Abstract
Increasing urbanisation reduces available habitat and increases human-wildlife interactions, presenting social and ecological challenges for many species; however, flexible generalist species, such as the vervet monkey, Chlorocebus pygerythrus, thrive under these pressures. In the urban mosaic, human-food sources represent clumped, monopolisable food that can increase contest competition. Social network analysis (SNA) is a powerful tool to monitor changes in social structure, yet it has rarely been used to study urban wildlife. Using SNA, we investigated the effect of anthropogenic food and human-wildlife interactions on social cohesion in five vervet monkey groups in urban KwaZulu-Natal, South Africa. Over six months, we conducted group scan samples every 30-min on each group and recorded all humans-vervet monkey interactions during dawn to dusk follows. We analysed the effect of foraging on natural and human-related food sources and human-vervet monkey interactions on social network metrics for grooming and aggression at group (density, clustering coefficient and distance) and individual (eigenvector centrality and degree) levels, using linear mixed models. Anthropogenic food influenced almost all social metrics. At the group level, foraging on anthropogenic food was related to increased density and cohesion in both grooming and aggression networks. At the individual level, increasing anthropogenic food affected high-ranking monkeys most: eigenvector centrality and outdegree in aggression networks increased with rank. Social network analysis can be a useful tool to document urban effects on wildlife groups, and aids our understanding of wildlife behavioural flexibility, a key tool in developing educated and effective management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The urban mosaic landscape is widely acknowledged as an important ecological system (Downs et al. 2021), yet our understanding of social adaptations within this ecological system is relatively limited (Lacy and Martins 2003; Thatcher et al. 2023). Evidence is accumulating that social network positions have fitness consequences that impact population dynamics (e.g. Royle et al. 2012), and therefore, an animal’s social network is expected to respond flexibly to environmental change (Snijders et al. 2017). Behavioural traits can affect effective population size through reproductive skew (Anthony and Blumstein 2000), and thus, increasing understanding of social behavioural processes can inform and contribute to wildlife conservation management.
Although research into anthropogenic wildlife social networks is steadily growing (e.g. elephants, Elephas maximus, Chiyo et al. 2012; moor macaques, Macaca maura, Morrow et al. 2019; spotted hyenas, Crocuta Crocuta, Belton et al. 2018; giraffes, Giraffa camelopardalis, Bond et al. 2021; bottlenose dolphins, Tursiops aduncus, Chilvers and Corkeron 2001), there are few assessments of peri-urban species (e.g. Macaca spp. Balasubramaniam et al. 2020; Balasubramaniam et al. 2021a, b; Chakraborty et al. 2023), and even fewer on urban species (e.g. domesticated dogs Canis lupus familiaris, Bhattacharjee and Bhadra 2020; sulphur-crested cockatoos, Cacatua galerita, Aplin et al. 2021). Understanding the interaction between anthropogenic food and ecological factors on social dynamics is important to developing our understanding of wildlife flexibility in the urban landscape (McKinney 2015; Balasubramaniam et al. 2021a, b), particularly in primate species (Thatcher et al. 2023).
Feeding competition is one of the most fundamental factors affecting fitness in animals (Chapman et al. 2012). Understanding how the distribution of food resources influences the nature of feeding competition is a central feature of ecological explanations of social structure and organisation (e.g. Wrangham 1980; van Schaik 1989; Sterck et al. 1997; Isbell and Young 2002). Changes in landscape profiles caused by anthropogenic pressures directly influence food distribution and availability, altering ecological and social challenges for many species (Chilvers and Corkeron 2001; Gilchrist and Otali 2002; Prange et al. 2011; Chakraborty et al. 2023). Anthropogenic suburbs provide habitats that are productive and well maintained, providing abundant dispersed resources (McKinney 2002), particularly in selectively maintained gate communities (Ballard and Jones 2011; Alexander et al. 2019a, b, c, 2021). Furthermore, anthropogenic environments also bring benefits such as increased access to high-value, patchy, monopolisable human food resources. Socioecological theory predicts that high-value monopolisable resource distribution gives rise to strong within-group contest competition (WGC) and when coupled with low between-group contest competition, should create more cohesive groups (Wrangham 1980; Sterck et al. 1997; van Schaik and Van Noordwijk 1998). Within such groups, small, supportive networks should form with key central individuals (van Schaik 1989), a more linear despotic hierarchy should be apparent, and females should form frequent coalitions to maintain rank-related benefits (Isbell 1991a, b; Sterck et al. 1997; Isbell and Young 2002). The effect of such ecological pressures on social structure has not yet been applied to intragroup variation in urban wildlife; nevertheless, using the rationale of WGC to test these ecological pressures allows a greater understanding of both group and individual adaptations to urbanisation.
Behavioural flexibility has desirable fitness benefits within the urban ecosystem (Sol et al. 2013), particularly for adaptive generalist species such as the vervet monkey, Chlorocebus pygerythrus (Chapman et al. 2016). Vervet monkeys are a highly social species that live in multimale-multifemale groups, are female philopatric and have strict female and male dominance hierarchies (e.g. Seyfarth and Cheney 1984; Borgeaud and Bshary 2015). They are highly adapted to an urban landscape (Saj et al. 2001; Patterson et al. 2017, 2018) and exhibit behavioural flexibility in response to anthropogenic disturbance (Chapman et al. 2016). Grooming and aggression are common behaviours among non-human primates (hereafter primates) that reflect cooperation and competition, respectively (Sueur et al. 2011) and are therefore, key metrics for social network analysis in primates. Previous social network studies have shown the strong influence of female philopatry and rank on vervet monkey social metrics (Henzi et al. 2013; Josephs et al. 2016; Young et al. 2017; Borgeaud et al. 2017). Vervet monkeys, therefore, provide a suitable model to test social flexibility in an urban matrix.
In our study, vervet monkeys had an abundant dispersed supply of natural food through the selectively maintained gardens and natural areas within their urban home range, whereas high-value human food was obtained by entering homes or raiding refuse (Thatcher, pers. obs.); these human foods were therefore opportunistic and clumped. Based on socioecological theory, we expected that greater exploitation of these high-value human foods and less dependence on natural food resources would increase WGC. We, therefore, made a group-level prediction: (1a) groups that foraged on these clumped human food resources more frequently would increase their grooming connections and aggressive interactions; thus, network density (both grooming and aggression) should increase, and distance should decrease compared with those that fed primarily on natural food resources, as group-living animals can adopt multiple competitive foraging strategies (Isbell et al. 1991). We further predicted (1b) that an increase in differentiated relationships should lead to increased sub-grouping. At the individual level, we predicted that (2a) because of the strict linear hierarchy associated with increased WGC, higher-ranked individuals would obtain more anthropogenic food and, therefore, be more central within their group grooming and aggression networks. Finally, because of female philopatry in vervet monkeys, we predicted that (2b) females would receive more grooming and aggression than males and be more central. We made no specific predictions about human aggression (e.g. chasing, or throwing of rocks); however, we included it as an additional level of anthropogenic disturbance to human food consumption.
Methods
Subjects and study site
We studied five groups of urban vervet monkeys (Table 1) in Simbithi Eco-estate, Ballito, Durban North-coast, KwaZulu-Natal, South Africa (S:29.3029, E:31.131). Simbithi Eco-estate is a gated housing estate converted from sugar cane farms to create a complex urban mosaic of urban built and green spaces (natural and managed) (Alexander et al. 2019a, b, c, 2021). The estate comprises a variety of urban complexes, structures and housing options, along with areas of human-made coastal forest and managed walking trails (Alexander et al. 2019a, b, c, 2021).
We habituated all groups to the presence of one observer at close proximity (10 m) over a two-month period, six months before data were collected. A group was deemed habituated when the observer could approach within 10 m proximity with no flight response.
We conducted our study from September 2016- February 2017. We studied only adult vervet monkeys, and all were individually recognisable from distinct markings. No population genetic data was available. The study period did not include the dispersal and mating seasons (April-July); therefore, the numbers of adults across groups were relatively stable. We collected data during the austral spring (September–October) and summer (November-March) in KwaZulu-Natal (SANBI 2017).
Behavioural data collection
HT collected vervet monkey behaviour data from dawn until dusk (~ 12 h in spring and ~ 17 h in summer). We conducted instantaneous group scans every 30 min for a 10-min period, recording both grooming and aggressive interactions, including the identity of the social partner/s. We also noted the occurrence of any dominance interactions and aggressive competition ad libitum. We collected all data using the Prim8 behavioural software (McDonald and Johnson 2014) on a handheld Lenovo tablet. We only present data on intragroup conflict, as our intergroup encounter rate was very low.
We used all occurrence sampling to record all interactions between humans and vervet monkeys during our dawn to dusk daily follows. We defined a human-related incident as any occasion when at least one vervet monkey interacted with humans directly or human-related possessions (e.g. houses, bins and cars). We classified incidents as positive (raiding, provisioning) or negative (human aggression towards monkeys). For positive human incidents, we included any form of human food consumption; human food consumption was considered a new event if the monkeys had not raided or been fed for the previous 20 min. We classed negative human incidents as any form of human-monkey aggression, independent of who initiated the attack. We recorded an incident as terminated once all parties were no longer in sight of each other, and recorded a new incident if there had been no human-monkey aggression in the previous 20 min. We recorded natural food consumption using focal animal sampling, noting all events when a monkey was seen consuming natural food following standard classification of foraging behaviour (Saj et al. 1999).
Association measures
We constructed directed weighted matrices for grooming and aggression per month. We calculated measures of network structure using UCInet (Borgatti et al. 2002). We used three commonly used network parameters to quantify group-level social associations: density, distance and a clustering coefficient (Croft et al. 2008) that could test overall connectedness and degree of sub-grouping within the group. Density is a measure of dyadic connections (ties) in a population with respect to the potential number of ties and reflects overall group cohesion. We expressed this as a percentage of social association: high scores represented a saturated network while low scores indicated a sparse network. We further assessed distance as a measure of direct social interaction. Distance reflects the shortest path of connections between two individuals and so, distance increases as networks become less cohesive. We calculated the average distance between pairs within a network, allowing us to assess how well connected a group was. The global clustering coefficient reflects the level of sub-grouping within the group, and measures how clustered a network is, e.g. how many ‘cliques’ are in a network.
We used three common network parameters to assess individual metrics: eigenvector centrality and degree centrality (Croft et al. 2008). Eigenvector centrality reflects an individual’s connection to other well-connected individuals (Croft et al. 2008), and has been found to be a better predictor of fitness than the strength of a relationship (Cheney 2016). Degree measures how many direct ties or relationships an individual has; an individual with more ties has higher centrality and thus, is a basic measure of connectedness. Outdegree refers to the number of ties originating from the focal (e.g. grooming given) whilst in-degree is the number of ties directed at the focal (e.g. grooming received). It is possible for individuals ro have the same number of ties (degree) but different eigenvector centrality because the connectedness of their partners to few or many others may vary. We used a weighted matrix to calculate eigenvector centrality and a binary matrix to assess degree. Again, we assessed both grooming and aggressive associations.
Statistical analyses
We conducted all statistical analyses using the R statistics programme (R Project 2013). We calculated a monthly rate (frequency/hour) for the two human incident measures (positive and negative) and natural food consumption. For brevity, we hereafter refer to the latter variable as natural food. We calculated both a group level and individual frequency for separate analyses of our individual and group level prediction. We assessed dominance rank monthly for each vervet monkey group as a combined male–female hierarchy. Although we acknowledge the value of Elo-ratings as a measure of rank overtime (Albers and de Vries 2001; Neumann et al. 2011), we also acknowledge the advantages of David’s score to allow a stronger comparison between individuals at a given time (Gammell et al. 2003; De Vries et al. 2006) and therefore used David’s score as a measure of rank in this study. David’s score measures the success of an individual winning relative to their opponents, hence providing a comparative measure of competitive ability. We calculated a normalised David’s score rank using the Steepness package (Leiva and de Vries 2011) in R.
Social network data is inherently non-independent because of the interconnectedness between individuals within the network. Whilst there are several options for analysing network data, recent research has demonstrated the benefit of using mixed effect models that address interdependence in the data and control for the effects of actor and receiver (Hart et al. 2022).
We created 12 linear mixed models (LMM) in total using the lme4 package (Bates 2010), with each social metric as the dependent variable. We created three models for our group metrics and three models for our individual metric models, and we ran these for aggression and grooming behaviours. Within each model, each row represented a mean monthly social metric calculated per group/individual. We constructed two separate model structures for group and individual metrics to address our questions separately. To test Predictions 1 and 2, we included predictor variables to reflect human-food resources (positive human incidents) and natural food resources, and because group size is known to affect intragroup contest competition in primates (e.g. Balasubramaniam et al. 2014) we included the number of adults in each group (group size). We ran an interaction between positive human incidents (clumped) and natural food (dispersed) to assess our group-level prediction of clumped food increasing WGC. To test our individual-level prediction, we additionally added sex and rank as predictors and ran an interaction between positive human incidents with sex and rank separately to assess the effect of linear hierarchy and WGC. Finally, we included negative human incidents (human-vervet monkey aggression) in all models as we previously found this factor affected time spent foraging in these groups (Thatcher et al. 2020).
Group level prediction
Group social metric ~ positive human incidents*natural food + negative human incidents + group size + (1|group identity).
Individual level prediction
Individual social metric ~ sex*positive human incidents + rank*positive human incidents + negative human incidents + natural food + group size + (1|group identity/monkey identity).
We used the boot package (Davison and Hinkley 1997; Canty and Ripley 2017) in R to run our models and bootstrap the confidence intervals of our model parameters to further avoid assumptions about the distribution from which the data were obtained. As we recorded active affiliative behaviours, we chose to run permutations on our parametric models (Hart et al. 2022) and ran all models using 1000 permutations. If the upper and lower CI straddled 0, then we did not consider the effect significant. We assessed the fit of each model graphically, checking residuals for normal distribution.
Results
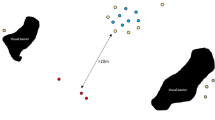
We observed each vervet monkey group for a minimum of 4 days and a maximuim of 6 days a month (mean 4.53 ± 0.63 (SD)). Over 6 months, we collected a mean (± SD) of 315 ± 25.34 scans per group with a monthly average of 52.5 ± 7.0. Networks showed clear variation between vervet monkey troop social metrics. We found consistent effects of positive human incidents increasing group cohesion and centrality measures (Fig. 1, Table 2 and 3).
Example sociogram representing the Ballito vervet monkey troop. (Note: Black circles represent female monkeys, and white squares represent male monkeys. The networks have been spring-embedded). Networks represent two separate months, where (a) is the month with the highest rate of positive human incidents and (b) is the month with the lowest positive human incidents
Group level predictions
Group Prediction 1a
We found a positive interaction effect between natural food and positive human incidents on grooming density, supporting the group-level prediction 1a for grooming. When the rate of positive human incidents and natural food was low, grooming density increased; however, this was offset by an increased rate of natural food decreasing grooming density (Table 3, Fig. 2a). Supportive to this, we found a negative interaction effect on distance (Table 3, Fig. 2b); distance decreased with an increased rate of positive human incidents, however as natural food availability increased distance plateaued. Aggression metrics also met our group-level prediction 1a, as we found that a greater rate of positive human incidents was significantly related to decreased distance within the network. Furthermore, we found a significant positive interaction between positive human incidents and natural food for the aggression network density. Increased frequency of positive human incidents was associated with a sharp increase in density, however, when the rate of natural food increased, density significantly decreased (Table 4, Fig. 2c).
Interaction between group natural food rate per hour and group positive human incidents rate per hour on group level social metrics of urban vervet monkeys at Simbithi Eco-estate, North Durban, KwaZulu-Natal, South Africa, where a) shows the positive effect on grooming density, b) shows the negative effect on grooming distance and c) shows the positive effect on aggression density
Group Prediction 1b
The clustering coefficient did not show a significant interaction. However, an increased clustering coefficient for both grooming (Table 2) and aggression (Table 3) were positively related to a higher rate of positive human incidents, supporting group-level prediction 1b for grooming.
Individual level predictions
We found partial support for our individual-level predictions (Table 5).
Individual Prediction 2a
At the individual level, prediction 2a was partially met for our grooming and aggression metrics (Table 6 and 7). Unexpectedly, we found a significant negative interaction between positive human incidents and rank for grooming indegree. Individuals’ grooming indegree increased with rank when rates of positive human incidents were low, however, as the rate of positive human incidents increased grooming indegree increased across ranks (Table 6, Fig. 3a). We found a positive interaction between positive human incidents and rank for both aggression eigenvector centrality and aggression outdegree (Table 7, Fig. 3b, c). When positive human incidents were high, higher-ranking monkeys’ aggressive connectedness (eigenvector centrality) increased; however, when positive human incidents were low, aggressive connectedness (eigenvector centrality) decreased. When the rate of positive human incidents increased, aggression outdegree increased with rank; however, when the rate of positive human incidents was low, the effect of rank weakened (Table 7).
Interaction between rank (normalised David’s score) and individual positive human incidents rate per hour on individual level social metrics of urban vervet monkeys at Simbithi Eco-estate, North Durban, KwaZulu-Natal, South Africa. Where a) shows the negative effect on grooming indegree, b) shows the positive effect on aggressive eigenvector centrality, and c) shows the positive effect on aggressive centrality outdegree
Individual Prediction 2b
In support of individual level prediction 2b, we found that females were significantly more central than males for all three measures of grooming centrality (Table 6); however, we found no differences between sex for aggressive metrics (Table 7).
Individual metrics and negative human incidents
Although we made no predictions for negative human incidents, we found that negative human incidents significantly affected all vervet monkey individual metrics, but no group metrics. A higher rate of negative human incidents significantly positively affected all three aggressive centrality measures (Table 6). Increased rates of negative human incidents had a positive significant effect on the grooming eigenvector centrality score; however, they had a negative significant effect on indegree and outdegree (Table 7).
Discussion
All social metrics, both group and individual, appeared to be influenced by either positive or negative aspects of urban living. We present data on intragroup associations only, as intergroup encounters were relatively rare, indicating aggressive intergroup competition was low.
Group level predictions
In support of Prediction 1a, all group-level metrics were influenced by the rate of clumped human food consumption, and in some cases, this was modulated by the interaction with dispersed natural food consumption. This interaction positively affected both grooming and aggression network density and, as expected, had a negative effect on grooming network distance. More connected networks typically show increased density and decreased distance. This suggests that when human food consumption was high, aggressive competition within the group increased and grooming connections also increased. These findings support established socioecological theories of alliance support over a clumped resource (van Schaik 1989; Hockings et al. 2012). This could also suggest that consuming human-derived food allows vervet monkeys to meet their daily energy requirements more efficiently and therefore have more time available to participate in grooming (Thatcher et al. 2019).
We also found a main effect of positive human incidents on the clustering coefficient in both grooming and aggression networks, indicating that increasing human food consumption increased sub-group formation, supporting our group-level prediction 1b. It is possible that increasing clique formation is beneficial to the formation of supportive alliances to obtain high-value resources (van Schaik 1989). Abundant high-value anthropogenic food availability has been shown to increase group formation in bushbabies (Scheun et al. 2015, 2019); however, further research would be necessary to look at the relationship between alliance support and human food consumption to support this hypothesis.
Overall, group metrics showed that greater human food consumption was related to increased group cohesion, supporting previous work by Hockings et al. (2012) that found chimpanzee (Pan troglodytes verus) groups became more cohesive during crop foraging. However, more recent research on chacma baboons (Papio ursinus) (Bracken et al. 2022) and macaque spp. (Marty et al. 2019; Morrow et al. 2019) have shown that peri-urban primate groups are less cohesive.These group metric results support the idea that group-living animals modify the nature of their social relationships to increase their competitive power over desirable food resources (Sterck et al. 1997). These findings develop our knowledge of the flexibility in the social structure of vervet monkeys in an urban setting. However, it is important to note that the effect sizes are small and that further research is needed to confirm these findings. Future studies investigating how such increased social cohesion relates to the spatial distribution of the group around human food sources can also be useful in informing urban management strategies.
Individual level predictions
For our individual level prediction 2a, the interaction between positive human incidents and rank significantly negatively affected the grooming indegree of vervet monkeys. Interestingly, positive human incidents reduced the effect of rank on grooming such that the grooming indegree of lower-ranking individuals increased to a comparable level with high-ranking individuals. There was no such effect on grooming outdegree, indicating that lower-ranking individuals became more attractive to groom, but themselves did not groom more partners. This result does not meet our expected directionality of individual-level prediction 2a of within-group competition; instead, results for grooming centrality suggest a more egalitarian relationship (Sterck et al. 1997). It could be argued that this increase in social value was because of potential coalitionary support and may have represented a social exchange (Schino and Nazionale 2007); however, we do not have the data to fulfil this statement within the realms of this study, but this provides an interesting note for further research.
Our aggression metrics did meet our individual-level prediction 2a. We found an interaction between positive human incidents and rank for aggressive eigenvector centrality, showing that the aggressive connections of higher-ranking vervet monkeys increased with a higher rate of positive human incidents. The same was true of aggression outdegree; higher rates of positive human incidents were related to an increased number of partners to whom high-ranking individuals gave aggression. These findings supported our individual-level prediction 2a based on socioecological theory that high-value resources (positive human incidents) increase despotism and the resource-holding potential of high-ranking individuals’ within the group (Isbell 1991a, b; Sterck et al. 1997; Marty et al. 2019). Furthermore, these results emphasise the importance of high-value resources to high-ranked urban vervet monkeys, highlighting the cost and benefits of these resources to the urban monkey, which is fundamental for management considerations.
Our individual-level prediction 2b was met. We found that females were more central in their grooming networks in vervet monkeys, having a higher centrality for all three individual metrics than males. This supports the idea of female centrality in a female philopatric species (Seyfarth and Cheney 1984; Canteloup et al. 2021). This also supports previous social network studies that have shown females are more central within their networks than males (Henzi et al. 2013; Josephs et al. 2016; Borgeaud et al. 2017; Young et al. 2017) and more recently in lion-tailed macaques (Macaca silenus) in the anthropogenic landscapes (Dhawale and Sinha 2022). Furthermore, the idea of female centrality endorses socioecological literature, suggesting females form coalitions under WGC (Isbell 1991a, b; Isbell and Young 2002; Sterck et al. 1997). However, it would be pertinent to explore individual rank further in future studies, notably if a males position and centrality changes within the mating period.
We found an unexpected yet consistent trend of negative human incidents across aggression and grooming metrics at the individual level. Negative human incidents positively influenced all aggressive measures. It is possible that increased aggression, both outdegree and indegree, between individuals was a result of redirected human aggression. Redirected aggression between primates is a relatively common behaviour where the individual that received aggression is more likely to be aggressive to its conspecifics (Cheney and Seyfarth 1989). Furthermore, we found that grooming eigenvector centrality increased, yet both grooming indegree and outdegree decreased, with a higher rate of negative human incidents. Previous primate literature has shown that grooming alleviates stress and anxiety (e.g. Wittig et al. 2008). If human-monkey conflict increased stress and anxiety in vervet monkeys, we would expect to see all grooming centrality measures positively increased. Nevertheless, previous literature has shown that under crowded conditions, female primates reduce their number of grooming partners; however, they maintain more selective grooming partners for future conflict avoidance (De Waal 1987; Koyama and Dunbar 1996; Judge and de Waal 1997; Judge et al. 2006). This literature focuses on captive primates under periods of short-term stress; however, their results could be applied to suggest that vervet monkeys in this study do maintain grooming rates (increased eigenvector centrality) but decrease grooming partners (decreased outdegree and indegree) under periods of short-term stress such as human-primate conflict. Furthermore, these results also support the social buffering hypothesis suggesting that under period of stress individuals may choose to groom select close associates (Wittig et al. 2008; Young et al. 2014). Overall, these results highlight the complex costs and benefits of the urban landscape (Thatcher et al. 2023), that the benefits of consuming human-derived foods override the costs of human-directed and within-group aggression.
This study presents findings from a six-month period, and the effect sizes are relatively small. Further long-term research is required to corroborate these findings and establish whether the trends reported are robust.
Conclusions
Overall, our group-level predictions were met. Group metrics assessed in this study followed socioecological predictions, suggesting increased access to anthropogenic food for vervet monkeys increases WGC. Our individual vervet monkey predictions were not so clearly met; nevertheless, this study reiterates the need for further research into the social flexibility of wildlife to anthropogenic pressures. We suggest that further research should focus on how increased social cohesion influences the spatial distribution of the group and on the direct consequences of raiding events, particularly coalition support. Furthermore, it was not possible in this study to examine the effects of intergroup encounters and competition as they were seldom witnessed because of field limitations and logistics. Nevertheless, it is likely that increasing encroachment of human populations and changes to landscape caused by urbanisation (McKinney 2006; Chakraborty et al. 2023) will affect inter-group encounters and would make an interesting topic for further research.
Considering the recent surge in ethnoprimatology and the acknowledgement of its concern for primate welfare and biodiversity (McLennan et al. 2017), we provide an important foundation for future research. We provide an assessment of five troops of vervet monkeys living in a highly anthropogenic mosaic landscape, of which they show social flexibility to adapt and thrive under these pressures. Our results largely comply with expected socioecological predictions, showing that groups from connected dominant structured groupings when are reliant upon clumped high-value anthropogenic resources. These results have important implications for urban wildlife management. Our findings demonstrate the social flexibility of urban vervet monkeys and the ways in which they dominate and exploit beneficial anthropogenic resources, highlighting the need for better public education and controlled refuse management.
Data availability
Data is available from the lead author upon reasonable request
References
Albers PC, de Vries H (2001) Elo-rating as a tool in the sequential estimation of dominance strengths. Anim Behav 61(2):489–495
Alexander J, Ehlers SD, Ehlers Smith Y, Downs CT (2019a) Drivers of fine-scale avian functional diversity with changing land use: an assessment of the effects of eco-estate housing development and management. Landsc Ecol 34(537–549):10. https://doi.org/10.1007/s10980-019-00786-y
Alexander J, Ehlers SD, Ehlers Smith Y, Downs CT (2019b) Eco-estates: diversity hotspots or isolated developments? Connectivity of eco-estates in the Indian Ocean Coastal Belt, KwaZulu-Natal, South Africa. Ecol Ind 103:425–433. https://doi.org/10.1016/j.ecolind.2019.04.004
Alexander J, Ehlers SD, Ehlers Smith Y, Downs CT (2019c) A multi-taxa functional diversity assessment of the effects of eco-estate development in the mixed land-use mosaic of the KwaZulu-Natal North Coast, South Africa. Landsc Urban Plan 192:103605. https://doi.org/10.1016/j.landurbplan.2019.103650
Alexander J, Ehlers SD, Ehlers Smith Y, Downs CT (2021) Urban land development for biodiversity: suggested development and management guidelines for eco-estates using case studies from coastal KwaZulu-Natal, South Africa. Urban For Urban Green 65:127347. https://doi.org/10.1016/j.ufug.2021.127347
Anthony LL, Blumstein DT (2000) Integrating behaviour into wildlife conservation: the multiple ways that behaviour can reduce. Biol Cons 95(3):303–315
Aplin LM, Major RE, Davis A, Martin JM (2021) A citizen science approach reveals long-term social network structure in an urban parrot, Cacatua galerita. J Anim Ecol 90(1):222–232
Balasubramaniam KN, Dunayer ES, Gilhooly LJ, Rosenfield KA, Berman CM (2014) Group size, contest competition, and social structure in Cayo Santiago rhesus macaques. Behaviour 151(12–13):1759–1798
Balasubramaniam KN, Marty PR, Samartino S, Sobrino A, Gill T, Ismail M, Saha R, Beisner BA, Kaburu SSK, Bliss-Moreau E (2020) Impact of individual demographic and social factors on human–wildlife interactions: a comparative study of three macaque species. Sci Rep 10(1):1–16
Balasubramaniam KN, Bliss-Moreau E, Beisner BA, Marty PR, Kaburu SSK, McCowan B (2021a) Addressing the challenges of research on human-wildlife interactions using the concept of Coupled Natural and Human Systems. Biol Cons 257:109095
Balasubramaniam KN, Kaburu SSK, Marty PR, Beisner BA, Bliss-Moreau E, Arlet ME, Ruppert N, Ismail A, AnuarMohdSah S, Mohan L (2021b) Implementing social network analysis to understand the socioecology of wildlife co-occurrence and joint interactions with humans in anthropogenic environments. J An Ecol 90(12):2819–2833
Ballard R, Jones GA (2011) Natural neighbors: Indigenous landscapes and eco-estates in Durban, South Africa. Ann Assoc Am Geogr 101(1):131–148. https://doi.org/10.1080/00045608.2010.520224
Bates DM (2010) lme4: Mixed-effects modeling with R. Springer
Belton LE, Cameron EZ, Dalerum F (2018) Social networks of spotted hyaenas in areas of contrasting human activity and infrastructure. Anim Behav 135:13–23. https://doi.org/10.1016/j.anbehav.2017.10.027
Bhattacharjee D, Bhadra A (2020) Humans dominate the social interaction networks of urban free-ranging dogs in India. Front Psychol 11:2153
Bond ML, Lee DE, Farine DR, Ozgul A, König B (2021) Sociability increases survival of adult female giraffes. Proc R Soc B 288(1944):20202770
Borgatti SP, Everett MG, Freeman LC (2002) Ucinet for windows: software for social network analysis. Harvard, MA: Anal Technol 6:12–15
Borgeaud C, Bshary R (2015) Wild vervet monkeys trade tolerance and specific coalitionary support for grooming in experimentally induced conflicts. Curr Biol 25(22):3011–3016. https://doi.org/10.1016/j.cub.2015.10.016
Borgeaud C, Sosa S, Sueur C, Bshary R (2017) The influence of demographic variation on social network stability in wild vervet monkeys. Anim Behav 134:155–165
Bracken AM, Christensen C, O’Riain MJ, Fürtbauer I, King AJ (2022) Flexible group cohesion and coordination, but robust leader–follower roles, in a wild social primate using urban space. Proc R Soc B 289(1967):20212141
Canteloup C, Puga-Gonzalez I, Sueur C, van de Waal E (2021) The consistency of individual centrality across time and networks in wild vervet monkeys. Am J Primatol 83(2):e23232
Canty A, Ripley B (2017) Package ‘boot.’ R package version: 1–3
Chakraborty B, Kaburu SSK, Balasubramaniam KN, Marty PR, Beisner B, Bliss-Moreau E, Mohan L, Rattan SK, McCowan B (2023) Intragroup sociality drives individual participation in intergroup competition in an urban-dwelling nonhuman primate. BioRxiv, 2023.09.06.556573. https://doi.org/10.1101/2023.09.06.556573
Chapman C, Rothman J, Lambert J (2012) Food as a selective force in primates. In: Mitani, John C., et al. (eds) The evolution of primate societies. University of Chicago Press, Chicago
Chapman CA, Twinomugisha D, Teichroeb JA, Valenta K, Sengupta R, Sarkar D, Rothman JM (2016) How Do Primates Survive Among Humans? In: Waller, M. (eds) Mechanisms Employed by Vervet Monkeys at Lake Nabugabo, Uganda. Springer, Cambridge
Cheney DL, Seyfarth RM (1989) Redirected aggression and reconciliation among vervet monkeys. Cercopithecus Aethiops Behav 110(1):258–275
Cheney DL, Silk JB, Seyfarth RM (2016) Network connections, dyadic bonds and fitness in wild female baboons. R Soc Open Sci 3(7):160255
Chilvers BL, Corkeron PJ (2001) Trawling and bottlenose dolphins’ social structure. Proc Royal Soc London B: Biol Sci 268(1479):1901–1905
Chiyo PI, Moss CJ, Alberts SC (2012) The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLoS ONE 7(2):e31382
Croft DP, James R, Krause J (2008) Exploring animal social networks. Princeton University Press, Oxford
Davison AC, Hinkley DV (1997) Bootstrap methods and their application (vol 1). Cambridge University Press, Cambridge
De Waal FBM (1987) Tension regulation and nonreproductive functions of sex in captive bonobos (Pan paniscus). Natl Geogr Res 3:318–335
De Vries H, Stevens JM, Vervaecke H (2006) Measuring and testing the steepness of dominance hierarchies. Anim Behav 71(3):585–592
Dhawale AK, Sinha A (2022) Female friendships: social network analysis as a tool to understand intra-group affiliation in semi-commensal lion-tailed macaques (Macaca silenus). BioRxiv, 2009–2022
Downs CT, Alexander J, Brown M, Chibesa M, Ehlers Smith YC, Gumede ST, Hart L, Josiah KK, Kalle R, Maphalala M (2021) Correction to: Modification of the third phase in the framework for vertebrate species persistence in urban mosaic environments. Ambio 50:1879–1881
Gammell MP, De Vries H, Jennings DJ, Carlin CM, Hayden TJ (2003) David’s score: a more appropriate dominance ranking method than Clutton-Brock et al.’s index. An Behav 66(3):601–605
Gilchrist JS, Otali E (2002) The effects of refuse-feeding on home-range use, group size, and intergroup encounters in the banded mongoose. Can J Zool 80(10):1795–1802
Hart JDA, Weiss MN, Brent LJN, Franks DW (2022) Common permutation methods in animal social network analysis do not control for non-independence. Behav Ecol Sociobiol 76(11):151. https://doi.org/10.1007/s00265-022-03254-x
Henzi SP, Forshaw N, Boner R, Barrett L, Lusseau D (2013) Scalar social dynamics in female vervet monkey cohorts. Philos Trans R Soc B 368(1618):20120351. https://doi.org/10.1098/rstb.2012.0351
Hockings KJ, Anderson JR, Matsuzawa T (2012) Socioecological adaptations by chimpanzees, Pan troglodytes verus, inhabiting an anthropogenically impacted habitat. Anim Behav 83(3):801–810. https://doi.org/10.1016/j.anbehav.2012.01.002
Isbell LA (1991a) Contest and scramble competition: patterns of female agression and ranging behaviour among primates. Behav Ecol 2(2):143–155. https://doi.org/10.1093/beheco/2.2.143
Isbell LA (1991b) Contest and scramble competition: Patterns of female aggression and ranging behavior among primates. Behav Ecol 2(2):143–155. https://doi.org/10.1093/beheco/2.2.143
Isbell LA, Young TP (2002) Ecological models of female social relationships in primates: similarities, disparities, and some directions for future clarity. Behaviour 139(2):177–202
Isbell LA, Cheney DL, Seyfarth RM (1991) Group fusions and minimum group sizes in vervet monkeys (Cercopithecus aethiops). Am J Primatol 25(1):57–65
Josephs N, Bonnell T, Dostie M, Barrett L, Peter Henzi S (2016) Working the crowd: Sociable vervets benefit by reducing exposure to risk. Behav Ecol 27(4):988–994. https://doi.org/10.1093/beheco/arw003
Judge PG, de Waal FBM (1997) Rhesus monkey behavior under diverse population densities: coping with long-term crowding. Anim Behav 54(3):643–662
Judge PG, Griffaton NS, Fincke AM (2006) Conflict management by hamadryas baboons (Papio hamadryas hamadryas) during crowding: A tension-reduction strategy. Am J Primatol: Official J Am Soc Primatol 68(10):993–1006
Koyama NF, Dunbar RIM (1996) Anticipation of conflict by chimpanzees. Primates 37(1):79–86
Lacy KE, Martins EP (2003) The effect of anthropogenic habitat usage on the social behaviour of a vulnerable species. Cyclura Nubila An Conserv 6(1):3–9. https://doi.org/10.1017/S1367943003003020
Leiva D, de Vries H (2011) Steepness: testing steepness of dominance hierarchies. by R. pv 0.2. R package version 0.2. Accessed May 2018: http://CRAN.R-project.org/package=steepness.
Marty PR, Balasubramaniam KN, Kaburu SSK, Hubbard J, Beisner B, Bliss-Moreau E, Ruppert N, Arlet ME, Sah SAM, Ismail A (2019) Individuals in urban dwelling primate species face unequal benefits associated with living in an anthropogenic environment. Primates 61:249–255
McDonald M, Johnson S (2014) ‘There’s an app for that’: a new program for the collection of behavioural field data. Anim Behav 95:81–87
McKinney ML (2002) Urbanization, biodiversity, and conservation: the impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. Bioscience 52(10):883–890
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127(3):247–260
Mckinney T (2015) A classification system for describing anthropogenic influence on nonhuman primate populations. Am J Primatol 77(7):715–726. https://doi.org/10.1002/ajp.22395
McLennan MR, Spagnoletti N, Hockings KJ (2017) The Implications of Primate Behavioral Flexibility for Sustainable Human-Primate Coexistence in Anthropogenic Habitats. Int J Primatol 38(2):105–121. https://doi.org/10.1007/s10764-017-9962-0
Morrow KS, Glanz H, Ngakan PO, Riley EP (2019) Interactions with humans are jointly influenced by life history stage and social network factors and reduce group cohesion in moor macaques (Macaca maura). Sci Rep 9(1):20162. https://doi.org/10.1038/s41598-019-56288-z
Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A (2011) Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. An Behav 82(4):911–921
Patterson L, Kalle R, Downs C (2017) A citizen science survey: perceptions and attitudes of urban residents towards vervet monkeys. Urban Ecosyst 20:617–628
Patterson L, Kalle R, Downs C (2018) Factors affecting presence of vervet monkey troops in a suburban matrix in KwaZulu-Natal, South Africa. Landsc Urban Plan 169:220–228
Prange S, Gehrt SD, Hauver S (2011) Frequency and duration of contacts between free-ranging raccoons: uncovering a hidden social system. J Mammal 92(6):1331–1342
R Project (2013) R: A language and environment for statistical computing. Vienna, Austria. Accessed May 2018. http://www.r-project.org/
Royle NJ, Pike TW, Heeb P, Richner H, Kölliker M (2012) Offspring social network structure predicts fitness in families. Proc Royal Soc B: Biol Sci 279(1749):4914–4922
Saj T, Sicotte P, Paterson JD (1999) Influence of human food consumption on the time budget of vervets. Int J Primatol 20(6):974–977. https://doi.org/10.1023/A:1020886820759
Saj TL, Sicotte P, Paterson JD (2001) The conflict between vervet monkeys and farmers at the forest edge in Entebbe, Uganda. Afr J Ecol 39(2):195–199
SANBI (2017) South African National Biodiversity Institute. ONLINE] Available at https://www.sanbi.org/. Accessed 10 Oct 2017
Scheun J, Bennett NC, Ganswindt A, Nowack J (2015) The hustle and bustle of city life: Monitoring the effects of urbanisation in the African lesser bushbaby. Sci Nature 102(9):1–24. https://doi.org/10.1007/s00114-015-1305-4
Scheun J, Greeff D, Nowack J (2019) Urbanisation as an important driver of nocturnal primate sociality. Primates 60(4):375–381
Schino G, Nazionale C (2007) Grooming and agonistic support : a meta-analysis of primate reciprocal altruism. Behavioural Ecology 18:115–120. https://doi.org/10.1093/beheco/arl045
Seyfarth RM, Cheney DL (1984) Grooming, alliances and reciprocal altruism in vervet monkeys. Nature 308(5959):541–543. https://doi.org/10.1038/308541a0
Snijders L, Blumstein DT, Stanley CR, Franks DW (2017) Animal social network theory can help wildlife conservation. Trends Ecol Evol 32(8):567–577. https://doi.org/10.1016/j.tree.2017.05.005
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85(5):1101–1112
Sterck EHM, Watts DP, VanSchaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41(5):291–309. https://doi.org/10.1007/s002650050390
Sueur C, Jacobs A, Amblard F, Petit O, King AJ (2011) How can social network analysis improve the study of primate behavior? Am J Primatol 73(8):703–719. https://doi.org/10.1002/ajp.20915
Thatcher HR, Downs CT, Koyama NF (2019) Anthropogenic influences on the time budgets of urban vervet monkeys. Landsc Urban Plan 181:38–44
Thatcher HR, Downs CT, Koyama NF (2020) Understanding foraging flexibility in urban vervet monkeys, Chlorocebus pygerythrus, for the benefit of human-wildlife coexistence. Urban Ecosystems 23(6):1349–1357
Thatcher HR, Downs CT, Koyama NF (2023) Primates in the Urban Mosaic: Terminology, Flexibility, and Management. In: McKinney T, Waters S, Rodrigues MA (eds) Primates in Anthropogenic Landscapes: Exploring Primate Behavioural Flexibility Across Human Contexts. Springer, Switzerland, pp 121–137
van Schaik CP, Van Noordwijk MA (1998) Scramble and contest in feeding competition among female long-tailed macaques (Macaca fascicularis). Behaviour 105(1):77–98
van Schaik CP (1989) The ecology of social relationships amongst female primates. Comp Socioecol 195–218
Wittig RM, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL (2008) Focused grooming networks and stress alleviation in wild female baboons. Horm Behav 54(1):170–177. https://doi.org/10.1016/j.yhbeh.2008.02.009
Wrangham RW (1980) An ecological model of female bonded primate groups. Behavior 75(3):262–300
Young C, Majolo B, Heistermann M, Schülke O, Ostner J (2014) Responses to social and environmental stress are attenuated by strong male bonds in wild macaques. Proc Natl Acad Sci 111(51):18195–18200
Young C, McFarland R, Barrett L, Henzi SP (2017) Formidable females and the power trajectories of socially integrated male vervet monkeys. Anim Behav 125:61–67. https://doi.org/10.1016/j.anbehav.2017.01.006
Acknowledgements
We thank Simbithi Eco-estate for permission to conduct this study. We acknowledge and thank Liverpool John Moores University (UK), the University of KwaZulu-Natal (ZA) and the National Research Foundation (ZA, grant 98404) for their support of this study. This study was financially supported by Liverpool John Moores University and Erasmus Mundus (AEuropean and South African Partnership on Heritage and Past (AESOP)) grant number: ES15CM0025). Ethical clearance was obtained from Liverpool John Moores University under permit number NK_HT/2017-6.
Funding
This work was supported by the European and South African Partnership on Heritage and Past (AESOP)) (grant number: ES15CM0025). Liverpool John Moores University matched funding.
Author information
Authors and Affiliations
Contributions
Harriet Thatcher: Conceptualisation, Methodology, Formal Analysis, Writing Original Draft; Colleen Downs: Supervision, Writing Original Draft, Funding acquisition, Resources; Nicola Koyama: Conceptualisation, Supervision, Methodology, Writing Original Draft.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thatcher, H.R., Downs, C.T. & Koyama, N.F. Using social networks to explore the social flexibility of urban vervet monkeys. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01539-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01539-9