Abstract
Urban watercourses are an essential part of blue-green infrastructure (BGI) that can mitigate the negative impact of habitat loss resulting from urbanization. Many studies on temperate urban bats activity did not concern its seasonal variations. Our work evaluated the bats’ activity and occurrence in different urban riverside environments throughout the vegetation period. Our results showed that long-range echolocators were represented mainly by Nyctalus noctula. Their activity peak occurred in late summer in open habitats of high anthropogenic pressure levels, and at least part of the population was present year-round in the city. Recently reported from Cracow: Hypsugo savii and Pipistrellus kuhlii were confirmed in the medium-range echolocators. The high occurrence of P. pygmeus with a relatively low P. pipistrellus was noteworthy and is probably a result of the low competitiveness of the latter in the presence of P. kuhlii. Two spring and late summer activity peaks on different rivers suggest different migration corridors from and to hibernacula. However, the autumn activity in the city center may also indicate partial hibernation in the old town. The activity peak of short-range echolocators occurred in spring, as expected - mainly in a narrow riverbed and low anthropogenic pressure environments. However, ambiguous spring and late summer activity in the open with the highest anthropogenic pressure environment may result from the rapidly progressing synurbization of M. daubentonii. Our results indicate the need to monitor the bat adaptation process to the cities in the context of dynamic climatic and environmental changes.
Similar content being viewed by others
Introduction
Urbanization involves changes in spatial use leading to the expansion and densification of urban areas. It is argued that these transformations lead to habitat loss or fragmentation, negatively affecting wild flora and fauna species (Fischer and Lindenmayer 2007). Factors inherent in urbanization include deforestation and increased built-up area, artificial lighting, river regulation, land drainage, excessive noise, and water, land, and air pollution. Expansion and densification of cities modifies natural local conditions, resulting in the urban heat island effect (Deilami et al. 2018).
Bats (Chiroptera) differ in reaction to anthropogenic pressure depending on the breadth of their ecological niches and the ability to adapt to the abovementioned environmental factors. On this basis, they were recommended as good bioindicators of the state of the environment, including transformations caused by urbanization (Russo and Ancillotto 2015; Jung and Threlfall 2018; Russo et al. 2021; Gutiérrez-Granados and Rodríguez-Zúñiga 2023). So far, it has been shown that species that hunt in open spaces have narrow wings, fast flight, and long echolocation ranges (LRE, e.g., Nyctalus, Eptesicus, and Vespertilio sp.) that best adapt to urban conditions. Bats that prefer cluttered spaces, with short sonar range, broad wings, and slow maneuverable flight (SRE, e.g., Myotis, Plecotus, and Barbastella sp.) are less likely to adapt. Between them, there are bats moving in semi-open spaces with medium sonar range (MRE, e.g., Pipistrellus and Hypsugo sp.) (Frey-Ehrenbold et al. 2013; Jung and Threlfall 2018). Apart from that, many individual species-specific preferences might be important, e.g., roost preferences.
As part of the blue-green infrastructure (BGI), rivers help mitigate habitat loss (and fragmentation) for urban wildlife (Fahrig 2003; Donati et al. 2022; Riva and Fahrig 2023). Bats may use them for orientation during migration, having constant access to water and food (Fukui et al. 2006; Furmankiewicz and Kucharska 2009). The riverbed within the city is usually regulated and has extensive hydro-technical infrastructure. These transformations often involve removing coastal vegetation that harbors insects, removing wooded areas and replacing them with buildings, and installing artificial lighting. In light of current knowledge, these transformations limit the free movement of bats and access to foraging and drinking sites (Azam et al. 2018; De Conno et al. 2018; Russo et al. 2019; Voigt et al. 2020).
Few studies focus on bats’ use of urban aquatic environments (Lintott et al. 2015; Li and Kalcounis-Rueppell 2018; Straka et al. 2020; Lehrer et al. 2021; Villarroya-Villalba et al. 2021; Lewanzik et al. 2022). To our knowledge, surveys considering different activities implemented in the annual life cycle of temperate bats are not available. Our work aimed to evaluate the intensity and nature of bat activity in different urban riparian environments during the growing season. We have chosen Cracow as a case study as this city consists of areas varying in human pressure levels and has watercourses crossing most of its territory. We hypothesize that bat activity will depend on river sections’ characteristics and will be different between seasons:
-
1)
LRE are expected mainly in open space habitats (wide riverbeds). Their activity will not be limited in environments of high anthropogenic pressure. The peak of activity will be observed in late summer if breeding colonies are present in the city or during spring and/or autumn if rivers serve as migratory corridors.
-
2)
MRE are expected mainly in edge habitats (greenery on riverbanks). Their activity may be limited in environments of high anthropogenic pressure. The peak of activity will be observed in late summer if breeding colonies are present in the city or during spring and/or autumn if rivers serve as migratory corridors.
-
3)
SRE are expected mainly in cluttered space habitats (narrow riverbeds with greenery). Their activity will be strongly limited in environments of high anthropogenic pressure. By assuming a very low probability of breeding colonies in the city, activity peaks may occur during spring and/or autumn migration.
Materials and methods
Study area
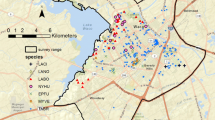
Research was conducted in 2015 in Cracow – the largest city in southern Poland, inhabited by over 750,000 people. It is within Polish Jurassic Highland, around 100 km from the Tatra Mountains. The city is located upon the Vistula, the longest river flowing through Poland (1047 km long). Several smaller rivers are within the city borders, including Rudawa – a 35 km long tributary of the Vistula. Rudawa flows into Cracow from the western outskirts and joins the Vistula in the city center. The western part of the city is characterized by a conspicuous urbanization gradient towards the outskirts and considerable landscape heterogeneity (Fig. 1). Areas within the city center represent dense, continuous urban cover with tall buildings and wide streets, resulting in a high percentage of impervious surfaces. The discontinuous urban cover is typical for suburban regions of the city and predominantly consists of individual single-family houses surrounded by gardens. Allotment gardens are a popular land use form in Cracow, where small plots of land are used for gardening and recreation. Green areas in the city are mainly deciduous tree stands with beech (Fagus silvatica), hornbeam (Caprinus betulus), and oak (Quercus sp.).
Study area in Cracow, Poland. River sections marked W1, W2, and W3 align with the Vistula River, and R1, R2, and R3 align with the Rudawa River. Further explanations on land use categories are included in the Methods section. Data from CORINE Land Cover 2012 and Google satellite images have been used to prepare this figure. The figure has been prepared and processed in QGIS 3.16.6 and Inkscape 1.0.1 software
Six river sections have been established along Vistula’s and Rudawa’s banks. (Fig. 1). Each section was characterized by environmental variables: mean riverbed width (RWidth), green riverbed cover (GCR), illumination (LIGHT), tree cover density (TCD), and impervious built-up cover density (IBD). The first two variables characterize the river sections themselves, while the latter three variables describe the level of human pressure on the adjacent environment and allow the classification of transects (also considering their location) as highly or little impacted (Table 1). Riverbed width was measured in three random locations within the watercourse in QGIS 2.16.1. Green riverbed cover refers to the percentage of riverbed area covered from the top by tree canopy and bushes, measured in QGIS 2.16.1. The measurements were based on the Google satellite images for these two environmental variables. Illumination here is a number of street lamps within 50 m from each river bank, recalculated per 100 m. Tree cover density and impervious built-up cover density were assessed using the zonal statistics algorithm run in QGIS 3.16.6 for the area within a 200 m radius of the transect course. For these analyses, raster Pan-European High-Resolution Layers from Copernicus Land Monitoring Service were used (tree cover density layer for TCD, imperviousness density layer for IBD).
Bat call sampling
Bats’ calls were recorded while cycling on the established transects. Pettersson D500 ultrasonic detector with a compatible external ultrasonic microphone was used to record full spectrum.wav format files. The equipment was transported in a backpack to protect it from shaking and recording excessive noises. The microphone was always directed towards the river. The recording process was continuous. Sampling occurred under favorable weather conditions (no rain, no strong wind) in the early evening hours (beginning from sunset). A single sampling session lasted around two hours. All river sections were sampled weekly from the 25th of March to the 12th of November 2015. The sampling period has been divided into four seasons that correspond to certain bat activities in their life cycle: (1) spring: migration, gathering in breeding colonies; (2) early summer: the breeding season; (3) late summer: colony dispersion; (4) autumn: migration and mating season. Season date intervals were the same as in Kohyt et al. (2021). Each transect was sampled eight times per season.
Call analysis
All the files were analyzed in the Kaleidoscope program (Wildlife Acoustics Inc.). Recorded echolocation call sequences with at least two subsequent calls were manually assigned to species or groups of species using reference data (Barataud 2015). The call’s parameters considered in identification include the duration, peak frequency, start frequency, end frequency, bandwidth, and interval between calls. When parameters’ values did not allow to recognize the species certainly, the sequence was assigned to one of the groups based on general call characteristics and echolocation range: (1) long-range echolocators (LRE): genera Nyctalus, Eptesicus and Vespertilio; (2) medium-range echolocators (MRE): genera Pipistrellus and Hypsugo; (3) short-range echolocators (SRE): genera Myotis, Plecotus and Barbastella (Frey-Ehrenbold et al. 2013). In some cases, sequences were identified to the genus level or species complex (e.g., Pipistrellus nathusi/kuhlii).
The number of passes (i.e., the number of 5-second long parts of recordings containing a single echolocation call sequence) serves as an indicator of bats’ activity. Since the sampling duration of subsequent transects was not equal, bats’ activity was further standardized per 10 min. Species occurrence is an additional indicator describing bat assemblages (Table 2). It is here understood as a minimum of one echolocation or social call sequence of the species per each river section and season. Based on recent literature, social calls were identified to the species level (Pfalzer 2002; Middleton et al. 2014). Foraging activity was assessed based on the number of feeding buzz sequences.
Statistical analysis
Bats differ regarding echolocation properties and detectability (Barataud 2015). For this reason, the analyses have been conducted separately for each group. Firstly, to test the differences in bat groups’ activity between river sections (six river sections), between seasons (four seasons), and the interaction of those two factors, a generalized linear model (GLM) was performed, as both factors were fixed. Because the data was right skewed and overdispersed the Tweedie distribution and log as link function were applied (Moshitch and Nelken 2014). Based on full models, a set of the model proposals was generated, then ranked by the corrected Akaike Information Criterion (AICc) (Burnham and Anderson 2002). In the case of MRE and SRE activity, due to zero activity in all (eight) transects made on river sections in some seasons, the transformation x + 1 was applied. Secondly, the Spearman correlation was employed to test whether bat groups’ activity is related to feeding. This analysis was performed for river sections where foraging behaviors were observed during at least two out of eight transects for each season. Finally, the influence of river section environmental characteristics on bat groups’ activity across the year was examined using multivariate analyses. In these models, bat group activity was summarised from eight transects, giving data for each section per season. First, detrended correspondence analysis (DCA) was performed to discover the data type and choose further analyses. As the greatest length of gradient presented by the first axis equals 1.277 SD - for the MRE model, gradients for LRE and SRE models equal respectively: 0.91 and 0.71. Therefore, the redundancy analysis (RDA) was employed (Lepš and Šmilauer 2003). Environmental factors included mean riverbed width (RWidth), green riverbed cover (GCR), tree cover density (TCD), and impervious buildup cover density (IBD). Illumination (LIGHT) was removed from the model due to a large value of the variance inflation factor (VIF) of all factors while present in the model or while present in the model after removing the IBD factor. After Naimi et al. (2014) we assumed the threshold for the VIF lower than 10. Multivariate analyses were performed in CANOCO ver. 4.5 (ter Braak and Šmilauer 2002). GLMs were performed using Statistica ver. 13.30 (Tibco Software Inc). Spearman tests were performed using PAST ver. 4.2 (Hammer et al. 2001). Figures in the results section were prepared using Inkscape 1.0.1 software. The significance level in the analyses was assumed to be p < 0.05, and in the case of multivariate analyses, trends at p ≤ 0.1 were additionally discussed.
Results
Species (groups of species) occurrence and social activity
The spatiotemporal occurrence for individuals that could be reliably identified to the species level is presented in detail in Table 2.
Bat activity in seasons and on river sections
Significant differences in the activity between seasons and river sections (i.e., spatiotemporal preferences) were present for each bat group, each factor, and their interaction (Table 3).
The most important observation for the long-range echolocators (LRE) is very high late summer activity, mainly on W1 and W2 sections (Fig. 2a). Large seasonal differences in activity between river sections were observed for medium-range echolocators (MRE). The highest means concern sections R1 (during spring) and W1 (during late summer), and they are significantly higher than the others except the high activity in W1 in spring and autumn which is also distinctive (Fig. 2b). Short-range echolocators (SRE) show the highest activity, mainly in spring, where the highest means are observed in sections R3 and R2. However, there are no statistical differences between those sections and R1 and W1 at this time (statistically significant differences are present for W2 and W3). Significantly, the highest activity of this group in the late summer period was also recorded in section W1 (Fig. 2c).
Predicted means of bat groups activity with 95% confidence interval (CI) on river sections (W1-W3: Vistula river; R1-R3: Rudawa river) in analyzed seasons (S – spring, Es – early summer, Ls – late summer, A – autumn). LRE: long-range echolocators, MRE: medium-range echolocators, SRE: short-range echolocators. MRE and SRE activity were transformed x + 1 (see: methods)
Foraging timing and place
The relationship between activity (echolocation) and feeding was analyzed for those sections where feeding buzz sequences were recorded on at least two transects in a season.
For LRE, only early summer (sections W1, W3, R1) and late summer (sections W1, W2, W3, R1) were tested. A positive correlation of activity and feeding was observed during late summer in sections W1 (rs = 0.95; p = 0.001) and W2 (rs = 0.78; p = 0.047). No correlation was found in any sections during the early summer. For MRE, spring (W1, R1, R3), early summer (W1) and late summer (W1, W2, R1) were tested. A positive correlation was observed in spring, in section W1 (rs = 0.86; p = 0.023), and during late summer, again in section W1 (rs = 0.93; p = 0.002). No correlation during early summer was observed. SRE were analyzed for spring and late summer, only section W1. A positive correlation was observed only during spring (W1; rs = 0.94; p = 0.0006). There was no correlation during late summer.
Impact of environmental factors on spatiotemporal activity
The impact of environmental factors on the spatiotemporal activity of each group of bats is presented in Table 4. Conditional effects for each model are also included.
In the model for LRE, the variability presented in the ordination diagram, which consists of the activity of bats on river sections and the relationship between activity and environmental factors, was explained in 93.3%. Impervious built-up cover density (IBD), correlated with the first axis, statistically significantly explains more than 67% of the variability observed (Table 4; Fig. 3a). This factor was positively correlated mainly with late summer activity (associated with W1).
In the case of MRE, the variability presented in the ordination diagram was explained in 97%. Both IBD and riverbed width (RWidth), correlated with the first and second axis, respectively, statistically significantly explained the variability in the ordination diagram in 58% and 27% (Table 4; Fig. 3b). IBD is strongly correlated with late summer and autumn activity on W1. RWidth additionally explains higher MRE activity during these seasons on Vistula’s sections.
For SRE, the variability presented in the ordination diagram was explained in 81.4%. The closest to statistical significance were IBD and green riverbed cover (GCR) (Table 4; Fig. 3c). Therefore, tendencies are discussed here. IBD explained 37% of the variability observed in the diagram. It was positively correlated with the late summer activity in W1. GCR was correlated with spring activity, especially in sections R2 and R3, and explained 30% of the variability.
Distribution of river sections of the Vistula (W1-W3) and Rudawa (R1-R3) in the redundancy analysis (RDA) ordination diagram for the model of (a) short-range echolocators (SRE); (b) medium range echolocators (MRE); (c) long-range echolocators (LRE), where GCR - green riverbed cover, RWidth - riverbed width, LIGHT - illumination, TCD - tree cover density, IBD - impervious built-up cover density
Discussion
Our research shows differences in the activity of bats in the city in riverside environments they use during the growing season. It has been shown that species belonging to different ecological groups distinguished by sonar range (LRE, MRE, SRE) reach the peak of their activity on different river sections depending on the season. It was also identified which environmental characteristics are responsible for these spatiotemporal differences in activity.
Long-range echolocators
The results regarding species occurrence in environments and seasons allow us to cautiously assume that most recorded individuals belonged to Nyctalus noctula (observed in all sections in all seasons). As Vespertilio murinus and Eptesicus serotinus were confirmed only from single locations, this may suggest that both species do not prefer urban riparian habitats. The latter’s case is consistent with previous observations (Gaisler et al. 1998).
The average LRE activity was relatively high during most seasons in all river sections (excluding autumn). It may indicate that at least part of the population has completed its annual life cycle in the city. These results support the observations of the adaptation process of noctules to urban conditions, where the reports of year-round colonies in cities are growing (Godlevska 2015; Lesiński and Janus 2019). The high activity of this group in late summer may be a result of long-distance flights from and to wintering areas (Petit and Mayer 2000; Ciechanowski et al. 2010) but also a doubling of the size of local populations due to the number of young individuals leaving the breeding colonies.
From all analyzed environmental characteristics, IBD explains the activity of this group to the greatest extent. Around the W1 and W2 sections (wide riverbed and high anthropogenic pressure), where LRE had their peak activity in late summer, the highest values of IBD were noted. In statistical procedures, the LIGHT factor had to be removed from the analyses as it was strongly correlated with IBD. However, it is possible that our result might be due to the „vacuum effect”: artificial light is responsible for attracting insects from their natural environments and attracting bats preying on them (Stone et al. 2015; Russo et al. 2019). Feeding and social activity also support this interpretation. A positive correlation between activity and feeding was recorded only in the river sections mentioned above in late summer. The social calls accompanying feeding emitted at that time may have resulted from communication on feeding grounds. Since social calls in late summer were also recorded on W3 without intensive feeding, likely mating occurred mainly along this section.
Medium-range echolocators
In our research, the high occurrence of Pipistrellus pygmaeus with a relatively low occurrence of P. pipistrellus is noteworthy. The latter was recorded as rarely as relatively new in the Polish fauna: Hypsugo savii. Often noted were P. nathusii, present in all sections at least once per season, and P. kuhlii, which was not confirmed only in the R3 section. Records of social calls belonging to P. kuhlii lepidus, suggest mating behavior in the city.
P. nathusii and P. pygmaeus are known to be associated with aquatic environments (Ciechanowski et al. 2010; Lewanzik et al. 2022). P. nathusii was previously defined as non-synanthropic (Ciechanowski 2015) and its influx into cities has only recently been recorded (Sachanowicz et al. 2019). Our data seem to confirm the generally observed trend for this species. Similarly, in the case of P. kuhlii, the available data suggest its rapid encroaching into cities (Sachanowicz et al. 2006). H. savii, like P. kuhlii, is of Mediterranean origin, slowly expanding its range northwards due to global warming rather than urbanization (Ancillotto et al. 2018). The most enigmatic are reports about P. pygmaeus’ presence in cities. Research from northern and central Europe indicates it is rather urban-avoidant (Gaisler et al. 1998; Lintott et al. 2015). However, in southern Spain, it predominates in city centers compared to the outskirts (Elena Tena, pers. comm.). The works mentioned above, and forecasts on range changes (Smeraldo et al. 2021) suggest difficulties in predicting the proportion of species within this group occurring in urban areas in the future. In our research, the observed low occurrence of P. pipistrellus, while high of P. pygmaeus, may result from P. kuhlii expansion to the north and the recent entry into southern Poland. In the absence of P. kuhlii (both historically and contemporaneously), P. pipistrellus was (and is) described as a generalist compared to P. pygmaeus (Gaisler et al. 1998; Nicholls and Racey 2006), showing a broader tolerance to habitat transformations caused by the urbanization process (Lintott et al. 2015). In Krakow, when P. pipistrellus co-occurs with P. kuhlii, the latter may win the competition, especially regarding feeding efficiency at street lamps (Tomassini et al. 2014; Salinas-Ramos et al. 2021). It is also possible that, as it is less adapted to hunting in riverine environments, it also loses the competition with P. pygmaeus in such habitats.
The most apparent differences in MRE activity concerned the seasons, where two peaks were observed. During spring, this group was the most active in R1 (narrow riverbed and high anthropogenic pressure), which was explained by a preference for an environment with a narrow riverbed (RWidth). Surprisingly, at that time, not R1 but W1 served as a feeding ground for this group. So, social calls recorded in this section may indicate the communication within breeding colonies (but unrelated to feeding). The other peak of activity was observed in W1 in late summer and was explained by the buildup area (IBD). Also, at this time, a strong positive correlation of activity with feeding was demonstrated only for W1. Then, social calls recorded in this section at this time may result from communication on feeding grounds and mating behavior. These observations support the hypothesis that despite using artificial light as a stable food supply, bats from the MRE avoid it during commuting flights and reproduction (Azam et al. 2016, 2018).
The two peaks of MRE activity observed in Cracow may indicate the use of different corridors while migrating from and to hibernacula, as some species (like P. nathusii) start migration early in the season (Jarzembowski 2003; Furmankiewicz and Kucharska 2009) and the autumn activity is still the highest in W1. Moreover, reports about autumn invasions and hibernation of this group in the city come from increasingly higher latitudes of the European continent (Flaquer et al. 2005; Nusová et al. 2019; Blomberg et al. 2021). It may indicate that some individuals, due to climate changes, likely overwinter in the city.
Short-range echolocators
As in most works concerning urban bats’ ecology, in our research, we did not attempt to assign SRE to a species level due to similar echolocation properties within this species group. We presume that M. daubentonii, as a species associated with water, may have the largest share in the activity of this group (Lintott et al. 2015).
Our results present the highest activity of SRE in spring, mainly in sections with a narrow riverbed and low anthropogenic pressure (R2 and R3). This result was expected and is consistent with the ecology of this group. According to statistical analyses, this activity may be explained with high probability by GCR, related to greenery and space clutter in the riverine environment. It could indicate the use of these sections for migration purposes. The winter-spring migration distance for M. daubentonii is about 150 km (Dietz et al. 2009). In Cracow, single individuals were recorded during winter cavern censuses (Wojciech J. Gubała, pers. comm). However, the high activity of SRE in spring and late summer on W1 is extraordinary, moreover, while explained by the IBD factor (p < 0.1). The negative relationship between M. daubentonii activity and the built-up area near the river was confirmed by Lintott (2015) and Langton et al. (2010). Especially surprising is the choice of foraging site: buildup over the broad (100 m) and lit surface of W1 in both seasons mentioned above. It is known that M. daubentonii hunts over large reservoirs (Ciechanowski 2002). Still, it would only partially explain the observed phenomenon, as section W3 seems to meet better the preferences of this species known so far. Previous research has shown that species belonging to this group are particularly threatened by processes related to urbanization; M. daubentonii, associated with water, was given as an example (Warren et al. 2000). Our results are in the contrary to this statement and indicate the need for a thorough analysis of the process of adaptation of this group to urban conditions.
Conclusion
Our work provides detailed information on bats’ temporal (seasonal) space use in temperate riparian habitats. The activity of different bat species (groups of species) varies greatly depending on anthropogenic pressure levels, environmental characteristics, and season. Although our study concerns local bat populations, its findings are of international importance for pan european conservation strategies. It constitutes a contribution to the growing body of knowledge about synurbization of temperate bats and indicates the need to take into account all seasons in their life cycle. More studies accounting for the abovementioned (and more) factors are needed to understand bats’ adaptation processes in the face of global climatic and land use changes.
Data availability
The datasets are available from the author on reasonable request.
References
Ancillotto L, Budinski I, Nardone V, Di Salvo I, Della Corte M, Bosso L, Conti P, Russo D (2018) What is driving range expansion in a common bat? Hints from thermoregulation and habitat selection. Behav Processes 157:540–546. https://doi.org/10.1016/j.beproc.2018.06.002
Anderson DR, Burnham KP (2002) Avoiding pitfalls when using information-theoretic methods. J Wildl Manage 66:912–918. https://doi.org/10.2307/3803155
Azam C, Le Viol I, Julien JF, Bas Y, Kerbiriou C (2016) Disentangling the relative effect of light pollution, impervious surfaces and intensive agriculture on bat activity with a national-scale monitoring program. Landsc Ecol 31:2471–2483. https://doi.org/10.1007/s10980-016-0417-3
Azam C, Le Viol I, Bas Y, Zissis G, Vernet A, Julien JF, Kerbiriou C (2018) Evidence for distance and illuminance thresholds in the effects of artificial lighting on bat activity. Landsc Urban Plan 175:123–135. https://doi.org/10.1016/j.landurbplan.2018.02.011
Barataud M (2015) Acoustic ecology of European bats. Species identification and studies of their habitats and foraging behaviour. Biotope Editions, Mèze; National Museum of Natural History, Paris
Blomberg AS, Vasko V, Salonen S, Pētersons G, Lilley TM (2021) First record of a Nathusius’ pipistrelle (Pipistrellus nathusii) overwintering at a latitude above 60°N. Mammalia 85:74–78. https://doi.org/10.1515/mammalia-2020-0019
Ciechanowski M (2002) Community structure and activity of bats (Chiroptera) over different water bodies. Mamm Biol 67:276–285. https://doi.org/10.1078/1616-5047-00042
Ciechanowski M (2015) Habitat preferences of bats in anthropogenically altered, mosaic landscapes of northern Poland. Eur J Wildl Res 61:415–428. https://doi.org/10.1007/s10344-015-0911-y
Ciechanowski M, Zając T, Zielińska A, Dunajski R (2010) Seasonal activity patterns of seven vespertilionid bat species in Polish lowlands. Acta Theriol 55:301–314. https://doi.org/10.4098/j.at.0001-7051.093.2009
De Conno C, Nardone V, Ancillotto L, De Bonis S, Guida M, Jorge I, Scarpa U, Russo D (2018) Testing the performance of bats as indicators of riverine ecosystem quality. Ecol Indic 95:741–750. https://doi.org/10.1016/j.ecolind.2018.08.018
Deilami K, Kamruzzaman M, Liu Y (2018) Urban heat island effect: a systematic review of spatio-temporal factors, data, methods, and mitigation measures. Int J Appl Earth Obs Geoinf 67:30–42. https://doi.org/10.1016/j.jag.2017.12.009
Dietz C, von Helversen O, Nill D (2009) Bats of Europe and Northwest Africa. Multico, Warsaw [in Polish]
Donati GFA, Bolliger J, Psomas A, Maurer M, Bach PM (2022) Reconciling cities with nature: identifying local Blue-Green infrastructure interventions for regional biodiversity enhancement. J Environ Manage 316:115254. https://doi.org/10.1016/j.jenvman.2022.115254
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Chang Biol 17:265–280. https://doi.org/10.1111/j.1466-8238.2006.00287.x
Flaquer C, Ruiz-Jarillo R, Torre I, Arrizabalaga A (2005) First resident population of Pipistrellus nathusii (Keyserling and Blasius, 1839) in the Iberian Peninsula. Acta Chiropterologica 7:183–188. https://doi.org/10.3161/1733-5329(2005)7[183:FRPOPN]2.0.CO;2
Frey-Ehrenbold A, Bontadina F, Arlettaz R, Obrist MK (2013) Landscape connectivity, habitat structure and activity of bat guilds in farmland-dominated matrices. J Appl Ecol 50:252–261. https://doi.org/10.1111/1365-2664.12034
Fukui D, Murakami M, Nakano S, Aoi T (2006) Effect of emergent aquatic insects on bat foraging in a riparian forest. J Anim Ecol 75:1252–1258. https://doi.org/10.1111/j.1365-2656.2006.01146.x
Furmankiewicz J, Kucharska M (2009) Migration of bats along a large river valley in Southwestern Poland. J Mammal 90:1310–1317. https://doi.org/10.1644/09-MAMM-S-099R1.1
Gaisler J, Zukal J, Rehak Z, Homolka M (1998) Habitat preference and fight activity of bats in a city. J Zool 244:439–445. https://doi.org/10.1017/S0952836998003148
Godlevska LV (2015) Northward expansion of the winter range of Nyctalus noctula (Chiroptera: Vespertilionidae) in Eastern Europe. Mammalia 79:315–324. https://doi.org/10.1515/mammalia-2013-0178
Gutiérrez-Granados G, Rodríguez-Zúñiga MT (2023) Bats as indicators of ecological resilience in a megacity. Urban Ecosyst. https://doi.org/10.1007/s11252-023-01462-5
Hammer Ø, Harper DAT, Ryan PD (2001) Past: Paleontological Statistics Software Package for Education and Data Analysis
Jarzembowski T (2003) Migration of the Nathusius’ pipistrelle Pipistrellus nathusii (Vespertilionidae) along the Vistula Split. Acta Theriol 48:301–308. https://doi.org/10.1007/BF03194170
Jung K, Threlfall CG (2018) Trait-dependent tolerance of bats to urbanization: a global meta-analysis. Proc R Soc Lond B 285:20181222. https://doi.org/10.1098/rspb.2018.1222
Kohyt J, Pierzchała E, Pereswiet-Soltan A, Piksa K (2021) Seasonal activity of urban bats populations in temperate climate zone—a case study from Southern Poland. Animals 11:1474
Langton SD, Briggs PA, Haysom KA (2010) Daubenton’s bat distribution along rivers - developing and testing a predictive model. Aquat Conserv Mar Freshw Ecosyst 20:45–54. https://doi.org/10.1002/aqc.1077
Lehrer EW, Gallo T, Fidino M, Kilgour RJ, Wolff PJ, Magle SB (2021) Urban bat occupancy is highly influenced by noise and the location of water: considerations for nature-based urban planning. Landsc Urban Plan 210:104063. https://doi.org/10.1016/j.landurbplan.2021.104063
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, New York
Lesiński G, Janus K (2019) Successful wintering of the noctule Nyctalus noctula on a balcony in Warsaw (Central Poland). Ecol Balk 11:291–294
Lewanzik D, Straka TM, Lorenz J, Marggraf L, Voigt-Heucke S, Schumann A, Brandt M, Voigt ChC (2022) Evaluating the potential of urban areas for bat conservation with citizen science data. Environ Pollut 297:118785. https://doi.org/10.1016/j.envpol.2021.118785
Li H, Kalcounis-Rueppell M (2018) Separating the effects of water quality and urbanization on temperate insectivorous bats at the landscape scale. Ecol Evol 8:667–678. https://doi.org/10.1002/ece3.3693
Lintott PR, Bunnefeld N, Park KJ (2015) Opportunities for improving the foraging potential of urban waterways for bats. Biol Conserv 191:224–233. https://doi.org/10.1016/j.biocon.2015.06.036
Middleton N, Froud A, French K (2014) Social calls of the bats of Britain and Ireland. Pelagic Publishing UK, Exeter
Moshitch D, Nelken I (2014) Using Tweedie distributions for fitting spike count data. J Neurosci Methods 225:13–28. https://doi.org/10.1016/j.jneumeth.2014.01.004
Naimi B, Hamm NAS, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37:191–203. https://doi.org/10.1111/j.1600-0587.2013.00205.x
Nicholls B, Racey PA (2006) Habitat selection as a mechanism of resource partitioning in two cryptic bat species Pipistrellus pipistrellus and Pipistrellus pygmaeus. Ecography 29:697–708. https://doi.org/10.1111/j.2006.0906-7590.04575.x
Nusová G, Uhrin M, Kaňuch P (2019) Go to the city: urban invasions of four pipistrelle bat species in eastern Slovakia. Eur J Ecol 5:23–26. https://doi.org/10.2478/eje-2019-0004
Petit E, Mayer F (2000) A population genetic analysis of migration: the case of the noctule bat (Nyctalus noctula). Mol Ecol 9:683–690. https://doi.org/10.1046/j.1365-294X.2000.00896.x
Pfalzer G (2002) Inter- und intraspezifische Variabilität der Soziallaute heimischer Fledermausarten. Dissertation, Universität Kaiserslautern
Riva F, Fahrig L (2023) Landscape-scale habitat fragmentation is positively related to biodiversity, despite patch-scale ecosystem decay. Ecol Lett 26:268–277. https://doi.org/10.1111/ele.14145
Russo D, Ancillotto L (2015) Sensitivity of bats to urbanization: a review. Mamm Biol 80:205–212. https://doi.org/10.1016/j.mambio.2014.10.003
Russo D, Cosentino F, Festa F, De Benedetta F, Pejic B, Cerretti P, Ancillotto L (2019) Artificial illumination near rivers may alter bat-insect trophic interactions. Environ Pollut 252:1671–1677. https://doi.org/10.1016/j.envpol.2019.06.105
Russo D, Salinas-Ramos VB, Cistrone L, Smeraldo S, Bosso L, Ancillotto L (2021) Do we need to use bats as bioindicators? Biology (Basel) 10:693. https://doi.org/10.3390/biology10080693
Sachanowicz K, Wower A, Bashta A (2006) Further range extension of Pipistrellus kuhlii (Kuhl, 1817) in central and eastern Europe. Acta Chiropterologica 8:543–548
Sachanowicz K, Ciechanowski M, Tryjanowski P, Kosicki JZ (2019) Wintering range of Pipistrellus nathusii (Chiroptera) in Central Europe: has the species extended to the north-east using urban heat islands. Mammalia 83:260–271. https://doi.org/10.1515/mammalia-2018-0014
Salinas-Ramos VB, Ancillotto L, Cistrone L, Nastasi C, Bosso L, Smeraldo S, Sánchez-Cordero V, Russo D (2021) Artificial illumination influences niche segregation in bats. Environ Pollut 284:117187. https://doi.org/10.1016/j.envpol.2021.117187
Smeraldo S, Bosso L, Salinas-Ramos VB, Ancillotto L, Sánchez-Cordero V, Gazaryan S, Russo D (2021) Generalists yet different: distributional responses to climate change may vary in opportunistic bat species sharing similar ecological traits. Mamm Rev 1–14. https://doi.org/10.1111/mam.12247
Stone EL, Harris S, Jones G (2015) Impacts of artificial lighting on bats: a review of challenges and solutions. Mamm Biol 80:213–219. https://doi.org/10.1016/j.mambio.2015.02.004
Straka TM, Lentini PE, Lumsden LF, Buchholz S, Wintle BA, van der Ree R (2020) Clean and green urbanwater bodies benefit nocturnal flying insects and their predators, insectivorous bats. Sustain 12:2634. https://doi.org/10.3390/su12072634
ter Braak CJF, Šmilauer P (2002) CANOCO Reference manual and user’s guide to Canoco for Windows: Software for Canonical Community Ordination (version 4.5)
Tomassini A, Colangelo P, Agnelli P, Jones G, Russo D (2014) Cranial size has increased over 133 years in a common bat, Pipistrellus kuhlii: a response to changing climate or urbanization? J Biogeogr 41:944–953. https://doi.org/10.1111/jbi.12248
Villarroya-Villalba L, Casanelles-Abella J, Moretti M, Pinho P, Samson R, Van Mensel A, Chiron F, Zellweger F, Obrist MK (2021) Response of bats and nocturnal insects to urban green areas in Europe. Basic Appl Ecol 51:59–70. https://doi.org/10.1016/j.baae.2021.01.006
Voigt CC, Scholl JM, Bauer J, Teige T, Yovel Y, Kramer-Schadt S, Gras P (2020) Movement responses of common noctule bats to the illuminated urban landscape. Landsc Ecol 35:189–201. https://doi.org/10.1007/s10980-019-00942-4
Warren RD, Waters DA, Altringham JD, Bullock DJ (2000) The distribution of Daubenton’s bats (Myotis daubentonii) and pipistrelle bats (Pipistrellus pipistrellus) (Vespertilionidae) in relation to small-scale variation in riverine habitat. Biol Conserv 92:85–91. https://doi.org/10.1016/S0006-3207(99)00062-2
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: J. Kohyt; Data curation: J. Kohyt, J. Karczmarz, E. Pierzchała; Formal analysis: J. Kohyt, J. Karczmarz, E. Pierzchała; Investigation: J. Kohyt, J. Karczmarz, A. Pereswiet-Soltan; Methodology: J. Kohyt, A. Pereswiet-Soltan; Project administration: J. Karczmarz; Resources: A. Pereswiet-Soltan; Supervision: J. Kohyt; Validation: J. Kohyt, J. Karczmarz, A. Pereswiet-Soltan; Visualization: J. Kohyt, E. Pierzchała; Roles/Writing - original draft: J. Kohyt; Writing - review & editing: J. Kohyt, J. Karczmarz, E. Pierzchała, A Pereswiet-Soltan.
Corresponding author
Ethics declarations
Ethical approval
No ethical approval was required as this was an observational study.
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kohyt, J., Karczmarz, J., Pereswiet-Soltan, A. et al. Spatiotemporal use of urban rivers by local bat populations in a large city (Cracow, Southern Poland). Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01545-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01545-x