Abstract
Urban greening has become an increasingly popular strategy to improve urban life and human health. However, there are indications that the presence and extent of urban greenness may increase the abundance of wild rats. Therefore, we investigated which environmental and socio-economic factors are associated with rat abundance, with a focus on factors related to urban greenness. We systematically trapped rats (222 Rattus norvegicus and 5 Rattus rattus) in parks and residential areas in three cities in the Netherlands. We modelled the relative abundance of rats against various environmental and socio-economic variables. In addition, we compared municipality rat complaint data with our trapping data and analysed trap success over time. We observed positive relationships between the relative abundance of rats and both greenness (NDVI) and different proxies for food resources (restaurants and petting zoos). In addition, there were more municipality rat complaints in residential areas compared to parks, while there was a higher relative abundance of rats in parks. Our findings corroborate that greenness is associated with a higher abundance of wild rats, and that municipality rat complaints may underestimate the abundance of rats in greener urban areas. This study provides new insights on factors affecting relative rat abundance in cities and can guide policy makers and city planners how to minimize rat nuisance in the greener parts of cities. By taking these potential effects of urban greenness on rat abundance into account, measures can be taken that on the one hand maintain the beneficial effects of urban greening, but at the same time reduce the carrying capacity for rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anthropophilic nature of wild brown rats (Rattus norvegicus) and black rats (Rattus rattus) has made them some of the most successful and abundant mammal species in urban areas (Feng and Himsworth 2014). They are considered pest species that need to be controlled to prevent gnawing damage, mental stress, and zoonotic pathogen transmission (Himsworth et al. 2013; Lederer et al. 2006; Murray et al. 2021). However, in practice rats are rather difficult to control, in part due to their neophobic behaviour towards new things, high adaptability to new environments, and level of cognition (Clapperton 2006; Feng and Himsworth 2014; Taylor and Thomas 1989). In order to prevent uncontrolled growth of rat populations and to optimize control measures, insight is needed in the environmental and socio-economic factors that promote rat abundance in urban areas.
Previous studies showed that rat abundance can be explained by the presence of (food) waste, the maintenance/age/ownership of buildings and houses, the presence of impervious surfaces, the number of restaurants in the area, socio-economic status of inhabitants, the type of sewage system present and human population density (Himsworth et al. 2014a; Tamayo-Uria et al. 2014; Traweger et al. 2006; van Adrichem et al. 2013). These environmental and socio-economic factors either directly or indirectly affect the availability of food and/or shelter. For example, income and the percentage of owner-occupied houses have been shown to be negatively related to rat abundance, probably because areas with higher socio-economic status have less (food) littering in public areas and improved residential upkeep, limiting shelter opportunities (Himsworth, Parsons, et al. 2014a, b; Masi et al. 2010). Shelter can vary from natural soil for making burrows to badly maintained buildings, or sewers (Traweger et al. 2006; van Adrichem et al. 2013). The condition of sewers can deteriorate over time, e.g., by cracks and blind ends, increasing the likelihood of rats using them as shelter. Combined sewage systems are particularly at risk, as they are generally older than separated sewage systems (van Adrichem et al. 2013). However, the relative importance of these environmental and socio-economic factors seems to vary even within cities over very short geographical distances (Himsworth et al. 2014a; Traweger et al. 2006).
Another potentially important but understudied factor for explaining rat abundance is the extent of vegetation or “greenness” of urban areas. Urban green spaces could provide food and shelter, two important requirements for rats, besides water (Masi et al. 2010; Sacchi et al. 2008). Previous studies found fewer rats when vegetation was absent and pavement was present (Masi et al. 2010; Tamayo-Uria et al. 2014; Traweger et al. 2006), which suggests that rat abundance is positively correlated with greenness, but detailed studies are lacking. A better understanding of the relationship between rat abundance and greenness is important, as urban greening is an increasingly popular strategy to increase mental health, biodiversity, and water retention, and to reduce urban heat island effects (Coutts and Hahn 2015; Green et al. 2016; Lohmus and Balbus 2015). However, its potential negative effects, such as potential increases in the abundance of pest species, have not been thoroughly investigated. We hypothesize that greener areas can provide a high availability of human food waste (which can get easily stuck or hidden in dense vegetation), and natural food resources (e.g., fruit and nut trees). Moreover, greener areas may provide a more natural environment for digging burrows, potentially increasing shelter opportunities for rats. This could in turn result in decreased predation risk, all of which may lead to a higher abundance of rats. Alternatively, predation risk might also be higher in greener urban areas due to higher predator abundance, which would negatively affect rat abundance. The net effect of these opposing forces remains unclear. Increasing our knowledge about the relationship between rat abundance and greenness, in relation to other environmental and socio-economic factors may enable smarter city design, and more effective preventive and control measures.
Here, we investigated the associations between rat abundance and various environmental and socio-economic factors in urban areas, with a focus on greenness. We systematically trapped rats in three cities, and modelled the relationship between these factors and relative rat abundance. Data on relative rat abundance was also compared with municipality rat complaint data to examine how well municipality rat complaint data reflects actual rat abundance. Furthermore, we investigated which factors affected trapping success over time to optimize rat trapping.
Materials & Methods
Ethics statement
This study was approved by the Dutch Animal Experiments Committee (DEC) (project number AVD3260020172104).
Study area & rat trapping
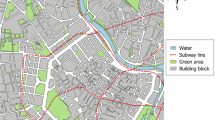
Rats were trapped in the cities of Amsterdam, Eindhoven, and Rotterdam, the Netherlands (Fig. 1). Amsterdam (219 km2) is the capital and the largest city of the Netherlands (41,850 km2), and is inhabited by approximately 870,000 people. Rotterdam (324 km2; approximately 590,000 inhabitants) and Eindhoven (89 km2; approximately 240,000 inhabitants) are respectively the second and fifth largest cities in the Netherlands (CBS 2023). Both Amsterdam and Rotterdam have a river running through the city, in contrast to Eindhoven. Brown rats are present in all three cities, but only in Eindhoven black rats are also present in the city. All cities have a similar temperate maritime climate and a similar composition of urban wildlife species. Trapping locations were divided into parks and residential areas. In each city, trapping locations were selected based on the percentages of greenness, according to the ‘Green map’ of the Netherlands (Atlas leefomgeving 2017). In residential areas, we selected locations with varying percentages of greenness (about half of the locations < 40% greenness and half > 40% greenness) to ensure sufficient variation in the percentage of greenness between trapping locations. The minimum distance between locations was 200m.
Wild brown rats (Rattus norvegicus) and black rats (Rattus rattus) were trapped using snap traps (AF Rat Box together with either the Snap-E Rat trap (Killgerm, the Netherlands) or the Gorilla rat trap (Futura, Germany)). Relative abundance of rats can also be assessed using live traps (Himsworth et al. 2014b; Traweger et al. 2006), but we chose to use snap traps because this study was part of a larger study in which rats were tested for zoonotic pathogens (de Cock et al. 2023). Rats were trapped alternatingly in Amsterdam and Eindhoven between May and October 2020, and one year later in Rotterdam in the same months as Amsterdam the year before. In total, rat trapping took place in 48 different locations (18 in Amsterdam, 18 in Rotterdam, and 12 in Eindhoven) divided over 16 parks and 32 residential areas (Fig. 1 and Table S1).
Per trapping location, 20 traps were placed within an area of approximately 100 × 100 m. In most cases, trapping took place in public areas. When public areas were not available, we used front gardens from private properties after consent from the owners. Traps were randomly placed and evenly distributed (minimum distance of 10 m between traps) along walls and fences or under vegetation. Furthermore, traps could only be positioned in places where they did not obstruct sidewalks and where they could be secured using ground anchors to prevent theft. One trapping period lasted four consecutive weeks, consisting of two weeks of pre-baiting to reduce neophobic behaviour (Taylor and Thomas 1989) followed by two weeks of trapping. The traps were pre-baited with a 1:1:1 mixture of oats, peanuts, and cocktail nuts. During the two weeks of trapping, traps were set and checked each weekday around the same time. When bait had been eaten, the traps were resupplied. On weekends, the traps were baited but non-active. On Mondays, the traps were activated again, resulting in 7 or 8 active trapping days per trap. For each rat, the date and location (GPS coordinates) of trapping was recorded. Rats were classified into age categories based on their bodyweight. For males we used: juvenile (< 100 g), subadult (101–200 g), and adult (> 200 g), and for females we used: juvenile (< 100 g), subadult (101-175 g), and adult (> 175 g) (Franssen et al. 2016).
Relative rat abundance
For each trapping day and location, we recorded the number of traps set, the number of rats trapped, and the number of traps triggered for other reasons (e.g., trapping non-target species or traps damaged by people). To compare wild rat abundance between locations, we calculated the relative rat abundance per location using a trap success index (Himsworth et al. 2014a). From now on, we will use the term relative rat abundance to refer to this trap success index. This index was calculated using the formulas below (Cavia et al. 2012; Nelson and Clark 1973). Stolen or damaged traps were excluded from these calculations. We subtracted half of the traps triggered by other reasons because it is not possible to know whether they were inactive from the beginning, during, or at the end of the trapping night. Therefore, it is assumed that on average these traps were inactive half of the time (Cavia et al. 2012).
Municipality rat complaint data
Besides quantifying relative rat abundance using snap or live traps, rat complaint data is used as a proxy for rat abundance (Murray et al. 2018; van Adrichem et al. 2013). While it has been shown that rat complaint data has the potential to adequately reflect rat abundance, it is prone to bias caused by various factors, such as the knowledge of citizens on how to file a rat complaint, and the tolerance level of citizens for rat nuisance in order to actively engage in filing a rat complaint (Murray et al. 2018). We wanted to test how well municipality rat complaint data reflected relative rat abundance measured using snap traps. We received rat complaint data from Amsterdam, Rotterdam and Eindhoven, which is comprised of the total number of rat complaints filed by citizens both online and by phone. For our analyses we used the total number of rat complaints in a radius of 150m around each trapping location three and six months prior to rat trapping.
Environmental and socio-economic variables
The environmental factors considered in this study included: greenness, presence of fruit- and nut-bearing vegetation, distance to the nearest water body, amount of (food) waste present, type of sewage system, number of restaurants, and presence or absence of petting zoos (Table 1). We quantified greenness using the Normalized Difference Vegetation Index (NDVI). NDVI quantifies vegetation greenness in a satellite image by measuring the difference between near-infrared (reflected by vegetation) and red light (absorbed by vegetation) in a range from 0 (no vegetation present) to 1 (only vegetation present). Water surfaces were excluded from the NDVI map and from subsequent NDVI calculations, using ArcGIS (ESRI ArcGIS™ version 10.8, CA, USA). NDVI was calculated using satellite maps from June 2020 and 2021, depending on the trapping year per location (Groenmonitor 2022). Fruit- and nut-bearing vegetation included apple (Malus domestica), pear (Pyrus communis), cherry (Prunus avium), plum (Prunus domestica), blackberry (Rubus plicatus), wild strawberry (Fragaria vesca), dog rose (Rosa canina), chestnut (Castanea spp.), hazel (Corylus avellana), and walnut (Juglans regia). We measured the number of (food) waste items, the number of waste bins, the number of restaurants, and the presence of petting zoos, as proxies for the availability of food resources (Table 1). Food waste items included actual food items, while waste items included all waste excluding food items (e.g., plastic and paper wrappings). The variables NDVI, number of restaurants, and the presence of petting zoos were calculated within a circular buffer with a 150 m radius around each trapping location, representing the average home range of rats (Badi et al. 1992; Davis 1953; Gardner‐Santana et al. 2009; Heiberg et al. 2012; Recht 1988) using QGIS version 3.16 (GIS Development Team 2022). To calculate the shortest distance between trapping sites and the nearest water body (m) in QGIS, we used a shapefile of national water bodies, which includes natural public water bodies such as rivers, canals, lakes, streams, ponds and ditches (pdok 2022). All other environmental variables were measured within the trapping locations (100 × 100 m). We also included the type of sewage system (combined versus separated) (van Adrichem et al. 2013).
The socio-economic variables considered in this study included: mean yearly income, human population density, and the percentage of owner-occupied houses (Table 1). To correct for trapping city and trapping season, we included city and season as fixed factors in the model. Seasons were defined as follows: spring (May), summer (June, July, and August) and autumn (September and October).
Data analysis
All statistical analyses were conducted in R studio version 4.0.3 (RStudio team 2015). All numerical variables were standardized using a z-transformation with two standard deviations (Gelman 2008). Collinearity between predictor variables was assessed using the corrplot package. In case of highly correlated predictor variables (rS > 0.7), only one of the two predictor variables was retained. Multicollinearity was tested using the variance inflation factor (VIF). Variables with a VIF score > 5 were excluded from the model. We checked the model assumptions with functions from both the DHARMa and performance package. Results were considered significant when p < 0.05.
Relative rat abundance model
We modelled the relationship between relative rat abundance and environmental and socio-economic predictor variables. As our data contained a lot of zero’s (42%), we analysed our data using zero-inflated negative binomial (ZINB) models with the glmmTMB package. ZINB models consists of a count part to model the values including true zero values (e.g., no rats present at the site), and a zero-inflated part to model the false zero values (e.g., when rats are present but not captured). The ZINB model used “the number of rats trapped per location” as the count outcome variable, offset by “the number of effective trapping nights”. All environmental and socio-economic predictor variables (Table 1) were included in the count part of the initial full multivariate model. We corrected for city and season by including them as fixed factors in the model. The zero-inflated part of the model accounts for the presence of both true zeros (no rats present) and false zeros (rats present but not captured). As the incentive of rats to enter traps can be influenced by the availability of food resources (e.g., rats being less prone to enter the traps when there are ample other food resources available) (Clapperton 2006; Taylor and Thomas 1989), we decided to include the food-related variables (e.g., number of restaurants, presence of waste, presence of petting zoos, and presence of fruit- and nut bearing vegetation) in the zero-inflated part of the model. Variables that were highly correlated (rS > 0.7 or VIF > 5) were excluded from the final model. Post-hoc tests were performed based on the Tukey method using the Emmeans package. For the trapping locations defined as “parks” (n = 16/48), we created an additional univariate negative binomial model to analyse the relationship between relative rat abundance and park size (km2; data from Google).
Comparing relative rat abundance and municipality rat complaint data
We used Kendall correlation tests to assess how well municipality rat complaint data reflected relative rat abundance. Similarities and discrepancies between both datasets were also tested and visualized per location type (park versus residential area).
Comparing rat trap success over time between parks and residential areas
In each trapping location, rats were trapped during eight trapping nights. We modelled the relationships between relative rat abundance per trapping night and the following variables: trapping night (night 1 – night 8), temperature, precipitation, season, and location type (park versus residential area). For temperature we included the minimum temperature (°C) of the day preceding the trapping night, and for precipitation we included the mean precipitation (mm) of the day preceding the trapping night (KNMI 2020–2021). This was modelled by using a ZINB model with “the number of rats trapped per location per trapping night” as count outcome variable, offset by “the number of effective traps per location per trapping night”. All variables were included in the count part of the initial full multivariate model. We included location type in the zero inflated part of the model, because we know from municipality pest controllers that control is less frequent in parks compared to residential areas. This might lead to an increased aversion of rats from residential areas to traps (e.g., false zeros) compared to rats from parks which have been less exposed to traps and might therefore more easily enter these traps (Clapperton 2006; Greaves 1994). We included city and trapping location as nested random factors in the model. Variables that were highly correlated (VIF > 5) were excluded from the final model. Post-hoc tests were performed based on the Tukey method using the Emmeans package.
Results
In total, 227 rats were trapped during this study, consisting of 222 (98%) brown rats and 5 (2%) black rats. Due to the low number of black rats trapped, we excluded black rats from subsequent analyses. In Amsterdam we trapped 137 brown rats, and in Rotterdam 85 brown rats. In Eindhoven we trapped only five black rats, no brown rats. Of the 222 trapped brown rats, 129 were female (59%), 91 were male (41%), while the sex could not be determined for two rats. There were 26% adults (58/222), 29% sub-adults (65/222), and 45% juveniles (99/222). The highest number of rats were trapped in two parks in Amsterdam (Fig. 1 and Table S1).
Relative brown rat abundance per trapping location. Triangles and dots represent trapping locations in parks and residential areas, respectively. The size of the grey circles around each dot represents the relative abundance of brown rats. Dots or triangles without circles represent a relative rat abundance of zero. The degree of greenness per location is visualized by the NDVI gradient ranging from 0.23 (purple) to 0.87 (green). An overview of the exact relative rat abundance per trapping location can be found in Table S1
Relative rat abundance is related to greenness
We tested the relationship between relative rat abundance and various socio-economic and environmental variables (Fig. 2). The variable “human population density” was excluded from the model due to high correlation with “greenness” (rS = -0.77, p < 0.001), and the variable “number of food waste items” was excluded due to high correlation with “number of waste items” (rS = 0.73, p < 0.001). The variable “number of waste bins” was also excluded from the count part of the model due to high multicollinearity (VIF > 5). The variable “fruit- and nut-bearing vegetation” was excluded from the zero-inflated part of the model due to model convergence problems. The final multivariate ZINB model included 11 variables in the count part of the model and three variables in the zero part of the model (Table 2). The final model showed positive relationships (in order of decreasing effect size) between relative rat abundance and the presence of petting zoos (β = 1.90, p = 0.019), greenness (β = 1.70, p = 0.003), and number of restaurants (β = 1.29, p < 0.001; Table 2 and Fig. 3). There were also positive trends, albeit not significant, between relative rat abundance and mean income (β = 0.81, p = 0.052) and the number of waste items (β = 0.77, p = 0.052; Table 2 and Fig. 3). There was a significant negative, albeit weak, correlation between the number of waste items and greenness (rS = -0.41, p = 0.003; Fig. S1), and no correlation between income and greenness (rS = 0.10, p = 0.480). In a separate model including only the rats trapped in parks as the outcome variable, we observed no significant relationship between relative rat abundance and park size (β = -0.60, SE = 0.90, p = 0.506; Table S1).
Distribution patterns of numeric predictor variables (e.g., greenness, distance to nearest water body, percentage of owner-occupied houses, mean yearly income, presence of petting zoos, number of restaurants, number of general waste items, number of food waste items, number of waste bins, and population density. The blue line represents the median value per predictor
Expected changes in relative rat abundance based on the probabilities of relative rat abundance and significant numerical predictor variables resulting from the model (e.g., greenness and number of restaurants; Table 2). Trendlines (blue) are added in the plots
Fewer rat complaints in parks
We compared the relative rat abundance per location with the total number of municipality rat complaints three and six months prior to trapping. We found significantly positive, albeit weak, correlations between relative rat abundance and the number of municipality rat complaints three and six months prior to trapping (τ = 0.23, p < 0.05, and τ = 0.30, p < 0.007, respectively). When we excluded the parks and only looked at the correlation between municipality rat complaints (6 months prior to rat trapping) and relative rat abundance in residential areas, we observed a significantly positive and slightly higher, albeit still weak, correlation (τ = 0.40, p < 0.004). For parks only, this relationship was slightly stronger compared to residential areas (τ = 0.49, p < 0.02). Overall, there seemed to be less municipality rat complaints in parks compared to residential areas (Fig. 4 and Table S1). Thus, the number of rat complaints in parks may be an underestimation of actual rat abundance.
Relationship between relative rat abundance per location and the number of municipality rat complaints in the six months prior to rat trapping per location type (e.g., parks in blue, and residential areas in red). Locations within different cities are visualized with different shapes (e.g. a dot for Amsterdam, a plus sign for Eindhoven, and a triangle for Rotterdam)
Trap success decreases over time and is higher in parks compared to residential areas
In total, we had 6846 effective trapping nights during this study. In 222 (3%) of these trapping nights a rat was trapped. With a ZINB model, we examined the relationship between relative rat abundance and the following predictor variables: trapping night (1–8), temperature (°C), precipitation (mm), and location type (park versus residential area). We observed a significantly lower relative rat abundance in residential areas compared to parks (β = -1.19, SE = 0.49, p = 0.015; Fig. 5B and Table S2). We also found that relative rat abundance significantly decreased after the second trapping night (Fig. 5A, Table S2 and S3).
A. Relative rat abundance per trapping night (1–8). Letters X and Y indicate significant differences between trapping nights (p < 0.05). B. Total relative rat abundance per location type (e.g., park and residential area). The asterisk (*) indicates a significant difference between location types (p < 0.05). The horizontal lines within bars show the median
Discussion
In this study, we investigated which environmental and socio-economic factors are related to urban rat abundance, with a focus on greenness. We observed significant positive relationships between relative rat abundance and both greenness and factors related to food availability. In addition, municipality rat complaints were lower in parks compared to residential areas, while the relative abundance of rats was higher in parks.
Positive relationship between relative rat abundance and greenness
We observed a strong positive relationship between relative rat abundance and greenness. This is in line with previous research that reported a higher abundance of rats in urban green spaces (Masi et al. 2010; Tamayo-Uria et al. 2014; Traweger et al. 2006). Our result suggests that greenness enables rat populations to grow into larger numbers, potentially by providing suitable habitat (e.g., food and shelter), and thereby allowing a higher rat carrying capacity. This, in combination with no or little pest control in public green spaces such as parks (personal communication with municipality pest control technicians), could facilitate the growth and maintenance of rat populations. However, these results should be interpreted with caution, as food availability and differences in avoidance behaviour of rats towards traps could differ between greener and less green areas, which we discuss in more detail below. Moreover, the term greenness encompasses a wide range of plant species and structural variations, which could have varying impacts on rat abundance but were not considered in this study. For example, certain vegetation types may provide rats with more coverage to hide, as rats avoid open space (Barnett 2017). Traweger et al. (2006) studied the relationship between rat abundance and vegetation types, and observed significant positive relationships between rat abundance and the presence of bushes, trees, ruderal vegetation, vegetation with fruits, riverbank vegetation, conifers, and deciduous trees, and a significant negative relationship between rat abundance and the presence of evergreens. In contrast, we did not observe a positive relationship between rat abundance and vegetation with fruits. We hypothesize that when ample other (human-provided) food resources are available, the abundance of rats will be influenced more by those food resources than by the presence of vegetation with fruits or nuts. In addition, fruit or nut vegetation only provides food during specific times of the year, which might be insufficient and too unreliable to support large rat populations whole year round.
Relationships between relative rat abundance and variables other than greenness
We observed significant positive relationships between relative rat abundance and the number of restaurants and the presence of petting zoos, and an almost significant relationship between relative rat abundance and the number of waste items. Previous studies showed a positive relationship between rat abundance and animal feed, which could explain the observed positive relationship with petting zoos (Masi et al. 2010; Miller and Bromley 1988; Traweger et al. 2006). The number of restaurants and waste items could also be proxies for food availability. The positive relationship with these variables is in line with previous studies, which found rat abundance to be associated with food waste (Masi et al. 2010; Traweger et al. 2006). Distance to water was not a significant variable in our model, but was significant in previous studies (Sacchi et al. 2008; Traweger et al. 2006). This could be explained by the fact that all of our parks contained water bodies within the 100 × 100 m trapping locations, and that Amsterdam and Rotterdam are both cities characterized by a significant presence of water, such as canals and rivers. As a result, distance to water was not a limiting factor, and therefore relatively less important when compared to the other variables.
It should be noted that rats were trapped in 2020 and 2021 during the Covid-19 pandemic, which could have slightly altered their abundance and distribution compared to other years. For example, the temporary closing of restaurants may have decreased food availability, forcing rats to seek food resources elsewhere. At the same time, people more frequently visited urban parks (Geng et al. 2021), which may have resulted in increased littering and thus food availability for rats in parks. Both of these effects may have contributed to higher rat abundances in parks.
Snap trap bias?
By systematically trapping rats, we were able to compare the relative abundance of rats between locations. However, as with most abundance measurements, the outcome needs to be interpreted with caution (Nottingham et al. 2021). From what we observed in the field together with previous research, we hypothesize that trap success is influenced by food availability and rat behaviour. When more food (waste) is available, there is less incentive for rats to enter the traps for food. Rats are neophobic animals, i.e. they avoid unfamiliar objects and will probably prefer food resources that are familiar or perceived as less dangerous (Clapperton 2006; Taylor and Thomas 1989). This could lead to relatively lower trap success and thus lower abundance estimates in areas with high food (waste) availability, and could therefore underestimate the relationship between waste and relative rat abundance. To what extent neophobia influences rat’ behaviour towards traps (e.g., avoidance) is difficult to predict, as it depends among other things on their previous experiences with traps (Clapperton 2006; Greaves 1994). Therefore, rats living in different environments (e.g., parks versus residential areas) may show different behaviour towards traps based on previous encounters, which could influence trap success and thus abundance estimates. For example, rats in parks might be more prone to enter traps because they have had less previous negative experiences with traps compared to rats in residential areas, as pest control is, in general, less intensive in parks compared to residential areas in the Netherlands. This could lead to an overestimation of rats in parks compared to residential areas. However, we did not observe significant differences between the response of rats to the traps (when looking at the trap success over time) in parks versus residential areas, which might indicate that the behaviour of rats from both areas is not that different. To account for the effect of neophobia in different locations, a suggestion for future research is to combine the use of snap traps with additional techniques to estimate rat abundance, such as chew cards or (short focal) camera traps (Nottingham et al. 2021; Sweetapple and Nugent 2011). In this study we tried to deploy camera traps in parallel to rat trapping, but this proved to be challenging. Especially in residential areas it was hard to install a camera trap without serious risk of theft(data not shown). Therefore, chew cards or deep learning-based systems to detect and analyse ultrasonic vocalizations, such as DeepSqueak, might be better alternatives (Coffey et al. 2019).
Fewer rat complaints yet more rats trapped in parks compared to residential areas
The number of rat complaints was lower than the number of rats trapped in parks compared to residential areas. Rats could be perceived less of a problem in public space compared to the home environment, or rats can hide better when there is more vegetation, which makes them less visible, and which could lead to fewer rat complaints. The relatively lower number of complaints in parks may lead to less intensive pest control, which may contribute to rats being more abundant in these greener urban areas. Nonetheless, it is important to be aware of a higher rat abundance in parks despite a low number of complaints, because we hypothesize that these rat populations might serve as source populations for neighbouring residential areas.
The correlation between the number of rat complaints and the number of rats trapped per trapping location was positive, but weak. In contrast, previous research in Chicago found a strong positive relationship between rat complaints and rat abundance (Murray et al. 2018). However, they used the total number of rat complaints in the last 12 months prior to trapping and in a 1 km radius of each trapping location, which is both a longer time period and larger area than we used here. It is known that rat complaints can be biased by certain factors such as knowledge on how to file a complaint and individual rat tolerance level or attitude (Murray et al. 2018, 2021). In addition, the municipality rat complaint dataset is probably not totally accurate, due to some misclassifications of citizens (e.g., filing a brown rat complaint while it is actually a mouse). Societal changes can also lead to changes in reporting complaints. For example, during the Covid-19 pandemic, various municipalities—including the municipality of Amsterdam – experienced surges in complaints about garbage piling up followed by surges of rat complaints in those same locations, mainly residential areas (personal communication with Amsterdam’s municipality pest control and (Sarkodie and Owusu 2021)). Although the additional garbage piling up could have attracted more rats, the increase in rat complaints might also be caused by the fact that people were at home much more, thereby increasing the chance of spotting the rats that were already there (Murray et al. 2021).
In addition, we observed that most rats were trapped in the first days of trapping, after which the number of trapped rats declined. This is in line with previous research (Himsworth et al. 2014a). These results suggest that for pest control it might be more effective to have a high trap effort for a few days instead of lower trap effort for a longer period.
In this study we trapped five black rats, but no brown rats in Eindhoven, while we know from local pest control technicians and from previous unpublished research that brown rats are present. Whether this difference reflects actual differences in rat abundance (a potential relationship between the size of both the city and the rat population), or is caused by behavioural differences, or other unknown differences between Eindhoven and the other cities remains unclear. The fact that no black rats were trapped in Amsterdam and Rotterdam is not surprising, since the distribution of black rats is mostly limited to the south of the Netherlands and to harbour areas.
Conclusions
We observed a significant positive relationship between the relative abundance of rats and both greenness and different proxies for food resources (restaurants, waste items, and petting zoos). This suggests that, in addition to greenness, food availability is one of the driving factors for rat abundance in Dutch cities, which is largely influenced by human behaviour. While this study shows that greenness may be important for rat abundance, there might be other factors contributing to this observed relationship, such as the altered behaviour of rats towards traps in greener versus less green areas, which might be influenced by variation in food resource availability and differences in avoidance behaviour towards traps based on previous encounters. In addition, greenness is a broad concept encompassing a wide range of vegetation types, which calls for more precise studies to disentangle the effects of different vegetation types on rat abundance. To minimize rat abundance, the general public, policy makers and city planners need to collaborate to limit the availability of food and shelter using a multifactorial approach with measures that take into account both human awareness and city design, especially in greener urban areas. Measures that can be implemented could focus on limiting the availability of food resources in greener urban areas, for example by avoiding easy access to food waste through increasing the number of waste bins, increasing the frequency of garbage collection, altering the design of waste bins to make them less accessible for rats to enter, and by increasing human awareness towards food waste. Measures could also focus on adapting urban green spaces by choosing different vegetation types (e.g., vegetation types that provide less shelter for rats), but this should first be evaluated in future studies. Likely, the absence of either or both food and shelter resources in greener urban areas will limit the abundance of rats. By taking measures to reduce the abundance of rats, the positive effects of urban greening can be retained, while its negative effects associated with rats and their risks posed to human health can be reduced.
Data availability
Data is available upon request.
References
Atlas leefomgeving (2017) Groenkaart van Nederland. https://www.atlasleefomgeving.nl/groenkaart-van-nederland
Badi M, Iliadis N, Sarris K (1992) Natural and experimental infection of rodents (Rattus norvegicus) with Salmonella gallinarum. Berl Munch Tierarztl Wochenschr 105(8):264–267
Barnett SA (2017) The rat: A study in behavior. Routledge
Cavia R, Cueto GR, Suárez OV (2012) Techniques to estimate abundance and monitoring rodent pests in urban environments. Integrated Pest Management and Pest Control—Current and Future Tactics. InTech, 147–172
CBS (2018) Percentage koopwoningen - 100 meter vierkant. https://cbsinuwbuurt.nl/#sub-vierkant100m2018_percentage_koopwoningen
CBS (2020) Aantal inwoners - 100 meter vierkant. https://cbsinuwbuurt.nl/#sub-vierkant100m2020_aantal_inwoners
CBS (2023) Bevolkingsteller. https://www.cbs.nl/nl-nl/visualisaties/dashboard-bevolking/bevolkingsteller
Clapperton BK (2006) A review of the current knowledge of rodent behaviour in relation to control devices (Vol. 263). Science & Technical Pub., Department of Conservation Wellington, New Zealand
Coffey KR, Marx RG, Neumaier JF (2019) DeepSqueak: a deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 44(5):859–868
Coutts C, Hahn M (2015) Green Infrastructure, Ecosystem Services, and Human Health. Int J Environ Res Public Health 12(8):9768–9798. https://doi.org/10.3390/ijerph120809768
Davis DE (1953) The characteristics of rat populations. Q Rev Biol 28(4):373–401
de Cock MP, de Vries A, Fonville M, Esser HJ, Mehl C, Ulrich RG, Joeres M, Hoffmann D, Eisenberg T, Schmidt K (2023) Increased rat-borne zoonotic disease hazard in greener urban areas. Sci Total Environ 896:165069
Feng AY, Himsworth CG (2014) The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus). Urban Ecosystems 17(1):149–162
Franssen F, Swart A, van Knapen F, van der Giessen J (2016) Helminth parasites in black rats (Rattus rattus) and brown rats (Rattus norvegicus) from different environments in the Netherlands. Infection Ecology & Epidemiology 6(1):31413
Gardner-Santana LC, Norris D, Fornadel C, Hinson E, Klein S, Glass GE (2009) Commensal ecology, urban landscapes, and their influence on the genetic characteristics of city-dwelling Norway rats (Rattus norvegicus). Mol Ecol 18(13):2766–2778
Gelman A (2008) Scaling regression inputs by dividing by two standard deviations. Stat Med 27(15):2865–2873
Gemeente Amsterdam (2022) Bomen - in beheer van Gemeente Amsterdam. https://maps.amsterdam.nl/bomen/?LANG=nl
Geng D, Innes J, Wu W, Wang G (2021) Impacts of COVID-19 pandemic on urban park visitation: a global analysis. Journal of Forestry Research 32:553–567
GIS Development Team (2022) QGIS Geographic Information System. In Q. Association (Ed.): https://www.qgis.org
Greaves J (1994) Resistance to anticoagulant rodenticides
Green OO, Garmestani AS, Albro S, Ban NC, Berland A, Burkman CE, Gardiner MM, Gunderson L, Hopton ME, Schoon ML (2016) Adaptive governance to promote ecosystem services in urban green spaces. Urban Ecosystems 19(1):77–93
Groenmonitor (2022) Groenindex (NDVI). https://www.groenmonitor.nl/groenindex
Heiberg A-C, Sluydts V, Leirs H (2012) Uncovering the secret lives of sewer rats (Rattus norvegicus): movements, distribution and population dynamics revealed by a capture–mark–recapture study. Wildl Res 39(3):202–219
Himsworth CG, Jardine CM, Parsons KL, Feng AY, Patrick DM (2014a) The characteristics of wild rat (Rattus spp.) populations from an inner-city neighborhood with a focus on factors critical to the understanding of rat-associated zoonoses. PLoS One 9(3), e91654. https://doi.org/10.1371/journal.pone.0091654
Himsworth CG, Parsons KL, Feng AY, Kerr T, Jardine CM, Patrick DM (2014b) A mixed methods approach to exploring the relationship between Norway rat (Rattus norvegicus) abundance and features of the urban environment in an inner-city neighborhood of Vancouver. Canada Plos One 9(5):e97776. https://doi.org/10.1371/journal.pone.0097776
Himsworth CG, Parsons KL, Jardine C, Patrick DM (2013) Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis 13(6):349–359. https://doi.org/10.1089/vbz.2012.1195
KNMI (2020–2021) Daggegevens van het weer in Nederland. https://www.knmi.nl/nederland-nu/klimatologie/daggegevens
Lederer W, Kinzl JF, Trefalt E, Traweger C, Benzer A (2006) Significance of working conditions on burnout in anesthetists. Acta Anaesthesiol Scand 50(1):58–63. https://doi.org/10.1111/j.1399-6576.2005.00867.x
Lohmus M, Balbus J (2015) Making green infrastructure healthier infrastructure. Infect Ecol Epidemiol 5:30082. https://doi.org/10.3402/iee.v5.30082
Masi E, Pino FA, Santos M, d. G. S., Genehr, L., Albuquerque, J. O. M., Bancher, A. M., & Alves, J. C. M. (2010) Socioeconomic and environmental risk factors for urban rodent infestation in Sao Paulo. Brazil Journal of Pest Science 83(3):231–241
Miller EJ, Bromley PT (1988) Wildlife damage control in Virginia. Controlling rodents in homes
Murray MH, Byers KA, Buckley J, Magle SB, Maffei D, Waite P, German D (2021) “I don’t feel safe sitting in my own yard”: Chicago resident experiences with urban rats during a COVID-19 stay-at-home order. BMC Public Health 21(1):1–14
Murray MH, Fyffe R, Fidino M, Byers KA, Ríos MJ, Mulligan MP, Magle SB (2018) Public Complaints Reflect Rat Relative Abundance Across Diverse Urban Neighborhoods. Front Ecol Evol 6:189
Nelson L, Clark FW (1973) Correction for sprung traps in catch/effort calculations of trapping results. J Mammal 54(1):295–298
Nottingham CM, Glen AS, Stanley MC (2021) Relative efficacy of chew card and camera trap indices for use in hedgehog and rat monitoring. New Zealand Journal of Zoology 48(1):32–46
Open Data Eindhoven (2022b) Riolering en aansluitingen. https://data.eindhoven.nl/explore/dataset/riolering-en-aansluitingen/export/
Open data van de overheid (2022) Bomen Rotterdam. https://data.overheid.nl/dataset/6098-bomen-rotterdam#panel-description
pdok (2022) BGT: Basisregistratie grootschalige topografie. https://www.pdok.nl/introductie/-/article/basisregistratie-grootschalige-topografie-bgt
Recht MA (1988) The biology of domestic rats: telemetry yields insights for pest control. Proceedings of the Vertebrate Pest Conference
RStudio team (2015) RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com, 42(14), 84
Sacchi R, Gentilli A, Pilon N, Bernini F (2008) GIS-modelling the distribution of Rattus norvegicus in urban areas using non toxic attractive baits. Hystrix 19(1)
Sarkodie SA, Owusu PA (2021) Impact of COVID-19 pandemic on waste management. Environ Dev Sustain 23:7951–7960
Statline CBS (2019) Kerncijfers wijken en buurten 2019. https://opendata.cbs.nl/#/CBS/nl/dataset/84583NED/table?ts=1661527812032
Sweetapple P, Nugent G (2011) Chew-track-cards: a multiple-species small mammal detection device. N Z J Ecol 153–162
Tamayo-Uria I, Mateu J, Escobar F, Mughini-Gras L (2014) Risk factors and spatial distribution of urban rat infestations. J Pest Sci 87(1):107–115
Taylor R, Thomas B (1989) Eradication of Norway rats (Rattus norvegicus) from Hawea Island, Fiordland, using brodifacoum. N Z J Ecol 23–32
Traweger D, Travnitzky R, Moser C, Walzer C, Bernatzky G (2006) Habitat preferences and distribution of the brown rat (Rattus norvegicus Berk.) in the city of Salzburg (Austria): implications for an urban rat management. J Pest Sci 79(3), 113–125
van Adrichem MH, Buijs JA, Goedhart PW, Verboom J (2013) Factors influencing the density of the brown rat (Rattus norvegicus) in and around houses in Amsterdam. Lutra 56(2):77–91
Acknowledgements
We would like to thank Jan Buijs (municipality of Amsterdam), Peter Muilwijk (municipality of Rotterdam) and Sierk Visser together with Ellen van Rosmalen (municipality of Eindhoven) for giving us permission to execute this research, for providing us with city-specific information, and for additional tips and help. We would also like to thank all residents who gave us permission to place a rat trap in their front yard. Lastly, we would like to thank groenmonitor.nl for providing us with the NDVI data, and Sara Wijburg for her help with coding the maps in R.
Funding
This work was supported by funding from both the Dutch Ministry of Health, Welfare and Sport and the European Union’s Horizon 2020 Research and Innovation Programme for the DESIRE project under grant agreement No 773830: One Health European Joint Programme.
Author information
Authors and Affiliations
Contributions
MdC, MM, HS and WvdP conceived the ideas and designed methodology; MdC and MM collected the data; MdC and HE analysed the data; MdC led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Cock, M.P., Esser, H.J., van der Poel, W.H.M. et al. Higher rat abundance in greener urban areas. Urban Ecosyst (2024). https://doi.org/10.1007/s11252-024-01513-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11252-024-01513-5