement cracks. It is not known whether hymenopterans inhabiting the subterranean part of pavements are also providing ecosystem services, e.g. by increasing permeability of urban soil and thus add to stormwater control and urban microclimate. But looking at the size and amount of their tunnels, this is not unlikely and should be investigated further. The need to integrate biodiversity in urban planning has been recognized by municipalities and city administrations around the globe (Nilon et al. 2017), not only because of their responsibility to protect nature (Oke et al. 2021), but also because urban nature provides a multitude of important ecosystem services, and potentially enhances residents’ health and wellbeing (Nieuwenhuijsen 2021).
To study the species nesting in Berlin pavements, we used classical monitoring techniques and combined it with eDNA analyses. The classical monitoring techniques involved visual surveys, sweep netting, and nest entrance counting of ground-nesting insects in the pavement habitats. We found that classical techniques were effective in detecting ground-nesting apoid insects in all three subsets of pavement plots. In contrast to the findings from Brussels recently reported by Noël et al. (2023), our investigation revealed three times as many identified species. Although the number of sites surveyed was lower in our study, the extended duration of observation increases the detection of additional species (McCabe 2012). Furthermore, the application of a repeated observation protocol at each site, spaced at one-month intervals, further augmented the species detection rate. After analysing the accumulation curves for the three locations over multiple sampling events, the observed taxa richness is, regarding the generated values calculated by Chao's index (Chao et al. 2005), probably underestimated. The actual species richness present in the streets may be more substantial than currently observed, underscoring the potential of an increased sampling effort by both classical, and if improved, also molecular methodologies.
Environmental DNA (eDNA) is a promising tool to complement traditional monitoring and detection methods (Taberlet et al. 2012). Recent studies have assessed different substrates as eDNA sources, including soil, and often report a more comprehensive assessment of species diversity, as well as an increased time- and cost-efficiency (Thomsen and Sigsgaard 2019; van der Heyde et al. 2020; Harper et al. 2021; Roger et al. 2022). Another advantage of eDNA-based species detection is the minimally invasive nature of sampling (Sickel et al. 2023), which is especially relevant for protected species, such as bees. Challenges for species detection via eDNA include, among others, the limited quantity and quality of target DNA (Bruce et al. 2021), and the occurrence of non-target DNA sources such as microorganisms (Ritter et al. 2019).
In our study, the eDNA approach only detected three hymenopteran species, and these were all dominant at the studied sites. These insufficient detection rates demonstrate that the method is still in its infancy. It is likely that the DNA extraction method was suboptimal, and possible that higher detection rates of ground-nesting insects based on soil eDNA could be achieved. For detecting ground-nesting insects based on samples collected from nest entrances, the target DNA exists as extracellular fragments, which may adhere to soil particles (Nagler et al. 2018a, b; Bairoliya et al. 2022). Thus, dissolving extracellular DNA fragments from soil particles via sample incubation with an alkaline buffer and submitting the DNA directly to PCR amplification (Recorbet et al. 1993; Ascher et al. 2009) may improve insect detection rates. Regarding PCR, soil as an eDNA substrate includes DNA traces of a variety of micro- and macro-organisms (Ritter et al. 2019), and the use of universal primers in this study may have been a sub-optimal choice for insect detection. We detected a high number of fungal ASVs, which demonstrates that non-target DNA was co-amplified and consequently, target DNA may have been under-amplified. This has been observed previously (Sickel al. 2023) and may be avoided by choosing more specific primers (Bleidorn and Heinze 2021). However, eDNA is known to exist at low quantity and quality and DNA fragments at nest entrances are additionally subjected to biotic and abiotic DNA degradation processes. Thus, collecting soil from nest mounts may not be the ideal sample approach for the detection of ground-nesting insects, and samples collected from inside the nest, e.g. by carefully extracting material from further down may lead to better results. This however needs to be tested further. The eDNA approach, although of limited success in this study, has great potential for insect species detections (Sickel et al. 2023) and comes with various advantages. Species can be detected via minimally invasive, non-lethal sampling, which can also be performed by volunteers (see Sickel et al. 2023). Taxonomic classification via DNA is observer-independent and backward compatible (Beentjes et al. 2019), and DNA metabarcoding has become a cost- and time-effective detection method.
Conclusion
In conclusion, our pilot study provides insights into the diversity and distribution of ground-nesting insects, in particular wild bees and wasps, in pavement habitats in Berlin. Our findings suggest that classical monitoring techniques, and nest entrance counting, are still highly effective in detecting ground-nesting insects, while further research is needed to refine and optimise the use of eDNA analysis for insect biodiversity monitoring in pavement habitats. We found that the proximity of pavement habitats to an insect-friendly flower garden, rather than the amount of green cover, was a significant factor in promoting the diversity and number of pavement nesting insects. Moderately sealed urban pavements, especially in the vicinity to biodiverse greenspaces, provide important nesting sites for wild bees and wasps and form an important ecosystem for urban insect wildlife. Our results are in line with other studies that show that human settlements can support high biodiversity (Ives et al. 2016, Hall et al. 2017 for pollinators), and a recently launched iNaturalis-project indicates that pavements have become a novel habitat for wild bees and wasps in multiple cities all over the world (https://www.inaturalist.org/projects/the-hidden-life-of-urban-pavements-cracks). A buzzing city is also a more liveable one, and the fluffy pantaloon bee assiduously shovelling the soil between two pavement tiles calls us to reimagine how we impact the world around us: it is making itself a home in the unlikeliest of all habitats—the urban concrete, constructed for human needs only. With two million species at risk of extinction (Hochkirch et al. 2023), it is time we rethink the way we reshape our environments, and include spaces for multispecies coexistence, right next to our doorsteps.
References
Ascher J, Ceccherini MT, Pantani OL, Agnelli A, Borgogni F, Guerri G, Nannipieri P, Pietramellara G (2009) Sequential extraction and genetic fingerprinting of a forest soil metagenome. Appl Soil Ecol 42:176–181. https://doi.org/10.1016/j.apsoil.2009.03.005
Bairoliya S, Xiang JKZ, Cao B (2022) Extracellular DNA in Environmental Samples: Occurrence, extraction, quantification, and impact on microbial biodiversity assessment. Appl Environ Microbiol 88(3):e01845–e01921. https://doi.org/10.1128/aem.01845-21
Banaszak-Cibicka W, Dylewski Ł (2021) Species and functional diversity. A better understanding of the impact of urbanization on bee communities. Sci Total Environ 774:145729
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.48550/arXiv.1406.5823
Beentjes KK, Speksnijder AGCL, Schilthuizen M, Hoogeveen M, Pastoor R, van der Hoorn BB (2019) Increased performance of DNA metabarcoding of macroinvertebrates by taxonomic sorting. PLoS ONE 14(12):e0226527. https://doi.org/10.1371/journal.pone.0226527
Bleidorn C, Henze K (2021) A new primer pair for barcoding of bees (Hymenoptera: Anthophila) without amplifying the orthologous coxA gene of Wolbachia bacteria. BMC Res Notes 14:427. https://doi.org/10.1186/s13104-021-05845-9
Bonthoux S, Voisin L, Bouché-Pillon S, Chollet S (2019) More than weeds: Spontaneous vegetation in streets as a neglected element of urban biodiversity. Landsc Urban Plan 185:163–172. https://doi.org/10.1016/j.landurbplan.2019.02.009
Bruce K, Blackman R, Bourlat SJ, Hellström AM, Bakker J et al (2021) A practical guide to DNA-based methods for biodiversity assessment. Pensoft Advanced Books, Sofia, Bulgaria
Buchholz S, Gathof AK, Grossmann AJ, Kowarik I, Fischer LK (2020) Wild bees in urban grasslands: Urbanisation, functional diversity and species traits. Landsc Urban Plan 196:103731. https://doi.org/10.1016/j.landurbplan.2019.103731
Cameron RWF, Brindley P, Mears M, McEwan K, Ferguson F, Sheffield D, Jorgensen A, Riley J, Goodrick J, Ballard L, Richardson M (2020) Where the wild things are! Do urban green spaces with greater avian biodiversity promote more positive emotions in humans? Urban Ecosystems 23:301–317. https://doi.org/10.1007/s11252-020-00929-z
Cervelli EW, Lundholm JT, Du X (2013) Spontaneous urban vegetation and habitat heterogeneity in Xi’an, China. Landsc Urban Plan 120:25–33. https://doi.org/10.1016/j.landurbplan.2013.08.001
Chao A, Chazdon RL, Colwell RK, Shen T (2005) A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett 8:148–159
Chithra SV, Nair MH, Amarnath A, Anjana NS (2015) Impacts of impervious surfaces on the environment. International Journal of Engineering Science Invention 4(5):27–31
Coombes MA, Viles HA (2021) Integrating nature-based solutions and the conservation of urban built heritage: Challenges, opportunities, and prospects. Urban Forestry & Urban Greening 63:127192. https://doi.org/10.1016/j.ufug.2021.127192
Daniels B, Jedamski J, Ottermanns R, Ross-Nickoll M (2020) A “plan bee” for cities: Pollinator diversity and plant-pollinator interactions in urban green spaces. PLoS ONE 15(7):e0235492. https://doi.org/10.1371/journal.pone.0235492
Del Tredici P (2014) The Flora of the Future. Places Journal. https://doi.org/10.22269/140417. Accessed 31 Oct 2023
Delahay RJ, Sherman D, Soyalan B, Gaston KJ (2023) Biodiversity in residential gardens: a review of the evidence base. Biodivers Conserv 32(13):4155–4179. https://doi.org/10.1007/s10531-023-02694-9
Dijon L, Dekoninck W, Colinet G, Francis F, Noel G (2023) They live under our streets: ant nests (Hymenoptera, Formicidae) in urban pavements. Biodivers Data J 11
Fagot M, De Cauwer B, Beeldens A, Boonen E, Bulcke R, Reheul D (2011) Weed flora in paved areas in relation to environment, pavement characteristics and weed control. Weed Res 51(6):650–660
Fini A, Frangi P, Mori J, Donzelli D, Ferrini F (2017) Nature based solutions to mitigate soil sealing in urban areas: Results from a 4-year study comparing permeable, porous, and impermeable pavements. Environ Res 156:443–454. https://doi.org/10.1016/j.envres.2017.03.032
Frazer L (2005) Paving paradise: the peril of impervious surfaces. Environ Health Perspect 113(7):456–462. https://doi.org/10.1289/ehp.113-a4
Garibaldi LA, Gomez Carella DS, Nabaes Jodar DN, Smith MR, Timberlake TP, Myers SS (2022) Exploring connections between pollinator health and human health. Philos Trans R Soc B 377(1853):20210158. https://doi.org/10.1098/rstb.2021.0158
Geslin B, Le Féon V, Folschweiller M, Flacher F, Carmignac D, Motard E, Perret S, Dajoz I (2016) The proportion of impervious surfaces at the landscape scale structures wild bee assemblages in a densely populated region. Ecol Evol 6:6599–6615. https://doi.org/10.1002/ece3.2374
Haeseler V (1982) Ameisen, Wespen und Bienen als Bewohner gepflasterter Bürgersteige, Parkplätze und Strassen (Hymenoptera: Aculeata). Drosera 82(1):17–32
Hall DM, Camilo GR, Tonietto RK, Ollerton J, Ahrné K, Arduser M, Threlfall CG (2017) The city as a refuge for insect pollinators. Conserv Biol 31:24–29. https://doi.org/10.1111/cobi.12840
Harper LR, Niemiller ML, Benito JB, Paddock LE, Knittle E, Molano-Flores B, Davis MA (2021) BeeDNA: microfluidic environmental DNA metabarcoding as a tool for connecting plant and pollinator communities. BioRxiv 2021.11.11.468290
Heikkinen MK, Iwachido Y, Sun X, Maehara K, Kawata M, Yamamoto S, Sasaki T (2023) Overlooked plant diversity in urban streetscapes in Oulu and Yokohama. Glob Ecol Conserv 46:e02621. https://doi.org/10.1016/j.gecco.2023.e02621
Hochkirch A, Bilz M, Ferreira CC, Danielczak A, Allen D, Nieto A, Zuna-Kratky T (2023) A multi-taxon analysis of European Red Lists reveals major threats to biodiversity. PLoS One 18(11):e0293083. https://doi.org/10.1371/journal.pone.0293083
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Hsieh TC, Ma KH, Chao A (2022) iNEXT: iNterpolation and EXTrapolation for species diversity. Rpackage version 3.0.0. http://chao.stat.nthu.edu.tw/wordpress/software-download/
Hülsmann M, von Wehrden H, Klein AM, Leonhardt SD (2015) Plant diversity and composition compensate for negative effects of urbanization on foraging bumble bees. Apidologie 46:760–770. https://doi.org/10.1007/s13592-015-0366-x
Hunter MR, Gillespie BW, Chen SY-P (2019) Urban nature experiences reduce stress in the context of daily life based on salivary biomarkers. Front Psychol 10:722. https://doi.org/10.3389/fpsyg.2019.00722
Ives CD, Lentini PE, Threlfall CG, Ikin K, Shanahan DF, Garrard GE, Bekessy SA, Fuller RA, Mumaw L, Rayner L, Rowe R, Valentine LE, Kendal D (2016) Cities are hotspots for threatened species. Glob Ecol Biogeogr 25(1):117–126. https://doi.org/10.1111/geb.12404
Jasprica N, Milović M (2020) Flora of the cobbled streets and pavements in the Mediterranean Old City of Dubrovnik during the COVID-19 lockdown. Natura Croatica: Periodicum Musei Historiae Naturalis Croatici 29(1):19–28. https://doi.org/10.20302/NC.2020.29.3
Kaluza BF, Wallace H, Heard TA, Klein AM, Leonhardt SD (2016) Urban gardens promote bee foraging over natural habitats and plantations. Ecol Evol 6(5):1304–1316. https://doi.org/10.1002/ece3.1941
Klausnitzer B (1987) Ökologie der Großstadtfauna. Gustav Fischer Verlag, Stuttgart, New York (ISBM 3-437-30563-8)
Klein AM, Boreux V, Fornoff F, Mupepele AC, Pufal G (2018) Relevance of wild and managed bees for human well-being. Curr Opin Insect Sci 26:82–88. https://doi.org/10.1016/j.cois.2018.02.011
Kowarik I (2023) Urban biodiversity, ecosystems and the city. Insights from 50 years of the Berlin School of urban ecology. Landsc Urban Plan 240:104877. https://www.sciencedirect.com/science/article/pii/S0169204623001962
Kratschmer S, Kriechbaum M, Pachinger B (2018) Buzzing on top: Linking wild bee diversity, abundance and traits with green roof qualities. Urban Ecosyst 21:429–446
Lanner J, Kratschmer S, Petrović B, Gaulhofer F, Meimberg H, Pachinger B (2020) City dwelling wild bees: how communal gardens promote species richness. Urban Ecosyst 23(2):271–288
Lepczyk CA, Aronson MFJ, La Sorte FA (2023) Cities as sanctuaries. Front Ecol Environ 21(5):251–259. https://doi.org/10.1002/fee.2637
Leray M, Yang JY, Meyer CP et al (2013) A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Front Zool 10:1–14. https://doi.org/10.1186/1742-9994-10-34
Li G, Fang C, Li Y, Wang Z, Sun S, He S, Liu X (2022) Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat Comm 13(1):1628. https://doi.org/10.1038/s41467-022-29324-2
Lowenstein DM, Matteson KC, Minor ES (2019) Evaluating the dependence of urban pollinators on ornamental, non-native, and ‘weedy’ floral resources. Urban Ecosyst 22:293–302. https://doi.org/10.1007/s11252-018-0817-z
MacIvor JS (2017) Cavity-nest boxes for solitary bees: a century of design and research. Apidologie 48:311–327. https://doi.org/10.1007/s13592-016-0477-z
Marselle MR, Bowler DE, Watzema J, Eichenberg D, Kirsten T, Bonn A (2020) Urban street tree biodiversity and antidepressant prescriptions. Sci Rep 10:22445. https://www.nature.com/articles/s41598-020-79924-5
Marselle MR, Lindley SJ, Cook PA, Bonn A (2021) Biodiversity and health in the urban environment. Curr Environ Health Rep 8(2):146–156
Mccabe D (2012) Sampling Biological Communities. Nat Educ Knowl 2(11):13
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Cons 127(3):247–260. https://doi.org/10.1016/j.biocon.2005.09.005
McMurdie PJ, Holmes S (2013) phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4):e61217. https://doi.org/10.1371/journal.pone.0061217
Melander B, Holst N, Grundy AC, Kempenaar C, Riemens MM, Verschwele A, Hansson D (2009) Weed occurrence on pavements in five North European towns. Weed Res 49(5):516–525
Nagler M, Podmirseg SM, Griffith GW, Insam H, Ascher-Jenull J (2018a) The use of extracellular DNA as a proxy for specific microbial activity. Appl Microbiol Biotechnol 102:2885–2989. https://doi.org/10.1007/s00253-018-8786-y
Nagler M, Insam H, Pietramellara G, Ascher-Jenull J (2018b) Extracellular DNA in natural environments: features, relevance and applications. Appl Microbiol Biotechnol 102:6343–6356. https://doi.org/10.1007/s00253-018-9120-4
Neame LA, Grisworld T, Elle E (2013) Pollinator guilds respond differently to urban habitat fragmentation in an oak-savanah ecosystem. Insect Conserv Divers 6:57–66
Nieuwenhuijsen MJ (2021) Green infrastructure and health. Annu Rev Public Health 42:317–328
Nilon CH, Aronson MF, Cilliers SS, Dobbs C, Frazee LJ, Goddard MA, Yocom KP (2017) Planning for the future of urban biodiversity: a global review of city-scale initiatives. BioScience 67(4):332–342. https://doi.org/10.1093/biosci/bix012
Noël G, Van Keymeulen V, Barbier Y, Smets S, Van Damme O, Colinet G, Lokatis S, Ruelle J, Francis F (2023) Nest aggregations of wild bees and apoid wasps in urban pavements: A ‘street life’ to be promoted in urban planning. Insect Conserv Divers. https://doi.org/10.1111/icad.12689
Oke C, Bekessy SA, Frantzeskaki N, Bush J, Fitzsimons JA, Garrard GE, Grenfell M, Harrison L, Hartigan M, Callow D, Cotter B, Gawler S (2021) Cities should respond to the biodiversity extinction crisis. Npj Urban Sustain 1:11. https://doi.org/10.1038/s42949-020-00010-w
Pardee GL, Philpott SM (2014) Native plants are the bee’s knees: Local and landscape predictors of bee richness and abundance. Urban Ecosyst 17:641–659. https://doi.org/10.1007/s11252-014-0349-0
Pescott OL (2016) A systematic florula of a disturbed urban habitat: Pavements of Sheffield, England. Biodivers Data J 4(1):e10658. https://doi.org/10.3897/BDJ.4.e10658
Pfeiffer V, Crowder DW, Silbernagel J (2023) Urban development reduces bee abundance and diversity. Urban Ecosyst 26(6):1535–1544. https://doi.org/10.1007/s11252-023-01393-1
Planchuelo G, Kowarik I, von der Lippe M (2020) Endangered plants in novel urban ecosystems are filtered by strategy type and dispersal syndrome, not by spatial dependence on natural remnants. Front Ecol Evol 8:18. https://doi.org/10.3389/fevo.2020.00018
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Recorbet G, Picard C, Normand P, Simonet P (1993) Kinetics of the persistence of chromosomal DNA from genetically engineered Escherichia coli introduced into soil. Appl Environ Microbiol 59(12):4289–4294
Ritter CD, Häggqvist S, Karlsson D, Sääksjärvi IE, Muasya AM, Nilsson RH, Antonelli A (2019) Biodiversity assessments in the 21st century: the potential of insect traps to complement environmental samples for estimating eukaryotic and prokaryotic diversity using high-throughput DNA metabarcoding. Genome 62:147–159. https://doi.org/10.1139/gen-2018-0096
Roger F, Ghanavi HR, Danielsson N, Wahlberg N, Löndahl J, Pettersson LB, Andersson GKS, Boke Olén N, Clough Y (2022) Airborne environmental DNA metabarcoding for the monitoring of terrestrial insects—A proof of concept from the field. Environ DNA 4(4):790–807. https://doi.org/10.1002/edn3.290
Rognes T, Flouri T, Nichols B, Quince C, Mahe F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Säumel I, Weber F, Kowarik I (2016) Toward livable and healthy urban streets: Roadside vegetation provides ecosystem services where people live and move. Environ Sci Policy 62:24–33. https://doi.org/10.1016/j.envsci.2015.11.012
Saure C (2004) Rote Liste und Gesamtartenliste der Bienen und Wespen (Hymenoptera part.) von Berlin mit Angaben zu den Ameisen. In: Der Landesbeauftragte für Naturschutz und Landschaftspflege, Senatsverwaltung für Stadtentwicklung Berlin. (Hrsg.): Rote Listen der gefährdeten Pflanzen und Tiere von Berlin
Scheuermann R, Wein K (1938) Die Gartenunkräuter in der Stadt Nordhausen. Hercynia 1(2):232–264. https://doi.org/10.25673/93534
Schmid-Egger C (2011) Rote Liste und Gesamtartenliste der Wespen Deutschlands. Hymenoptera, Aculeata: Grabwespen (Ampulicidae, Crabronidae, Sphecidae), Wegwespen (Pompilidae), Goldwespen (Chrysididae), Faltenwespen (Vespidae), Spinnenameisen (Mutillidae), Dolchwespen (Scoliidae), Rollwespen (Tiphiidae) und Keulhornwespen (Sapygidae). – In: Binot-Hafke M, Balzer S, Becker N, Gruttke H, Haupt H, Hofbauer N, Ludwig G, Matzke-Hajek G, Strauch M (Red.): Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 3: Wirbellose Tiere (Teil 1). – Münster (Landwirtschaftsverlag). – Naturschutz und Biologische Vielfalt 70(3):419–465
Schueller SK, Li Z, Bliss Z, Roake R, Weiler B (2023) How informed design can make a difference: Supporting insect pollinators in cities. Land. https://doi.org/10.3390/land12071289
Senatsverwaltung für Stadtentwicklung und Wohnen (2016) Berliner Umweltatlas. Versiegelung https://www.berlin.de/umweltatlas/boden/versiegelung/2016/karten
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci USA 109:16083–16088
Sickel W, Kulow J, Krüger L, Dieker P (2023) BEE-quest of the nest: A novel method for eDNA-based, nonlethal detection of cavity-nesting hymenopterans and other arthropods. Environ DNA 5:1163–1176. https://doi.org/10.1002/edn3.490
Sikorska D, Ciężkowski W, Babańczyk P, Chormański J, Sikorski P (2021) Intended wilderness as a Nature-based Solution: Status, identification and management of urban spontaneous vegetation in cities. Urban For Urban Green 62:127155. https://doi.org/10.1016/j.ufug.2021.127155
Somme L, Moquet L, Quinet M, Vanderplanck M, Michez D, Lognay G, Jacquemart AL (2016) Food in a row: urban trees offer valuable floral resources to pollinating insects. Urban Ecosyst 19:1149–1161. https://doi.org/10.1007/s11252-016-0555-z
Sonti NF, Campbell LK, Svendsen ES, Johnson ML, Novem Auyeung DS (2020) Fear and fascination: Use and perceptions of New York City’s forests, wetlands, and landscaped park areas. Urban For Urban Gree 49 https://doi.org/10.1016/j.ufug.2020.126601
Sukopp H, Weiler S (1988) Biotope mapping and nature conservation strategies in urban areas of the Federal Republic of Germany. Landsc Urban Plan 15(1–2):39–58
Svenningsen CS, Bowler DE, Hecker S, Bladt J, Grescho V, van Dam NM, Dauber J, Eichenberg D, Ejrnæs R, Fløjgaard C, Frenzel M, Bonn A (2022) Flying insect biomass is negatively associated with urban cover in surrounding landscapes. Divers Distrib 28:1242–1254. https://doi.org/10.1111/ddi.13532
Taberlet P, Coissac E, Hajibabaei M, Rieseberg LH (2012) Environmental DNA. Mol Ecol 21:1789–1793
Thomsen PF, Sigsgaard EE (2019) Environmental DNA metabarcoding of wild flowers reveals diverse communities of terrestrial arthropods. Ecol Evol 9:1665–1679
Turo KJ, Gardiner MM (2021) Effects of urban greenspace configuration and native vegetation on bee and wasp reproduction. Conserv Biol 35:1755–1765. https://doi.org/10.1002/cobi.13753
Turrini T, Knop E (2015) A landscape ecology approach identifies important drivers of urban biodiversity. Glob Change Biol 21:1652–1667
UN-DESA (United Nations, Department of Economic and Social Affairs, Population Division) (2019) World Urbanization Prospects. World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420). Demographic Research. New York. https://doi.org/10.4054/demres.2005.12.9
van der Heyde M, Bunce M, Wardell-Johnson G, Fernandes K, White NE, Nevill P (2020) Testing multiple substrates for terrestrial biodiversity monitoring using environmental DNA metabarcoding. Mol Ecol Resour 20(3):732–745. https://doi.org/10.1111/1755-0998.13148
Westrich P, Frommer U, Mandery K, Riemann H, Ruhnke H, Saure C, Voith J (2011) Rote Liste und Gesamtartenliste der Bienen (Hymenoptera, Apidae) Deutschlands. 5. Fassung, Stand Februar 2011. Naturschutz und Biologische Vielfalt 70(3):S. 373–416. Bundesamt für Naturschutz
Wheater CP (2020) The invasion of walls, pavements, and building surfaces by organisms. In: Douglas I, Goode D, Houck MC, Wang R (eds) The Routledge handbook of urban ecology. Routledge
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York, USA
Wieland SE (2022) The hidden life of a pavement crack. JAWS: Journal of Arts Writing by Students 8:23–30. https://doi.org/10.1386/jaws_00040_1
Yu B, Lu Q (2014) Estimation of albedo effect in pavement life cycle assessment. J Clean Prod 64:306–309
Acknowledgements
We thank Alexander Fürst von Lieven for guiding our sight to the ecosystem below our feet. We greatly appreciate the assistance of Sophia Kaschper, Sarah Schweier, Corinna Hartling, Waleed Ghilan, Lydia Jentsch and Kathrin Bramke during field work. Our work was kindly supported by Jonathan M. Jeschke (Freie Universität Berlin and IGB) and Frédéric Francis (Université de Liège) and their research groups. We thank Christian Schmid-Egger for the verification and identification of insects. We gratefully acknowledge the support of the German Centre for Integrative Biodiversity Research (iDiv) funded by the German Research Foundation (DFG-FZT 118, 202548816).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SL, CW and MM contributed to the study conception and design. Sampling was performed by CW and SL, and Material preparation and analysis were performed by CW and WS. The first draft of the manuscript was written by CW and SL, GN, AB, WS and MM contributed significantly on previous versions, as well as the final version, of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file5 (MP4 32.0 mb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weber, C., Noël, G., Sickel, W. et al. Urban pavements as a novel habitat for wild bees and other ground-nesting insects. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01569-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01569-3