Abstract
Invasive alien plants (IAPs) are rapidly increasing around the world, mainly due to land transformation, climate change, and urbanisation. Whilst urban areas are highly susceptible to invasion by IAPs, crucial information on the distribution and abundance of woody invaders in small towns is limited. Here, we determined the distribution and abundance of woody IAPs across different suburbs and land use types in 12 small towns in the Eastern Cape province of South Africa. We enumerated 38 427 individual woody IAPs, and their abundance and distribution varied across surveyed towns. Although species such as Melia azedarach, Pinus elliotti, Jacaranda mimosifolia, Eucalyptus camaldulensis, and Ligustrum lucidum were the most distributed, species density varied across towns. More than half of the woody IAPs were identified in affluent (56%) suburbs compared to the townships (23%), Central business district (CBD) (16%), and low-income housing neighbourhoods (5%). There were significant differences in woody IAPs distribution across different land use types, with more than half (54%) found in the residential areas compared to road verges (32%) and public urban green spaces (14%). Some socio-economic and environmental predictors such as town population, mean temperature, unemployment rate, and Gross domestic product (GDP) showed significant and positive correlations with woody IAPs species richness and density. Our findings suggest an uneven spatial distribution and diversity of woody IAPs across the different small towns, suburbs, and land use types in the region. It is recommended that small towns in South Africa need financial and human resources support to effectively manage IAPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent reports by the Intergovernmental Science Policy Platform on Biodiversity and Ecosystem Services (IPBES) indicated that more than 37 000 alien species have been introduced to new habitats globally, mainly through increased human activities such as travel and trade (IPBES 2023). The rate of new introductions remains high, with close to 200 new alien species being introduced annually in different regions and biomes of the world (IPBES 2023). With regards to plant species, approximately 6% of the introduced alien species tend to become invasive (IPBES 2023), usually resulting in severe negative socio-economic and environmental effects once their populations expand over large areas (Rai and Singh 2020; Eschen et al. 2021; IPBES et al. 2023). Impacts of invasive alien plants (IAPs) on ecosystems tend to vary depending on the species and the landscape (Rai and Singh 2020). For example, the introduction of IAPs such as Australian Acacias and Eucalyptus species to South African riparian systems is associated with severe negative effects on biodiversity and water resources with significant economic implications e.g., reduced water supply (Le Maitre et al. 2016; Maitre et al. 2020). Similarly, although the distribution of A. dealbata, A. melanoxylon, and A. longifolia in southern Europe is influenced by factors such as soil pH, frost and precipitation, Acacias are generally associated with negative environmental impacts in Spain and Portugal (Vieites-Blanco and González-Prieto 2020). Negative impacts of IAPs on ecosystems can include displacement of endemic native species, alteration of fire regimes, changes in soil properties, (Richardson et al. 2020) and loss of rangelands, water resources, and agricultural productivity (O’Connor and van Wilgen 2020; Eschen et al. 2021). It is estimated that the global costs of invasive alien species (IAS) exceed US$423 billion per year, with the bulk of the costs being associated with negative impacts on human livelihoods and expenditure to control invasions (IPBES 2023). Zenni et al. (2021) conducted a 50-year global economic cost assessment of IAS and reported that invasion is responsible for approximately US$1.3 trillion in ecosystem damages (based on 2017 US$ value). In Africa alone, it is estimated that invasion by IAS costs the agricultural sector approximately US$ 65 billion annually through weeding labour costs, and loss of crop yields and livestock income (Eschen et al. 2021). Although some IAS have benefits, such as providing food, firewood, and timber in the forestry industry (Shackleton et al. 2020), these benefits rarely outweigh the above-mentioned negative costs (Zenni et al. 2021; Fantle-Lepczyk et al. 2022) and thus significant effort is being put to manage IAS. However, the balance between costs and benefits is dependent on the species and the phase of invasion (Shackleton et al. 2007). With urban areas being hotspots for IAS introductions (Gaertner et al. 2017), many of the above-mentioned invasion effects and monetary costs will be felt in cities, although information on urban invasions remains scant.
More than half of the global human population currently lives in urban areas, and projections suggest that it will grow to 68% by 2050 (Kookana et al. 2020). Gaertner et al. (2016) suggested that increased urban populations, environmental disturbance, and fragmentation in urban areas make cities more susceptible to invasion by IAS. For example, the increased movement of humans across urban areas for tourism purposes and the movement of goods between and across urban ports, rail, and roads will create pathways for IAS introduction (Gaertner et al. 2016; Faulkner et al. 2020). Padayachee et al. (2017) reported that roads and rivers in urban areas act as invasion conduits due to several factors such as disturbance and increased human movement. Recently, Borden and Flory (2021) suggested that urban evolution (population heritable genetic changes in response to urban development and urban anthropogenic activities) of IAS can enhance their establishment and spread, this is likely to increase in future due to global change. Once IAS have arrived in urban areas, they tend to encounter disturbed and fragmented habitats where biotic (soil bacteria, species composition and traits) and abiotic (urban climate) conditions have been altered by human activities, thus promoting their establishment and spread (Kowarik 2010; Gaertner et al. 2016). For example, Boscutti et al. (2022) reported that urban fragmentation and landscape change can facilitate the dispersal and spread of IAPs in cities. Furthermore, Géron et al. (2021) reported that the urban heat-island effect can favour the growth of some alien compared to native Asteraceae species because of their ability to tolerate heat. Using Pieris rapae as an example, Borden and Flory (2021) attributed urban invasion to urban evolutionary adaptation, e.g., P. rapae has developed large wings to facilitate its spread in fragmented urban habitats. The above-mentioned examples suggest that urban areas are highly susceptible to invasion by IAS, yet information regarding the abundance and distribution in urban areas remain limited (Gaertner et al. 2016; Boscutti et al. 2022).
Studies that have examined the distribution and density of IAPs in urban areas have reported that they are widespread (Chance et al. 2016; McLean et al. 2018; Mabusela et al. 2021). For example, a study conducted in five small towns located in the Eastern Cape province of South Africa reported that woody IAPs are widespread but unevenly distributed across different land use types, with high-income suburbs having more IAPs compared to low-income suburbs (Mabusela et al. 2021). The authors suggested that some of the IAPs distribution patterns in urban areas can be linked to introduction pathways, town planning, and colonial legacies (Mabusela et al. 2021). McLean et al. (2018) reported that most of the identified IAPs in Riebeek Kasteel in the Western Cape province of South Africa were in residential gardens compared to industrial areas. Elsewhere, Chance et al. (2016) used remote sensing to show that both Rubus armeniacus and Hedera helix invade roadsides, open areas, and parks in Surrey, Canada, although their abundance decreases with increasing distance from the roads. In the USA, Staudhammer et al. (2015) reported that invasive tree distribution and abundance increased towards the coastal areas of Miami-Dade Metropolitan Area, whereas in San Juan Metropolitan Area, invasive tree abundance was more concentrated inland. Several ecological and socio-economic factors can explain the distribution and abundance of IAPs in urban areas. For example, the introduction of IAPs for agricultural and ornamental purposes could explain why certain species dominate in urban gardens as compared to road verges (Staudhammer et al. 2015). Similarly, benefits provided by some IAPs, such as fruits and shade, could explain why some species dominate certain urban spaces (e.g., residential gardens and parks) as compared to other areas (e.g., road verges). In the South African context, the legacy of colonial era laws that promoted urban greening in some areas compared to other areas could explain the current distribution of IAPs in urban areas (Venter et al. 2020). Urban size, shape, and human population density can also influence the distribution and abundance of IAPs in urban areas (Liu et al. 2005; Ricotta et al. 2017). In China, Liu et al. (2005) reported that highly populated areas tend to have more IAPs, most likely because of increased human movement and subsequent seed dispersal. In Europe, Pyšek (1998) reported that urban settlement size and population are predictors of IAPs abundance and richness. Although several studies have shown positive correlations between socio-economic (e.g., population size) and environmental (e.g., rainfall) variables in predicting urban IAPs distribution and abundance (Appalasamy et al. 2020; Rejmanek 2000; Lubeck et al. 2019), some studies have reported the opposite (Clements and Moore 2003). Clements and Moore (2003) found no correlation between town size and alien species abundance and richness in eight USA cities, although this was not the case for town age. From an urbanisation standpoint, Burton et al. (2005) reported an inverse relationship between alien species diversity and urbanisation. These mixed results point to the need to conduct more studies to understand invasion patterns in urban areas, particularly how environmental and socio-economic factors influence alien species abundance and diversity. Such information is needed to inform effective management and control of alien species in urban areas.
While some IAPs may provide important ecosystem services to some urban dwellers, they also provide a plethora of ecosystem disservices to human wellbeing in general (Potgieter et al. 2020). Within urban areas, IAPs are linked with damage to property (Reis et al. 2008), reduction in native biodiversity (Shochat et al. 2010), human health issues related to allergens (Gaertner et al. 2016), increased fire hazards (Potgieter et al. 2019), and security risks (Potgieter et al. 2019). Using face-to-face interviews, Potgieter et al. (2019) reported that Cape Town (South Africa) urban residents are concerned with ecosystem disservices caused by IAPs, particularly displacement of native species, water losses, health impacts, and increased fire risks. Previous studies in urban areas have reported that IAPs disrupt important ecosystem services such as water infiltration and flood attenuation (Gaertner et al. 2016; Potgieter et al. 2020). In some cases, IAPs change the aesthetics of the urban environments, can clog water canals and streams, and increase soil erosion (Gaertner et al. 2016; Padayachee et al. 2017; Potgieter et al. 2020). Strategies to manage IAPs in urban areas to reduce the above-mentioned ecosystem disservices require information regarding species abundance, distribution and diversity in urban areas. Besides documenting abundance and diversity in urban areas, studies that document environmental and socio-ecological predictors that explain urban IAP occurrence are needed to better understand invasion patterns in urban areas. Such studies need to include small towns since they present a unique set of urban conditions, such as a relatively larger urban-wildland interface and area ratio that favours plant invasion (McLean et al. 2018). The South African National Environmental Management: Biodiversity Act (NEM: BA Act 10 of 2004) Alien and Invasive Species Regulations of 2020 (NEM: BA A&IS regulations) requires all municipalities to develop invasive species control plans, yet most small towns struggle financially and do not have the resources to gather data on identity, distribution, and densities of IAPs (Irlich et al. 2017).
Considering the above, our aim was to determine the distribution and abundance of woody IAPs in 12 small towns in the Eastern Cape province of South Africa. The study further assessed how IAPs differ within and between towns. The three research questions were: (i) what is the distribution and abundance of IAPs within each town? (ii) How does urban land use type (parks, road verges, residential areas) and suburb (affluent, township, CBD, Reconstruction and Development Programme (RDP)) influence the diversity, distribution, and abundance of IAPs? (iii) How does the abundance, richness, and density of IAPs relate to town environmental and socio-economic attributes?
Methods
Study area
This study was conducted in 12 small towns in the Eastern Cape province of South Africa, namely Adelaide, Alexandria, Barkly East, Bedford, Burgersdorp, Cathcart, Kirkwood, Middelburg, Paterson, Somerset East, St Francis Bay, and Willowmore (Fig. 1; Table 1). We defined small towns as urban areas that contain between 1 000 and 20 000 inhabitants according to Statistics South Africa (2016). The small towns were randomly selected from a pool of 25 small towns that were identified from the city population database for 2016 South African Census (City population database 2016). The Eastern Cape province is the second largest province in South Africa, covering approximately 13.8% of the country (Statistics South Africa 2016). It has the third largest population in South Africa (approximately 6.9 million people), and it is divided into six district and 31 local municipalities (Gwedla and Shackleton 2019). The urban population in the province is very diverse, ranging from more than one million inhabitants in Nelson Mandela Bay to just above 1 000 in Hogsback (Statistics South Africa 2016). Most of the people in the province are black Africans (86%) who speak isiXhosa (Statistics South Africa 2016). The province has high poverty levels (61%) and unemployment rates, mostly being triggered by low formal education levels (Statistics South Africa 2016).
Although the province has a great diversity of vegetation types, with eight of the nine South African biomes occurring in the province, the selected small towns occur in the Albany thicket biome (Alexandria, Kirkwood, Paterson, Somerset East), Fynbos biome (St Francis Bay), Succulent karoo biome (Willowmore), Nama-Karoo biome (Burgersdorp, Middelburg), and Grassland biome (Adelaide, Barkly East, Bedford, Cathcart) (Mucina and Rutherford 2006). The Fynbos biome is in the Western Cape and parts of Eastern Cape provinces of South Africa and has a Mediterranean climate (Mucina and Rutherford 2006). The biome is known for high species endemism, mostly dominated by the evergreen sclerophyllous plants (Mucina and Rutherford 2006). The Succulent karoo is in the coastal strip of Northern Cape province and inland into Western Cape province of South Africa. Due to low winter rainfall and extreme summer aridity, vegetation in the succulent karoo biome is predominantly dwarf and succulent shrubs (Mucina and Rutherford 2006). The Nama-Karoo biome is a semi-arid biome located in the central plateau of the western parts of South Africa (Mucina and Rutherford 2006). Most rain in the Nama-Karoo falls in summer and vegetation is dominated by perennial dwarf shrubs and grasses (Mucina and Rutherford 2006). The Grassland biome is in the high central plateau of the country, mostly inland of KwaZulu-Natal and Eastern Cape provinces (Mucina and Rutherford 2006). Extensive grazing, fire, and frost tends to exclude trees, making grasses and forbs the dominant plant growth forms in the biome (Mucina and Rutherford 2006).
The small towns located close to the coast such as Alexandria, Paterson and St Francis Bay have mild temperatures and receive rainfall throughout the year (Table 1), whereas the towns further inland, such as Willowmore, Burgersdorp and Middelburg are associated with warm and dry climates, receiving most of the rain in the austral summer (Mucina and Rutherford 2006). Population sizes in the 12 towns ranged from 4 933 in St Francis Bay to 18 824 in Somerset East (Table 1). Similarly, town size ranged between 5.7 km2 in Alexandria to 72.8 km2 in Somerset East. Like most South African towns, the spatial structure of the sample towns reflects the former racially based apartheid period, with affluent suburbs dominated by large plots and dwellings that were previously reserved for white inhabitants, compared to townships which have small houses and are mostly occupied by black South Africans. The Reconstruction and Development Programme (RDP) neighbourhoods, characterized by rows of government built, uniform small houses on small plots, were introduced post-apartheid to address apartheid social injustices, and these houses are mainly occupied by poor residents (Mabusela et al. 2021). In comparison to the affluent suburbs, both townships and RDP suburbs are associated with high poverty levels and limited basic municipal services (Mabusela et al. 2021).
Data collection
We conducted a street-based road survey of all public roads in the 12 small towns between October and December 2020. The street-based road surveys were conducted using a moving vehicle with two field assistants at the back of the vehicle observing each side of the road, as described by McLean et al. (2018) and Mabusela et al. (2021). Henderson and Wilson (2017) suggested that street-based road surveys are effective at identifying visible plant growth forms like woody trees and shrubs, which justifies the use of the method in this study.
Within each small town we used recent Google Earth images to categorise the land uses alongside each road as residential, road verges, or public urban green spaces (PUGS), and four suburbs, namely affluent, township, Central business district (CBD), RDP. Public urban green space included areas such as parks, public open spaces, schools, churches, cemeteries, and servitudes (as suggested by Cilliers et al. 2012). The latest aerial images of each study town were obtained from Google Earth and digitised before the classification of the different land uses was done. Suburb delineation on Google Earth was based on housing size and density as well as land parcel. The delineation of land use type within each town was based on land cover data obtained from the Department of Environmental Affairs website (https://egis.environment.gov.za/gis_data_downloads). Within each small town, land use, suburb, and all the publicly accessible streets were identified and surveyed. By driving along each street slowly, all woody IAPs that were visible on each side of the road were counted, identified and recorded. The street-based road survey allowed all woody IAPs along the road verge and immediate surrounding to be detected and identified (Baard and Kraaij 2019). If the woody IAPs was a continuous hedge it was counted as one unit, and if there was a break in between the hedges, these were counted separately. An IAPs photo catalogue was used to help with the plant identification, and records from previous studies by Kuruneri-Chitepo and Shackleton (2011) and Shackleton and Shackleton (2016) were used to create the species photo catalogue. We acknowledge that other species identification methods like iNaturalist could have been useful, however, the creation of our own species identification catalogue was preferred since it is based by plant taxonomic observation that were already published in above-mentioned studies. Additionally, iNaturalist records for small rural towns and poor suburbs are generally low. For each identified IAP observed, the following information was captured, plant name, suburb and land use, as well as National Environmental Management: Biodiversity Act (NEM: BA Act 10 of 2004) Alien and Invasive Species Regulations of 2020 (NEM: BA A&IS regulations) (available on https://www.dffe.gov.za/legislation/actsregulations). We acknowledge that street-based road surveys have their own biases e.g., they fail to differentiate planted from self-seeded wild species and the possibility of underestimating the counts in areas with high walls, fences or hedges that obscure the view. However, the method was chosen because it is reliable, produces reasonably accurate data and it is fast, allowing greater road distance to be covered within each town (Marco et al. 2010; Rejmánek et al. 2016). Similarly, the differentiation of the identified woody species based on cultivation status as suggested by Gildenhuys et al. (2024) could have provided robust data, however this was not done due to the limitations linked to street-based road surveys. Despite these methodological limitations, the value of our study is in providing useful insights on the relative distribution and abundance of woody IAPs in small towns.
Data analysis
Invasive alien plant species counts were used to calculate species diversity indices per town, namely species richness, Shannon-Wiener, Simpson’s diversity index, and species evenness. Species density was calculated using the number of enumerated woody IAPs divided by the area of a town, suburb, or land use type. The counts and proportional distribution of woody IAPs per suburb and land use type were tested within each town using a Chi-squared test. Principal Component Analysis (PCA) was used to determine the variation of woody IAPs across suburbs (affluent, township, CBD, and RDP) and land use types (residential, road verge, and PUGS). Multiple regression analysis was conducted to test for relationships between environmental and socio-demographics indicators on the abundance, species richness, and density of woody IAPs. The environmental and socio-economic indicators used were town size, area, population, mean annual rainfall, unemployment rate, education, GDP-R (GDP by region) and altitude (Table S1). Data for these indicators was obtained for each town from the city population database from Statistics South Africa (2016). We acknowledge that the 2022 South African statistical data is now available, however we used the 2016 data since it was the one available when the study commenced in 2020. These indicators were used because previous studies have shown that the distribution, abundance, and diversity of IAPs may be influenced these environmental and socio-demographic factors (Appalasamy et al. 2020; Fuentes et al. 2015; McKinney 2004). All statistical analyses were conducted using the STATISTICA version 13 (TIBCO Software Inc 2020).
Results
Woody IAP species composition and diversity across towns
In total 38 427 individual woody IAPs were enumerated from 56 different species across the sampled towns (Table 2 and S2). Middleburg had the highest number of woody IAPs with 5 573 individuals, which was five times more than Paterson which had the least (947 individuals) (Table 2). Melia azedarach was the most common IAP species with an average of 365 ± 105 individuals per town (Table S2). Other species in the top five were Pinus elliotti (338 + 58), Jacaranda mimosifolia (303 ± 122), E. camaldulensis (290 ± 77) and L. lucidum (179 ± 38) (Table S2). Five species P. patula, Spathodea campanulate, Ardisia crenata, Parkinsonia aculeata and Leucaena leucocephala had less than ten individuals across the 12 towns (Table S2). The density of woody IAPs in Kirkwood (445 plants per km2) was more than seven times greater than in Somerset East and Cathcart with 56 IAPs per km2 (Table 2).
Species richness of woody IAPs varied across all the towns, ranging from 43 in Somerset East to 21 in St. Francis Bay (Table 2). Middleburg which recorded the highest number of woody IAP individuals had a species richness of 40, whereas Paterson which had the lowest abundance had a species richness of 24 (Table 2). Both Shannon-Wiener and Simpson’s index of diversity were high in Burgersdorp (3.04 and 0.94, respectively) and low in St Francis Bay (1.91 and 0.83, respectively) (Table 2). In contrast, St Francis Bay had the highest species evenness of 0.27, compared to Bedford and Somerset East which recorded the lowest species evenness of 0.23 (Table 2).
Woody IAP species abundance per suburb and land use type
Overall, more than half (56%) of the IAPs were identified in the affluent suburbs with 23% in the townships, 16% in the CBD and 5% in the RDP areas (Tables 3 and 4). These findings were more visible in St Francis Bay and Cathcart, were the number of woody IAPs was more than five times greater in the affluent suburb compared to other areas. In Barkley East, Middleburg, St Francis Bay, and Willowmore, the number of woody IAPs was 20 times higher in the affluent suburbs than the RDP areas. Across all towns, there was a significant (χ2 = 1134.4; p < 0.05) difference in the proportion of woody IAPs per town area. However, the proportional size of the different suburbs is not equal (Table 4). Taking size into account, results shows that the affluent and CBD areas have a disproportionately high occurrence of woody IAPs, whilst the RDP and township have lower than expected from a random distribution.
More than half (54%) of the woody IAPs were counted in residential areas, followed by road verges (32%) and PUGS (14%) (Tables 3 and 4). These results were more visible in St Francis Bay and Adelaide where the number of woody IAPs was double in the residential areas compared to road verges. For Kirkwood, Paterson and Adelaide the number of woody IAPs was six times higher in the residential areas than in PUGS. There were significant (χ2 = 850.0; p < 0.05) differences on woody IAPs distribution across the different land use types. However, although the residential areas had the highest proportion (68%) of woody IAPs relative to land area contribution, the ratio was higher in road verges (ratio = 1.52) and PUGS (ratio = 1.27) than residential areas (ratio = 0.79) (Table 4).
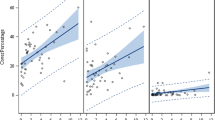
The results from PCA show that Salix babylonica, P. canariensis, L. lucidum and Celtis australis were more abundant in the affluent suburbs (Fig. 2A). In contrast, M. azedarach, A. mearnsii, Caesalpinia decapetala and Schinus molle were more abundant in the townships, whereas species such as Syzygium paniculatum, Prunus serotina, A. saligna, A. melanoxylon and Hakea sericea commonly occurred in the CBD (Fig. 2A). The RDP areas were associated with species such as Callistemon viminalis, S. mauritianum, A. crenata, and Prosopis velutina. Generally, most of the species were found in both affluent and township suburbs (Fig. 2A). With regards to land use type, PUGS were associated mostly with species such as A. crenata, Solanum mauritianum, Bauhinia purpurea, and Triplaris americana, whereas road verges were characterised by P. patula, P. canariensis, and Spartium junceum (Fig. 2B). Generally, the residential areas were associated with more IAPs than road verges and PUGS, but species such as S. babylonica, Grevillea robusta, and Morus alba were found across the residential and road verge land use types (Fig. 2B).
Principal Component Analysis (PCA) of the distribution of woody invasive alien plant species across (A) suburbs and (B) land uses. Plant names are the first letter of the genus and first three letters of the specific epithet. Full names of the plant names are provided in Table S1
Relationship between species and socio-economic and environmental attributes
Multiple regression analysis between woody IAP species abundance and socio-demographic and environmental variables showed no significant (F = 9.93, p > 0.05, R2 = 0.890) relationships (Table 5). In contrast, a significantly (F = 12,991, p < 0.01, R2 = 0.999) positive relationship was shown between woody IAPs richness and the predictor variables collectively (Table 5). For the species richness, eight of the ten indicator variables namely, mean annual rainfall, town size, population density, altitude, population, unemployment rate, tertiary education, and secondary education all showed a significant (p < 0.05) relationship (Table 5). However, of the above-mentioned predictor variables, mean annual rainfall, altitude, and secondary education showed a negative regression coefficient, meaning IAP species richness increases as the value of these predictor variables decreases. The remainder of the above-mentioned predictor variables showed a positive regression coefficient (Table 5). Only mean temperature and GDP-R showed no significant (p > 0.05) relationship with the IAP species richness. Collectively, there was a significant (F = 3,009,797, p < 0.001, R2 = 0.999) relationship between species density and the socio-demographic as well as environmental variables (Table 5). Except for town size, population density, and tertiary education, all other predictor variables showed significantly (p < 0.05) positive relationships, meaning, species density increased as these predictor variables increased (Table 5).
Discussion
Results of this street-based road survey show that the abundance and distribution of woody IAPs was not uniform across the surveyed small towns, with some towns such as Middelburg, St. Francis Bay, Burgersdorp, and Somerset East having a high species abundance and diversity compared to other towns such, as Cathcart and Paterson. Similarly, species density varied across the towns, an indication that town size might not have an influence on woody IAPs abundance. Our results contradict previous results that have reported a strong positive correlation between human population density and IAP abundance and diversity (Liu et al. 2005; Aronson et al. 2014; Ariori et al. 2017; McLean et al. 2017). For example, McLean et al. (2017) reported that bigger towns, particularly those with large affluent areas tend to have a higher number of IAPs, an observation that was only noted in St Francis Bay. However, it is important to note that the above-mentioned study surveyed all IAPs compared to our study that surveyed woody IAPs only. In contrast, our results concur with studies that have reported uneven distribution of IAPs across and within small towns (Mabusela et al. 2021). Although not assessed in this study, a potential explanation for the observed uneven distribution could be environmental (climatic adaptation) and human-driven (human movement) factors (Gaertner et al. 2017). For example, different urban climatic conditions could favour proliferation of some IAPs compared to others. For example, Mabusela et al. (2021) noted that higher densities of IAPs were observed in coastal towns as compared to inland towns of Eastern Cape province, South Africa, however, this was not the case in our study.
Although most woody IAPs were noted in the affluent suburbs, the proportional distribution relative to suburb size revealed that the CBD was similar to the affluent areas, both of which were markedly higher than the poorer townships and RDP areas. Indeed, previous studies have shown that IAPs tend to be distributed differently across different residential suburbs, although such distributions could be species specific (Mabusela et al. 2021). The above-mentioned study noted that some species e.g., C. laevigatum and M. azedarach were most common in townships, whereas some like Acacia and Eucalyptus species were more concentrated in affluent suburbs. In our study we noted similar patterns of different species assemblages in different suburbs, such as S. babylonica, P. canariensis, and L. lucidum concentrated more in the affluent suburbs as compared to A. saligna, A. melanoxylon and H. sericea which dominated the CBD, whilst C. viminalis, S. mauritianum, A. crenata, and P. velutina commonly appeared in RDP suburbs. However, it is important to note that these patterns are not universal across all sampled small towns. In the South African context, such patterns could be linked to colonial history and apartheid policies that influenced the abundance, distribution, species and quality of urban green infrastructure generally (Gwedla and Shackleton 2017; Venter et al. 2020; Shackleton and Gwedla 2021). Indeed, recent reports have acknowledged that South Africa’s colonial history could explain some of the current invasion distribution patterns, particularly for plant species that were introduced in urban areas for ornamental purposes or for specific cultural and personal benefits (Richardson et al. 2020). For example, preferred ecosystem benefits such as shade, wind breaks, and hedges could explain why some species are preferred in some suburbs and land use types as compared to others. Apart from that, suburb age could explain some of our results, particularly the high proportional distribution of woody IAPs in affluent and the CBD compared to townships and RDP areas. For example, in most South African towns the CBD tends to be older than RDP areas, since the CBD is the nerve centre of urban development and growth (Dauskardt 1993; Das 2022), which could subsequently be associated with IAPs introduction by European settlers (Richardson et al. 2020). Additionally, the CBD is the urban node of transportation, human activities, and housing development, which are likely to increase IAPs introductions in the CBD compared to the outskirts where townships and RDP areas are located. There is a need for more studies in urban areas to understand IAP distribution patterns in different suburbs based on ecosystem services provided, cultural and personal preferences.
Although the proportion of woody IAPs was greatest in the residential areas, when proportional area of the different land uses was considered, it was revealed that road verges have the highest per unit area. Indeed, road verges are regarded as corridors of IAPs due to increased disturbance caused by mainly by construction and traffic movement (Son et al. 2024). Štajerová et al. (2017) reported that verges such as road margins, railway lines, and public areas tend to have high richness of invasive species, however this decreased with increasing distance from the city centre. Our results show that IAPs in small towns are not distributed uniformly. This could be explained by various factors such as human mediated introduction, disturbance, and municipal management strategies. For example, Potgieter et al. (2020) noted that increased human movement in urban public spaces, coupled with high disturbance, particularly in small towns where management of public green spaces is limited, could explain the occurrence and dominance of IAPs in public areas. Mabusela et al. (2021) noted that the inability of South African small towns to manage PUGS could explain the high abundance of IAPs in these areas. Small municipalities in South Africa do struggle with financial and human resources, and in such contexts the control and management of IAPs in public spaces is not a priority (McConnachie et al. 2008; Ruwanza and Shackleton 2016). There is a need to equip small town municipalities with the necessary information and resources to manage IAPs in their jurisdiction, or for higher tiers of government to take over the responsibility (Ruwanza and Shackleton 2016; McLean et al. 2017).
The most abundant woody IAPs were M. azedarach, P. elliotti, J. mimosifolia, E. camaldulensis and L. lucidum, which were dominant in almost all towns except in St Francis Bay where counts were below 150 individuals except for L. lucidum. Although we did not interrogate reasons why these particular woody IAPs dominate, their occurrence could possibly be linked to two factors, namely the ecosystem services that they provide to urban communities and species-specific traits that facilitate their proliferation. For example, M. azedarach is a fast-growing tree that dominates, and it is preferred for its aesthetic value and medicinal properties (Khan et al. 2011), yet its ability to produce numerous, bird dispersed seeds and reproduce vegetatively explain its proliferation in urban areas (Bhatt et al. 2021). On the other hand, Hirsch et al. (2020) reported that E. camaldulensis is widely used for shade and windbreaks; however, it is regarded as a conflict species (species with both costs and benefits) which makes management in urban areas difficult as some urban residents favour it and oppose management actions to remove it. The high occurrence of J. mimosifolia in South African towns is linked to deliberate introductions by Europeans as street trees for the shade and beautiful flowers (Newete et al. 2022). Past introductions of J. mimosifolia were viewed as a symbol of modernity and beautification of urban areas (Newete et al. 2022). Ligustrum lucidum is a common hedge plant and is also dispersed by birds (Mabusela et al. 2021). However, it is important to note that some hedge species (e.g. Ligustrum species) could have been under sampled due to limitations associated with the street-based road survey, therefore, their abundance and distribution in urban areas could be higher than reflected by our results.
The multiple regression analysis showed different relationships with socio-economic and environmental variables, depending on which measure of woody IAPs was assessed. Spatial variability in the relationship between IAPs and socio-economic as well as environmental predictors has been reported in the past (Reynolds et al. 2020). However, a closer examination of the data shows that only three indicators, namely town population, temperature, and unemployment were significantly and positively correlated with woody IAP species richness and density. Indeed, there is evidence that densely populated urban areas tend to have more IAPs, possibly linked to increased seed dispersal caused by increased human movement (Liu et al. 2005). Similarly, temperature variabilities have been shown to influence IAPs. For example, Mabusela et al. (2021) observed more IAPs in temperate coastal towns, compared to ones in drier and climatically harsher regions. Besides temperature influencing plant establishment and growth, it can also influence soil nutrient availability and uptake by IAPs (Skálová et al. 2015). Although a positive relationship was found between GDP and all measured IAP variables, it was only significant for species density. Previous studies have reported positive relationships between GDP and invasions (Sharma et al. 2010). However, this is more evident at global and national scales than at town scales, given evidence that developed nations with high GDP and increased movement of goods tend to receive more IAPs than less developed countries (Levine and D’Antonio 2003; Sharma et al. 2010). Perhaps at a town scale, a positive relationship between GDP and IAPs density speaks to the financial ability of a municipal authority to manage IAPs. If this proposition is correct, then poorer municipalities with low financial resources (e.g., Paterson) should be assisted by national government when it comes to management of IAPs.
In conclusion, despite the limitations associated with street-based road survey, our results provide insight on the abundance and distribution of woody IAPs that were detected in Eastern Cape small towns. Our results shown an uneven spatial distribution and diversity of woody IAPs across different towns, suburbs, and land use types in 12 Eastern Cape small towns. We have provided evidence that some towns have a high abundance, diversity, and densities of IAPs whilst others do not. Nevertheless, there were at least 21 different woody IAPs species in every town and as many as 43. We found evidence that some socio-economic and environmental predictors such as town population, temperature, unemployment, and GDP show significant and positive correlation with IAP species richness and density, but not abundance. Based on these results we recommend the following, firstly, municipalities should prioritise the problem of IAPs in urban areas. Our results reaffirm the importance of monitoring woody IAPs in urban areas and such monitoring information can be used to develop effective methods to manage invasives in urban areas. Secondly, human and financial resources should be allocated to manage IAPs in suburbs (affluent) and land use (residential) areas that have the most species. Thirdly, municipalities should develop control plans that aim to manage IAPs in their jurisdiction. Although this is a national legislative requirement in South Africa, there is evidence that small town municipalities are struggling to develop control plans, thus the need for national government support (Zengeya and Wilson 2020). Lastly, is the development and distribution of appropriate and local language information and awareness materials on IAPs to be available in public green spaces and facilities (like municipal offices, schools, libraries, clinics).
Data availability
The data that support the findings of this study are available on request from the corresponding author S.R.
References
Appalasamy M, Ramdhania S, Sershenc B (2020) Aliens in the city: towards identifying non-indigenous floristic hotspots within an urban matrix. Flora 269:151631
Ariori C, Lammens-Aiello ME, Silander JA (2017) Plant invasion along an urban-to-rural gradient in northeast Connecticut. J Urban Ecol 3:1–13
Aronson MF, La Sorte FA, Nilon CH, Katti M, Goddard MA, Lepczyk CA, Warren PS, Williams NS, Cilliers S, Clarkson B, Dobbs C, Dolan R, Hedblom M, Klotz S, Kooijmans JL, Kuhn I, Macgregor-Fors I, McDonnell M, Mortberg U, Pyšek P, Siebert S, Sushinsky J, Werner P, Winter M (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Pro Royal Soc B: Biol Sci 281:20133330
Baard JA, Kraaij T (2019) Use of a rapid roadside survey to detect potentially invasive plant species along the Garden Route, South Africa. Koedoe 61(1)
Bhatt A, Gairola S, Govender Y, de Souza–Filho M PR (2021) The invasive Melia azedarach in Durban (South Africa): impacts on tree community structure. Folia Geobot 56:139–147
Borden JB, Flory SL (2021) Urban evolution of invasive species. Front Ecol Environ 19(3):184–191
Boscutti F, Lami F, Pellegrini E, Buccheri M, Busato F, Martini F, Sibella R, Sigura M, Marini L (2022) Urban sprawl facilitates invasions of exotic plants across multiple spatial scales. Biol Invasions 24:1497–1510
Burton ML, Samuelson LJ, Pan S (2005) Riparian plant diversity and forest structure along an urban-rural gradient. Urban Ecosyst 8:93–106
Chance CM, Coops NC, Plowright AA, Tooke TR, Christen A, Aven N (2016) Invasive shrub mapping in an urban environment from Hyperspectral and LiDAR-Derived attributes. Front Plant Sci 7:1528
Cilliers SS, Cilliers J, Lubbe R, Siebert S (2012) Ecosystem services of urban green spaces in African countries-perspectives and challenges. Urban Ecosyst 16:681–702
City Populations Database (2016) South Africa: Eastern Cape urban areas and places. http://www.citypopulation.de/php/southafrica-easterncape.php. Accessed 14 June 2020
Clements DR, Moore AD (2003) Patterns of species richness in eight North-eastern United States Cities. Urban Habitats 1(1), ISSN1541- 7115. http://www.urbanhabitats.org. Accessed 23 July 2021
Das DK (2022) A perspective for transforming central areas of South African cities into great place. Int J Urban Sustain Dev 14:441–459
Dauskardt RPA (1913) Reconstructing South African cities: contemporary strategies and processes in the urban core. GeoJournal 30:9–20
Department of Environmental Affairs, DEA National Landcover (TIFF) (2015) 2015 [Raster] 2015. Available from the Biodiversity GIS website, https://egis.environment.gov.za/gis_data_downloads. Accessed on 20 September 2021
Eschen R, Beale T, Bonnin JM, Constantine KL, Duah S, Finch EA, Makale F, Nunda W, Ogunmodede A, Pratt CF, Thompson E, Williams F, Witt A, Taylor B (2021) Towards estimating the economic cost of invasive alien species to African crop and livestock production. CABI Agric Biosci 2:1–18
Fantle-Lepczyk JE, Haubrock PJ, Kramer AM, Cuthbert RN, Turbelin AJ, Crystal-Ornelas R, Diagne C, Courchamp F (2022) Economic costs of biological invasions in the United States. Sci Total Environ 806:151318
Faulkner KT, Hulme PE, Pagad S, Wilson JRU, Robertson MP (2020) Classifying the introduction pathways of alien species: are we moving in the right direction? In: Wilson JR, Bacher S, Daehler CC, Groom QJ, Kumschick S, Lockwood JL, Robinson TB, Zengeya TA, Richardson DM (eds) Frameworks used in Invasion Science. NeoBiota 62:143–159
Fuentes N, Saldaña A, Kühn I, Klotz S (2015) Climatic and socio-economic factors determine the level of invasion by alien plants in Chile. Plant Ecol Divers 8:371–377
Gaertner M, Larson BMH, Irlich UM, Holmes PM, Stafford L, van Wilgen BW, Richardson DM (2016) Managing invasive species in cities: a framework from Cape Town, South Africa. Landsc Urban Plan 151:1–9
Gaertner M, Wilson JRU, Cadotte MW, Maclvor J, Zenni RD, Richardson DM (2017) Alien plant species in urban environments: patterns, processes, impacts and challenges. Biol Invasions 19:3461–3469
Géron C, Lembrechts JJ, Nijs I, Monty A (2021) Shielded environments reduce stress in alien Asteraceae species during hot and dry summers along urban-to-rural gradients. Ecol Evol 11:10613–10626
Gildenhuys CP, Potgieter LJ, Richardson DM (2024) The genus Quercus (Fagaceae) in South Africa: introduction history, current status, and invasion ecology. S Afr J Bot 167:150–165
Gwedla N, Shackleton CM (2017) Population size and development history determine street redistribution and composition within and between Eastern Cape towns, South Africa. Urban Urban Green 25:11–18
Gwedla N, Shackleton CM (2019) Perceptions and preferences for urban trees across multiple socio-economic contexts in the Eastern Cape, South Africa. Landsc Urban Plan 189:225–234
Henderson L, Wilson JRU (2017) Changes in the composition and distribution of alien plants in South Africa: an update from the southern African plant invaders Atlas (SAPIA)’. Bothalia 47(2):a2172
Hirsch H, Allsopp MH, Canavan S, Cheek M, Geerts S, Geldenhuys CJ, Harding G, Hurley BP, Jones W, Keet J-H, Klein H, Ruwanza S, van Wilgen BW, Wingfield MJ, Richardson DM (2020) Eucalyptus camaldulensis in South Africa - past, present, future. Trans R Soc S Afr 75:1–22
IPBES (2023) In: Roy HE, Pauchard A, Stoett P, Renard Truong T, Bacher S, Galil BS, Hulme PE, Ikeda T, Sankaran KV, McGeoch MA, Meyerson LA, Nuñez MA, Ordonez A, Rahlao SJ, Schwindt E, Seebens H, Sheppard AW, Vandvik V (eds) Summary for policymakers of the thematic assessment report on invasive alien species and their control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES secretariat, Bonn, Germany
Irlich UM, Potgieter LJ, Stafford L, Gaertner M (2017) Recommendations for municipalities to become compliant with national legislation on biological. Bothalia 47:1–11
Khan AV, Ahmed QU, Mir MR, Shukla I, Khan AA (2011) Antibacterial efficacy of the seed extracts of Melia azedarach against some hospital isolated human pathogenic bacterial strains. Asian Pac J Trop Biomed 1(6):452–455
Kookana RS, Drechsel P, Jamwal P, Vanderzalm J (2020) Urbanisation and emerging economies: issues and potential solutions for water and food security. Sci Total Environ 732:139057
Kowarik I (2010) Novel urban ecosystems, biodiversity, and conservation. Environ Pollut 159:1974–1983
Kuruneri-Chitepo C, Shackleton CM (2011) The distribution, abundance and composition of street trees in selected towns of the Eastern Cape, South Africa. Urban Urban Green 10:247–254
Le Maitre DC, Forsyth GG, Dzikiti S, Gush MB (2016) Estimates of the impacts of invasive alien plants on water flows in South Africa. Water SA 42:659–672
Le Maitre DC, van Wilgen BW, Gelderblom CM, Bailey C, Chapman RA, Nel JA (2020) Invasive alien trees and water resources in South Africa: case studies of the costs and benefits of management. Ecol Manag 160:143–159
Levine JM, D’Antonio CA (2003) Forecasting biological invasions with increasing international trade. Conserv Biol 17(1):322–326
Liu J, Liang S, Liu F, Wang R, Dong M (2005) Invasive alien plant species in China: regional distribution patterns. Divers Distrib 11:341–347
Lubeck AA, Metcalf AL, Beckman CL, Yung L, Angle JW (2019) Collective factors drive individual invasive species control behaviours evidence from private lands in Montana, USA. Landsca Ecol 28:1203–1212
Mabusela A, Shackleton CM, Gwedla N (2021) The distribution of selected invasive alien species in small towns in the Eastern Cape, South Africa. S Afr J Bot 141:290–295
Marco A, Lavergne S, Thierry D, Bertaudière-Montes V (2010) From the backyard to the backcountry: how ecological and biological traits explain the escape of garden plants into Mediterranean old fields. Biol Invasions 12:761–779
McConnachie MM, Shackleton CM, McGregor G (2008) Extent of public green space and alien species in ten small towns in the thicket biome, South Africa. Urban Urban Green 7:1–13
McKinney ML (2004) Citizens as Propagules for exotic plants: measurement and management implications. Weed Technol 18:1480–1483
McLean P, Wilson JR, Gaertner M, Kritzinger-Klopper S, Richardson DM (2018) The distribution and status of alien plants in a small South African town. S Afr J Bot 117:71–78
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland. Strelitizai 19. South African National Biodiversity Institute, Pretoria
Newete SW, Abutaleb K, Byrne MJ (2022) Mapping the distribution and tree canopy cover of Jacaranda mimosifolia and Platanus × acerifolia in Johannesburg’s urban forest. Sci Rep 12:5998
O’Connor TG, van Wilgen BW (2020) The impact of alien plants on rangelands in South Africa. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, German, pp 457–486
Padayachee AL, Irlich UM, Faulkner KT, Gaertner M, Procheş S, Rouget M, Wilson JRU (2017) How do invasive species travel to and through urban environments? Biol Invasions 19:1596–1599
Potgieter LJ, Gaertner M, O’Farrell P, Richardson DM (2019) Perception of impact: invasive alien plants in the urban environment. J Environ Manag 15:379–391
Potgieter LJ, Douwes E, Gaertner M, Measey J, Paap T, Richardson DM (2020) Biological invasions in South Africa’s urban ecosystems: patterns, processes, impacts, and management. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, German, pp 275–309
Pyšek P (1998) Alien and native species in central European urban floras: a quantitative comparison. J Biogeogr 25:155–163
Rai PK, Singh JS (2020) Invasive alien plant species: their impact on environment, ecosystem services and human health. Ecol Indic 111:106020
Reis RB, Ribeiro GS, Felzemburgh RDM, Santana FS, Mohr S, Melendez AXTO, Queiroz A, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI (2008) Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis 2:e228
Rejmanek M (2000) Invasive plants: approaches and predictions. Austral Ecol 25:497–506
Rejmanek M, Huntley BJ, Le Roux JJ, Richardson DM (2016) A rapid survey of invasive alien plants in western Angola. Afr J Ecol 55:56–69
Reynolds C, Venter N, Cowie BW, Marlin D, Mayonde S, Tocco C, Byrne MJ (2020) Mapping the socio-ecological impacts of invasive plants in South Africa: are poorer households with high ecosystem service use most at risk? Ecosyst Serv 42:101075
Richardson DM, Foxcroft FC, Latombe G, Le Maitre DC, Rouget M, Wilson JRU (2020) The biogeography of South African terrestrial plant invasions. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, German, pp 67–96
Ricotta C, Rapson GL, lrlich S, Pyšek P, Kühn I, La Sorte FA, Thompson K (2017) British plants as aliens in New Zealand cities: residence time moderates their impact on the beta diversity of urban floras. Biol Invasions 19:3589–3599
Ruwanza S, Shackleton CM (2016) Incorporation of environmental issues in South Africa’s municipal integrated development plans. Int J Sustain Dev World Ecol 23:28–39
Shackleton CM, Gwedla N (2021) The legacy effects of colonial and apartheid imprints on urban greening in South Africa: spaces, species and suitability. Front Ecol Evol 8:710549
Shackleton CM, Shackleton RT (2016) Knowledge, perceptions and willingness to control designated invasive tree species in urban household gardens in South Africa. Biol Invasions 18:1599–1609
Shackleton CM, McGarry D, Fourie S, Gambiza J, Shackleton SE, Fabricius C (2007) Assessing the effects of invasive alien species on rural livelihoods: case examples and a framework from South Africa. Hum Ecol 35:113–127
Shackleton RT, Novoa A, Shackleton CM, Kull CA (2020) The Social dimensions of Biological invasions in South Africa. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological invasions in South Africa. Springer, Berlin, German, pp 701–729
Sharma GP, Esler KJ, Blignaut JN (2010) Determining the relationship between invasive alien species density and a country’s socio-economic status. S Afr J Sci 2010:106–112
Shochat E, Lerman SB, Anderies JM, Warren PS, Faeth SH, Nilon CH (2010) Invasion, competition, and biodiversity loss in urban ecosystems. Bioscience 60:199–208
Skálová H, Moravcová L, Dixon AFG, Kindlmann P, Pyšek P (2015) Effect of temperature and nutrients on the growth and development of seedlings of an invasive plant. AoB Plants 7:plv044
Son D, Chu Y, Lee H (2024) Roads as conduits for alien plant introduction and dispersal: the amplifying role of road construction in Ambrosia trifida dispersal. Sci Total Environ 912:169109
štajerová K, šmilauer P, Brůna J, Pyšek P (2017) Distribution of invasive plants in urban environment is strongly spatially structured. Landsc Ecol 32:681–692
Statistics South Africa (Stats SA) (2016) Statistics by place. http://www.statssa.gov.za/. Accessed on 15 June 2020
Staudhammer CL, Escobedo FJ, Holt N, Young LJ, Brandeis TJ, Zipperer W (2015) Predictors, spatial distribution, and occurrence of woody invasive plants in subtropical urban ecosystems. J Environ Manag 155:97–105
TIBCO Software Inc (2020) Statistica (data analysis software system), version 14, http://tibco.com. Accessed 26 March 2021
Venter ZS, Shackleton CM, van Staden F, Selomane O, Masterson VA (2020) Green apartheid: urban green space remains unequally distributed across income and race geographies in South Africa. Landsc Urban Plan 203:103–889
Vieites-Blanco C, González-Prieto SJ (2020) Invasiveness, ecological impacts and control of acacias in southwestern Europe – a review. Web Ecol 20:33–51
Zengeya TA, Wilson JR (2020) The status of biological invasions and their management in South Africa in 2019. South African. National Biodiversity Institute, Kirstenbosch and DSI-NRF Centre of Excellence for Invasion Biology, Stellenbosch, South Africa
Zenni RD, Essl F, García-Berthou E, McDermott SM (2021) The economic costs of biological invasions around the world. In: Zenni RD, McDermott S, García-Berthou E, Essl F (eds) The economic costs of biological invasions around the world. NeoBiota 67:1–9
Acknowledgements
This paper has resulted from research work completed for Master’s Thesis at Rhodes University, South Africa in 2022, entitled “The distribution and perceptions of invasive alien plants in small towns in the Eastern Cape province, South Africa”. We wish to thank Ms. Andiswa Mabusela and Dr. Nhanamhla Gwedla for assisting with the field work. This work was funded by the DSI-NRF Centre of Excellence for Invasion Biology (CIB) and National Research Foundation (NRF) South Africa, grant number 137789. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
Funding
This work was funded by DSI-NRF Centre of Excellence for Invasion Biology (CIB) and National Research Foundation (NRF) South Africa, grant number 137789.
Open access funding provided by Rhodes University.
Author information
Authors and Affiliations
Contributions
Conceptualisation was done by CS. All authors contributed to the study design. Data collection and analysis were performed by TCS. The first draft of the manuscript was written by SR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seboko, T.C., Ruwanza, S. & Shackleton, C. The distribution and abundance of woody invasive alien plants in small towns in Eastern Cape Province, South Africa. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01563-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01563-9