Abstract
Urbanisation is widely considered as having a large impact on most native species. However, the species response to urbanisation varies among taxonomic groups, and its generalisation might lead to contradictory or incorrect management decisions in urban planning. Land snails, as an understudied group in this sense, are good subjects to study the impact of urbanisation due to their low dispersal capabilities and mobility. The study aimed to determine how land snail diversity patterns and community structure are influenced by urbanisation through an urban-to-rural gradient. A total of 59 terrestrial gastropod species and more than 4600 individuals were recorded at 24 alluvial sites distributed along an urban-to-rural gradient in three Slovak cities (Bratislava, Zvolen and Prešov). Most species belonged to euryecious, hygrophilous and forest specialist ecological groups; seven species were non-native to Slovakia. The results clearly highlight the negative effect of densely built areas on land snail community structure, since the proportion of indigenous and specialist species decreased progressively towards urban sites. The highest mean number of species was found in suburban zones and the lowest in urban zones, suggesting that most species favour intermediate levels of urbanisation. Some species (e.g. Arion vulgaris) were evenly distributed along the urbanisation gradient, supporting the importance of river floodplains as bio-corridors for the dispersal of gastropods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, 55% of the world’s population lives in urban areas, a proportion that is expected to increase to 68% by 2050 (United Nations 2018). To ensure that urban areas are planned both for the well-being of city dwellers and urban nature, knowledge of ecosystem responses to the influence of urbanisation is needed (McDonnell and Pickett 1990; Niemelä 1999). The effects of urbanisation may be illuminated through investigations of biotic and abiotic changes across urban-to-rural gradients (McDonnell et al. 1997; Niemelä 1999, 2000). Such gradients, from densely built city cores to increasingly rural surroundings, reflect diminishing intensities of human intervention on originally similar land bases. Such a gradient occurs worldwide and provides a useful framework for comparative work on a global scale, because it reflects similar anthropogenic patterns and processes (Niemelä 2000; Niemelä et al. 2002). Terrestrial gastropods are good subjects to study the impact of urbanisation, because organisms with low dispersal capabilities, like snails and slugs, are very susceptible to anthropogenic activities (Ström et al. 2009). Microsnails (< 5 mm in diameter), in particular, are often more vulnerable to disturbance because of their very limited mobility and dispersal and their strong dependence on microhabitats (Baur and Baur 1988). For these reasons, land-snail community composition, especially where microsnails are included, is a good indicator of the overall health of an ecosystem (Frest 2002). A review by Yanes (2012) concludes that three main anthropogenic factors affect land snails: habitat loss through urban and agricultural development, the introduction of non-native predators, and the introduction of non-native snail species. As cities are well-known “hot spots” for introducing non-native species (Bergey and Figueroa 2016), urban areas strongly affect land snails via all three of these factors. There are many ways to study urban impacts on biodiversity (Adler and Tanner 2013), but one of the most common methods is to compare biological communities along disturbance gradients or different levels of urban disturbance. McKinney (2008) found 57 urban gradient studies on invertebrate species, but none of them examined land snails. Indeed, we know of only four such studies in European cities (Penev et al. 2008; Horsák et al. 2009, 2013b; Lososová et al. 2011) and one in Tennessee, USA (Hodges and McKinney 2018), that examined the gradations of urban impacts on land snails in a systematic way. We chose alluvial habitats for research because they are important line corridors along which terrestrial gastropods spread. In general, river floodplains are among the most biologically productive and diverse ecosystems on Earth. They are also remarkable for their bio-corridor functions and dynamic succession due to periodic flood activity, especially in relatively preserved and almost natural environment sections. For these reasons, scientists pay a lot of attention to these habitats in ecological, landscape function and biodiversity studies (e.g. Ward et al. 1999; Tockner and Stanford 2002). There are also numerous studies on alluvial land snail communities in Europe (e.g. Ložek 1947; Bába 1977; Frank 1984, 1985; Obrdlík et al. 1995; Čejka 2003, 2022; Horsák 2000; Vašátko et al. 2002; Čejka et al. 2008; Ilg et al. 2009; Čejka and Hamerlík 2009; Horáčková et al. 2011a, b, 2014; Myšák and Horáčková 2011). On the other hand, alluvia/floodplains have not yet been systematically studied in urban areas, despite being one of the few habitats inhabited by species-rich snail communities. In addition, these corridors can be an important landscape feature in the spread of non-native and invasive species.
This study analyses the effects of urbanisation on land snail communities in three cities across an urban-to-rural (UTR) disturbance gradient. We hypothesise that (i) there will be more specialists in less disturbed (suburban to rural) sites and more generalists in the urban section of the UTR gradient. Another hypothesis is that (ii) there will be higher species diversity towards the urban sections of the UTR, as a larger proportion of non-native and/or euryoecious species increases it.
Materials and methods
Surveyed area, sampling design
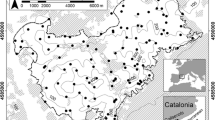
Sampling was conducted in three cities (Prešov, Zvolen and Bratislava) located evenly across Slovakia (Fig. 1).
The town of Prešov is situated in the Košice basin and is surrounded by the Slanské vrchy Mts and Šarišská vrchovina Upland (48.9978°N, 21.2399°E). The elevation of the historic city centre is approximately 255 m a.s.l. Prešov town has a population of 83,897 (as of 1 January 2021) and covers an area of 70.4 km2 (http://datacube.statistics.sk). Significant international road and railway lines lead through the city to Poland and Ukraine. The rivers Torysa and Sekčov flow through the town. The section of the Torysa River that flows through the built-up part of the city is 8.5 km long.
The town of Zvolen lies in the southwestern part of the Zvolen basin at the confluence of the Hron and Slatina rivers and is surrounded by the Kremnické vrchy Mts and Javorie Mts. The elevation ranges from 278 to 420 m a.s.l. with the town centre at an elevation of approximately 295 m a.s.l. Zvolen has a population of 40,637 (as of 1 January 2021) and covers an area of 98.7 km2 (http://datacube.statistics.sk).
The City of Bratislava lies in three orographic units – the Záhorská nížina Lowland, the Little Carpathian Mts and the Danube Lowland, where the Danube River flows through it for a 30-km stretch. With an area of 853.15 km² (85,315 ha), Bratislava is the largest city in Slovakia, with 475,500 inhabitants (http://datacube.statistics.sk).
We stratified the alluvial study areas of each city and its close surrounding into three zones of decreasing urbanisation (urban, suburban and rural). The urbanisation zones were primarily determined using the Corine Land Cover (CLC, reference year 2018) obtained from www.copernicus.eu. Specifically, the assignment of sites to urbanisation zones was based on the proportion of artificial surfaces (CLC class codes 1.1. − 1.3.) within a 300 m radius of each site. Sites in the urban zones were defined as highly urbanised areas where the proportion of artificial surfaces exceeded 52%. The suburban zones included moderately urbanised areas where the proportion of artificial surfaces ranged from 8 to 52% (with one exception - a suburban site in the city of Bratislava had 7.34% of artificial surfaces), and the rural zone was represented by peripheral areas with less than 8% of artificial surfaces. To investigate the way the urban-to-rural gradient influences the snail species richness and community structure, we chose 24 study sites: 9 urban, 6 suburban and 9 rural sites (8 sites in each city, Fig. 1). The number of individual sites was lower in the suburban zone, since this zone was the narrowest in each study city compared to the urban and rural zones. Most sites were located on the alluviums of the main rivers flowing through the cities (i.e. the Torysa River in Prešov, the Danube in Bratislava and the Hron in Zvolen). Since the Hron flows along the edge of the town of Zvolen, we additionally chose the alluviums of two other water flows – the Slatina River and the Zolná Brook to complete the sampling design.
A sampling of communities, extraction of shells
The mollusc communities were sampled from May to June 2019, when temperatures and moisture levels were moderate. The individual sites were usually about 600 to 1800 m apart (the largest distance in one case reached 7 km). Each sampling site was a ca. 400 m2, chosen to represent a specific part of the surrounding vegetation, avoiding edges or transitions to different habitats. Within each site, molluscs were searched by eye. One person searched this way in all appropriate microhabitats for 1.5-h at each site (see Cameron and Pokryszko 2005). Slugs are also an important component of urban molluscan communities, so we included them in the analyses. As the activity of slugs depends largely on weather conditions, individual searching was also conducted on rainy days, early mornings or late evenings in order to find the most slug species possible. Three-litre samples (10 pooled subsamples) were collected during each sampling period within a site; all litter, twigs, vegetation and loose soil were sieved (10 mm mesh size) down to a depth where the soil became difficult to remove (app. 4 cm). The coarse fraction was searched by eye in the field and discarded. The residue was bagged and taken to the laboratory. All shells extracted were identified and counted, excluding very old or unidentifiable remains. Identification was based on morphology using taxonomic keys by Ložek (1956); Wiktor (2004); Horsák et al. (2013a); Rowson et al. (2014). Species nomenclature follows Horsák et al. (2022); for authorities, see the Supplementary Data. Species were classified into basic ecological groups according to Lisický (1991) and Juřičková et al. (2014), with correction based on the authors’ own long-term field experience from the Slovak river floodplain areas. The samples are kept in the collection of TČ, MČ and PM.
Explanatory variables
Four explanatory variables characterising the urban-to-rural gradient and approximating human impacts were established for each site (Table 1). The distance from the city centre was measured as the linear distance from the city centre (specifically the Presidential (Grassalkovich) Palace in Bratislava, the Co-Cathedral of Saint Nicholas in Prešov and the railway junction close to Zvolen Castle in Zvolen) to the centre of each site using orthophoto mosaic and Measure tool in the ArcGIS 10.3 software (ESRI 2015). High-resolution (10 m, reference year 2018) maps of impervious surfaces (%), referring to the percentage of imperviousness degree per raster cell, built-up area and the vector layer of Corine Land Cover (CLC) were obtained from www.copernicus.eu. While imperviousness represents a continuous range of surface imperviousness (1–100%), the built-up area is a binary product, expressed as built-up (1) or non-built-up areas (0) per pixel. Therefore, surface imperviousness (%) was calculated as the average imperviousness degree and built-up area (%) as a percentage of the total number of pixels (i.e. 1/1 + 0) within an area of 300 m around the centre of each site (circular area with a radius of 300 m). Within the CLC classes, the proportion of artificial surfaces (CLC class codes 1.1. − 1.3.) was calculated for each circular area. All analyses were performed using ArcGIS 10.3 software (ESRI 2015). Furthermore, tree cover (%) was estimated at each of the 24 sites directly in the field.
Statistical analyses
The effect of the urbanisation zone on snail species richness and proportion of indigenous and specialist species was evaluated using linear mixed-effects models (LMMs). LMMs involved fixed effects of the urbanisation zone with three levels and random effects of the city. The mixed model approach was applied with respect to an experimental design, since the sites representing three urbanisation zones were grouped within a particular city. Residuals of the models were checked for normality and homogeneity of variances. The proportion of indigenous and specialist species was arcsine-transformed to meet the assumptions. The statistical significance of the fixed effect was assessed using F tests. When global tests of the fixed effect yielded significant results (p < 0.05), pairwise comparisons among the habitat types were performed using Tukey’s test.
We assembled a species-by-sites matrix to investigate the main gradients of compositional change in snail communities among the zones. We performed partial principal component analysis (pPCA) to reduce the overall compositional variation into a smaller dimension of the main compositional changes. The city was used as a co-variable to partial out its effect on species composition. Before the pPCA, the data on species abundances in the species-by-sites matrix were log-transformed to reduce the effect of dominant species (and thus focus the analyses on the entire community composition). Subsequently, we employed detrended correspondence analysis (DCA) to assess the length of the environmental gradient. DCA yielded a short gradient length (2.9 SD units), and thus, we preferred the linear method (pPCA) to the unimodal one (Lepš and Šmilauer 2003). The broken stick model was used to determine the number of principal components (PCs) representing non-trivial (significant) variation in the species-by-sites matrix. Only the first two PCs had eigenvalues larger than values generated by the broken stick model; these were chosen and used for further analyses. We characterised the relationships between the environmental variables and major compositional gradients in snail assemblages by calculating Spearman’s rank correlations between the pPCA plot scores (on the first two axes) and the measured environmental variables.
A multivariate analogue of analysis of variance with permutations – perMANOVA (Anderson 2001) – was applied to test for the effect of urbanisation zone on the composition of snail assemblages. To be consistent with the pPCA, the Euclidean distance was used to measure (dis)similarity. Species data were log-transformed before the analysis to reduce the effect of dominant species. The calculation of p-values was based on 10,000 permutations (McArdle and Anderson 2001). Due to the sampling design, we restricted the permutation schemes to free permutations of sites within the cities to obtain proper probability values.
All analyses were performed in R language (version 4.1.1, R Core Team 2021) using the vegan (Oksanen et al. 2020), nlme (Pinheiro et al. 2021) and multcomp (Hothorn et al. 2008) packages.
Results
A total of 59 terrestrial gastropod species and 4635 individuals were found at the 24 sites (see Supplementary Data). Species richness varied from 8 to 21, with a median value of 15 species per site and from 34 (Prešov) to 46 (Zvolen) per city. In some cities or urbanisation zones, several species were represented by only one individual, suggesting that the species inventory was incomplete, but this was not the aim of this study (see Discussion). The similarity index (Jaccard’s index) also showed differences in species composition found in the studied cities. The Jaccard’s similarity index between Bratislava and Zvolen reached the value of 0.5, while between Bratislava and Prešov it was 0.44. The similarity of species composition between the Prešov and Zvolen was 0.51. The total number of species represented 33.5% of Slovak terrestrial molluscan fauna (Horsák et al. 2022). Of the total number of species found, 23.7% were euryecious, and 11.9% were non-native.
The most frequent species were Helix pomatia (90%), Monachoides incarnatus (80%), Arion vulgaris and Cepaea hortensis (both 77%). Another six species (Fruticicola fruticum, Euomphalia strigella, Alinda biplicata, Succinea putris and Caucasotachea vindobonensis) were present in at least half of the sampling sites. Most species belong to the euryecious, hygrophilous and forest specialist ecological groups.
We found the highest mean number of snail species in suburban sites (Fig. 2a); however, they were not statistically different from the mean number of species observed in urban and rural sites. The proportion of indigenous and specialist species decreased progressively towards the urban sites (Fig. 2b, c), with the urban sites being significantly different from the rural and suburban ones.
Comparison of the mean (black dots) number of snail species (a), the proportion of indigenous (b) and specialist (c) species among the three urbanisation zones. Error bars represent 95% confidence intervals. Different letters above the bars indicate significant mean differences (p < 0.05) based on Tukey’s tests
Partial PCA, in which the effect of the city was separated out, revealed one main environmental gradient that represented urbanisation zones (Fig. 3). The compositional gradient related to the urban-to-rural gradient was represented by ordination axis 1 (pPCA 1, 25.3% of the variation explained) and further corroborated by axis 1 scores that were positively correlated with surface imperviousness and built-up area (high in urban sites) and negatively with distance from the city centre (Fig. 3a; Table 2). No statistically significant relationships were found between the first two axis scores and tree cover (%), suggesting that site score positions on pPCA axis 1 were related to the urban-rural gradient rather than to differences in tree cover between the sites. Two distinctive clusters became apparent along the first pPCA axis: the rural sites at one end overlapped only a little with the urban sites at the opposite end. Suburban sites are placed in between them (Fig. 3a).
Snail community composition varied considerably among the three urbanisation zones (i.e. along the UTR gradient, perMANOVA, pseudo-F = 1.37, p < 0.01). While Arianta arbustorum, Aegopinella nitens, Clausilia pumila, Petasina unidentata, Urticicola umbrosus and Semilimax semilimax tended to prefer rural and suburban sites, respectively, Arion distinctus, Truncatellina cylindrica, Tandonia kusceri and Oxychilus draparnaudi were typically associated with the urban sites (Fig. 3b). Species such as Zonitoides nitidus, Succinea putris and Cochlicopa lubrica did not prefer any position along the UTR disturbance gradient.
Discussion
Species richness, UTR gradient
The species richness values found in this study (59 species) were smaller than in other studies carried out in this geographical area – 125 terrestrial species in Prague, Czech Republic (Juřičková 1995); 67 terrestrial species in Hradec Králové, Czech Republic (Juřičková 1998); 100 species in Bratislava (Čejka et al. 2020). It should be emphasised here that our research did not include a faunistic inventory (or finding the more or less complete species spectrum), as reported, for example, by Cameron and Pokryszko (2005), it is evidenced by the abundance structure of the molluscan communities in the individual cities (several species were represented by only one individual in particular cities and urbanisation zones). Lososová et al. (2011) found 87 species of terrestrial gastropods during a biodiversity survey of thirty European cities; the main differences in species composition were observed between strongly urbanised sites in city centres and early successional and mid-successional sites. The number of species for vascular plants was lowest in city squares and boulevards and highest at successional sites and residential areas with compact building patterns.
As for non-European sites, the terrestrial molluscan fauna of the greater Sydney area is surprisingly diverse due to the wide range of vegetation communities present, with over 80 species of native snails and slugs recorded (Clark 2004). The above-mentioned results are not surprising, since we focused on studying the importance of the UTR gradient within a limited number of sites in the floodplain, not on the inventory of malacofauna in particular cities. Differences between the studied cities (e.g. size, geography, geomorphology, population density, length of the UTR gradient, etc.) were manifested in the malacofauna composition and diversity (highest in Zvolen, lowest in Prešov). On the other hand, clear patterns in the UTR gradient were observed after excluding the city’s influence on the species composition in statistical analyses. Although we found the highest species richness in the suburban part of the gradient, our results clearly confirm the negative impact of urbanisation on the proportion of native species and specialists. The highest number of species in the suburban parts of the gradient and a decrease in species richness of forest specialists and shade-loving species on the UTR gradient towards the city centre were found in research by Hungarian arachnologists (Tajthi et al. 2017). One of the reasons for the higher diversity in suburban sites is the higher proportion of non-native and xenocenous species (part of the so-called “density-diversity paradox” sensu Shochat et al. 2010). However, there is also a view that non-native species should also be considered part of biodiversity, especially in urban environments (e.g. Schlaepfer et al. 2018). Another reason for the higher species richness of the suburban zones could be due to the increased landscape heterogeneity and habitat diversity in areas with intermediate levels of urbanisation, which is in accordance with the intermediate disturbance hypothesis (Connell 1978), which is also consistent with recent research by Hungarian arachnologists (Tajthi et al. 2017, as mentioned above). In a more diverse environment (in terms of diverse habitats and heterogeneous land cover), one might expect species diversity to increase (cf. Marzluff 2005).
In general, previous studies showed increased snail richness from the city centre to the outskirts (Juřičková 1995; Tappert 1996; Dedov and Penev 2008), but the results of Horsák et al. (2009) showed that this is more a function of habitat degradation than the effect of the distance from the centre. Species-poor assemblages are more frequent near city centres due to the lower frequency of preserved natural habitats in them when compared to rural zones. In all cities, there was a greater frequency of more degraded habitats nearer the centre. However, a study of the impact of urbanisation on spiders in Hungary (Horváth et al. 2012) did not support the increasing disturbance hypothesis, as overall species richness decreased from rural to urban sites. As predicted by the authors, both open habitat and generalist species increased towards urban sites. The number of forest specialist species was higher in the suburban part of the gradient than in the rural and urban sites. Another general result of previous studies was finding a higher proportion of slug species in urban snail assemblages (e.g. Klausnitzer 1993), such as in the study of Horsák et al. (2009). Since slug species represented the majority of synanthropic species records, the observed increase of synanthropic species to the city centre was de facto an increase of slug species towards the centre. In the case of our study, this clear trend does not follow for all species of slugs, as even some generalists from the slug group, surprisingly Arion vulgaris, were evenly distributed along the entire length of the UTR gradient (Supplementary Data). The occurrence of this invasive slug along the entire length of the gradient confirms the importance of river corridors for the dispersal of gastropods, even slugs, which cannot use a shell as temporary protection from drowning.
Gastropods can actively disperse along the alluvia even upstream, with the dispersal rate depending on various environmental factors, such as the connectivity, quality and structure of riparian vegetation and its canopy or land use. This type of dispersal is slow but can be accelerated by vectors, most often humans and other mammals or even birds (e.g. Baur and Bengtsson 1987; Gittenberger et al. 2006), as it has been proven that gastropods can pass through the digestive tract of birds without damage (Wada et al. 2012). Highly adaptable and large species (Arion vulgaris in our case) are less constrained by landscape barriers to dispersal than smaller specialist species. As confirmed, for example, by Lososová et al. (2011), another passive dispersal strategy also enables snails to colonise city centres. Both native and alien snail species can spread passively with garden soil in the most urbanised habitats, as a result of which they often occur in flowerpots with ornamental plants in city squares. Piano et al. (2020) did not observe any significant declines along the urbanisation gradient for snails, because they are small, passively dispersing organisms (Fontaneto 2019; Gianuca et al. 2018). As such, they do not need large habitat patches to thrive, and, at the same time, being passive dispersers, they cannot avoid cities during their dispersal process. On the other hand, snails include several species that prefer common habitats in cities, such as moist patches of soil because they are covered with debris, stones and other building material. Horsák et al. (2009) found that the response of local snail communities along a gradient of habitat degradation can be generalised as a gradual decline in species richness, especially of rare and anthropophobic species. Furthermore, this decline was independent of the location of sites within the city, highlighting the importance of natural habitats for maintaining snail diversity in urban environments. Other authors also confirmed the effect of urbanisation on land snail communities. Hodges and McKinney (2018) found that urbanisation significantly alters land snail community structure in city parks. More natural sites farther from the centre (downtown) had substantially greater species richness, diversity and evenness than the more degraded ones closer to commercial city centres. Synanthropic snail species accounted for a significant percentage of species in increasingly urbanised habitats. In Sofia (Bulgaria), the effect of urbanisation was reflected in the diversity of snail communities. In urban areas, this was half as much as in suburban areas (Penev et al. 2008). Plant diversity shows a similar pattern in Kazakhstan – it increased with increasing distance to the city centre and was also influenced by the type of land use (Vakhlamova et al. 2014). Turnbull and Scott (2012) found a greater abundance of molluscs in habitats with less anthropogenic influence.
Impact of urbanisation on species richness and the response of land snails
Even though urbanisation can dramatically reduce the number of native species (e.g. Pauleit and Duhme 2000; Kühn and Klotz 2006), the urban environment often supports a high species diversity (e.g. Gilbert 1989; Wang et al. 2007). Several factors can cause this paradox, with the situation varying among taxonomic groups. In many studies that have shown that cities harbour more plant species than their surroundings, it has been argued that the increase in species is caused by many non-indigenous species (e.g. McKinney and Lockwood 2001). Lososová et al. (2011) found that for snails, alpha and especially gamma diversity at mid-successional sites (abandoned for 5–15 years, dominated by perennial grassland, with scattered shrubs and young trees) was considerably higher than in other habitat types. More than 83% of all snail species recorded in their study were found at mid-successional sites. Furthermore, only this habitat type harboured rare and endangered snail species, and several species typical of natural habitats were diagnostic for this habitat type. Although this wilder habitat type contributes greatly to urban biodiversity, increasing urbanisation often destroys it. Therefore Knapp et al. (2008) proposed expanding conservation strategies in urban areas to protect similar habitat hotspots. Within our research, similar habitats included parts of suburban zones that were characterised by a high proportion of indigenous and specialist species. Piano et al. (2020), who studied the effect of urbanisation on several model animal groups, found that group-specific responses were highly heterogeneous, but, except for bdelloid rotifers and cladocerans, all other terrestrial groups showed a significantly negative response to increasing local- and/or landscape-scale urbanisation for at least one of the diversity components. Increased local urbanisation primarily decreased the local α–diversity of butterflies and orthopterans and decreased (additive) variation in species composition of ground beetles, snails and orthopterans. Piano et al. (2020), by applying a multiscale approach across multiple animal groups, demonstrated an overall negative effect of urbanisation on insect abundance and diversity of a range of terrestrial and (semi)aquatic taxa. In particular, they highlighted how passively dispersing taxa tend to be less sensitive to urbanisation than actively dispersing taxa. However, they also highlight that the responses to urbanisation strongly depend on the examined group, the scale of urbanisation and the scale at which diversity was assessed. Our results suggest that gathering information on how individual taxonomic groups respond to urbanisation is important, as generalisations of species responses might lead to contradictory or poor management decisions in urban planning. Our study fully contributes to that purpose by pointing out the negative impact of urbanisation on specialist and native species of land snails. Nowadays, understanding the habitat-related biodiversity patterns in an urbanised landscape is crucial. It can contribute to optimised management strategies for achieving more sustainable urban areas and allow predictions of future impacts of urban land-use changes on the biota in a rapidly urbanising world.
Data availability
The data that support the findings of this study are available on request from the corresponding author [MČ].
References
Adler F, Tanner CJ (2013) Urban ecosystems. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511981050
Anderson M (2001) A new method for non-parametric analysis of variance in ecology. Aust Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Bába K (1977) Die kontinentalen Schneckenbestände Der Eichen-Ulmen- Eschen-Auwäldern (Fraxino Pannonicae-Ulmetum pannonicum Soó) in Der Ungarischen Tiefebene. Malacologia 16:51–57
Baur A, Baur B (1988) Individual movement patterns of the minute land snail Punctum Pygmaeum (Draparnaud) (Pulmonata: Endodontidae). Veliger 30:372–376
Baur B, Bengtsson J (1987) Colonizing ability in land snails on Baltic uplift archipelagos. J Biogeogr 14:329–341. https://doi.org/10.2307/2844941
Bergey EA, Figueroa LL (2016) Residential yards as designer ecosystems: effects of yard management on land snail species composition. Ecol Appl 26:2538–2547. https://doi.org/10.1002/eap.1378
Cameron RAD, Pokryszko BM (2005) Estimating the species richness and composition of land mollusc communities: problems, consequences and practical advice. J Conchol 38:529–548
Čejka T (2003) Molluscs (Mollusca). In: Stanová V, Viceníková A (eds) Biodiversity of Abrod - State, Changes and Restoration. Daphne - Institute of Applied Ecology, Bratislava, pp 187–190
Čejka T (2022) Diversity and classification of the terrestrial molluscan fauna in the Danube Plain. Slovakia Biol 77:739–748. https://doi.org/10.1007/s11756-022-01013-x
Čejka T, Hamerlík L (2009) Land snails as indicators of soil humidity in danubian woodland (SW Slovakia). Pol J Ecol 57:741–747
Čejka T, Horsák M, Némethová D (2008) The composition and richness of danubian floodplain forest land snail faunas in relation to forest type and Flood frequency. J Mollusc Stud 74:37–45. https://doi.org/10.1093/mollus/eym041
Čejka T, Čačaný J, Dvořák L (2020) Mäkkýše Bratislavy. Slovenské národné múzeum, Bratislava
Clark SA (2004) Native snails in an urban environment-conservation from the ground up. In: Lunney D, Burgin S (eds) Urban Wildlife: More Than Meets the Eye, Royal Zoological Society of New South Wales, Mosman, Australia, pp 78–81 https://doi.org/10.7882/FS.2004.084
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310. https://doi.org/10.1126/science.199.4335.1302
Dedov I, Penev L (2008) Species composition and origins of the terrestrial gastropod fauna of Sofia City. Bulgaria Ruthenica 10:121–131
ESRI (2015) ArcGIS Desktop: Release 10.3.1. Environmental Systems Research Institute, Redlands, CA
Fontaneto D (2019) Long-distance passive dispersal in microscopic aquatic animals. Mov Ecol 7:10. https://doi.org/10.1186/s40462-019-0155-7
Frank C (1984) Aquatische und terrestrische Mollusken Der niederösterreichischen Donau - Auengebiete Und Der Angrenzenden Biotope. VI. Die Donau Von Wien bis Zur Staatsgrenze. Teil. Z Angew Zool 3 1:257–303
Frank C (1985) Aquatische und terrestrische Mollusken Der niederösterreichischen Donau - Auengebiete Und Der Angrenzenden Biotope. VI. Die Donau Von Wien bis Zur Staatsgrenze. Teil 2. Z Angew Zool 4:405–457
Frest TJ (2002) Native snails: indicators of ecosystem health. In: Wuerthner G, Matteson M (eds) Welfare ranching. Island Press, Sausalito, pp 211–215
Gianuca AT, Engelen J, Brans KI, Hanashiro FT, Vanhamel M, van den Berg EM, Souffreau C, Meester LD (2018) Taxonomic, functional and phylogenetic metacommunity ecology of cladoceran zooplankton along urbanization gradients. Ecography 41:183–194. https://doi.org/10.1111/ecog.02926
Gilbert OL (1989) The Ecology of Urban habitats. Chapman & Hall, London
Gittenberger E, Groenenberg DS, Kokshoorn B, Preece RC (2006) Molecular trails from hitch-hiking snails. Nature 439:409. https://doi.org/10.1038/439409a
Hodges MN, McKinney ML (2018) Urbanization impacts on land snail community composition. Urban Ecosyst 21:721–735. https://doi.org/10.1007/s11252-018-0746-x
Horáčková J, Ložek V, Juřičková L (2011a) Nivní malakofauna řeky Ohře - její minulost a současnost [The floodplain molluscan fauna of the Ohře River (Czech Republic) – its past and present]. Malacol Bohemoslov 10:51–64. Available from http://mollusca.sav.sk (accessed August 2022)
Horáčková J, Ložek V, Juřičková L (2011b) Měkkýši v nivě Milešovského potoka [Molluscs of the Milešovský potok floodplain (Northwest Bohemia, Czech Republic)]. Malacol Bohemoslov 10:24–34. Available from http://mollusca.sav.sk (accessed August 2022)
Horáčková J, Horsák M, Juřičková L (2014) Land snail diversity and composition in relation to ecological variations in central European floodplain forests and their history. Community Ecol 15:44–53. https://doi.org/10.1556/ComEc.15.2014.1.5
Horsák M (2000) Měkkýši (Mollusca) navrhované NPR Oderský Luh v CHKO Poodří (Česká Republika) [The molluscs of the Oderský Floodplain Forest proposed National Nature Reserve in the Poodří Protected Landscape Area (Czech Republic)]. Čas. Slez Muz Opava (A) 49:183–187
Horsák M, Juřičková L, Kintrová K, Hájek O (2009) Patterns of land snail diversity over a gradient of habitat degradation: a comparison of three Czech cities. Biodivers Conserv 18:3453–3466. https://doi.org/10.1007/s10531-009-9654-y
Horsák M, Juřičková L, Picka J (2013a) Měkkýši České a Slovenské republiky. Molluscs of the Czech and Slovak republics. Kabourek, Zlín
Horsák M, Lososová Z, Čejka T, Juřičková L, Chytrý M (2013b) Diversity and biotic homogenization of urban land-snail faunas in relation to habitat types and macroclimate in 32 central European cities. PLoS ONE 8:e71783. https://doi.org/10.1371/journal.pone.0071783
Horsák M, Čejka T, Juřičková L, Beran L, Horáčková J, Hlaváč JČ, Dvořák L, Hájek O, Divíšek J, Maňas M, Ložek V (2022) Check-list and distribution maps of the molluscs of the Czech and Slovak Republics. Available from http://mollusca.sav.sk/malacology/checklist.htm (accessed August 2022)
Horváth R, Magura T, Tóthmérész B (2012) Ignoring ecological demands masks the real effect of urbanization: a case study of ground-dwelling spiders along a rural–urban gradient in a lowland forest in Hungary. Ecol Res 27:1069–1077. https://doi.org/10.1007/s11284-012-0988-7
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in General Parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Ilg C, Foeckler F, Deichner O, Henle K (2009) Extreme Flood events favour floodplain mollusc diversity. Hydrobiologia 621:63–73. https://doi.org/10.1007/s10750-008-9632-5
Juřičková L (1995) Měkkýší fauna Velké Prahy a její vývoj pod vlivem urbanizace. Natura Pragensis. Český ústav pro ochranu přírody, Praha
Juřičková L (1998) Měkkýši Hradce Králové (Mollusca of Hradec Králové, East Bohemia, Czech Republic). Acta Musei Reginaehradecensis 26:101–172
Juřičková L, Horsák M, Horáčková J, Abraham V, Ložek V (2014) Patterns of land-snail succession in central Europe over the last 15,000 years: main changes along environmental, spatial and temporal gradients. Quat Sci Rev 93:155–166. https://doi.org/10.1016/j.quascirev.2014.03.019
Klausnitzer B (1993) Ökologie Der Großstadtfauna, 2. Auflage edn. Gustav Fischer, Stuttgart
Knapp S, Kühn I, Mosbrugger V, Klotz S (2008) Do protected areas in urban and rural landscapes differ in species diversity? Biodivers Conserv 17:1595–1612. https://doi.org/10.1007/s10531-008-9369-5
Kühn I, Klotz S (2006) Urbanization and homogenization–comparing the floras of urban and rural areas in Germany. Biol Conserv 127:292–300. https://doi.org/10.1016/j.biocon.2005.06.033
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge university press. https://doi.org/10.1017/CBO9781139627061
Lisický MJ (1991) Mollusca Slovenska. Veda, Bratislava
Lososová Z, Horsák M, Chytrý M, Čejka T, Danihelka J, Fajmon K, Hájek O, Juřičková L, Kintrová K, Láníková D, Otýpková Z, Řehořek V, Tichý L (2011) Diversity of central European urban biota: effects of human-made habitat types on plants and land snails. J Biogeogr 38:1152–1163. https://doi.org/10.1111/j.1365-2699.2011.02475.x
Ložek V (1947) Měkkýši dolního Povltaví [Mollusca of the Vltava River down-stream]. ČNM 2:135–148
Ložek V (1956) Klíč československých měkkýšů. Vyd. Slov. Akad. vied, Bratislava
Marzluff JM (2005) Island biogeography for an urbanizing world: how extinction and colonization may determine biological diversity in human-dominated landscapes. Urban Ecosyst 8:157–177. https://doi.org/10.1007/s11252-005-4378-6
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance based redundancy analysis. Ecology 82:290–297. https://doi.org/10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2
McDonnell MJ, Pickett STA (1990) Ecosystem structure and function along urban-rural gradients: an unexploited opportunity for ecology. Ecology 71:1232–1237. https://doi.org/10.2307/1938259
McDonnell MJ, Pickett STA, Groffman P, Bohlen P, Pouyat RV, Zipperer WC, Parmelee RW, Carreiro MM, Medley K (1997) Ecosystem processes along an urban-to-rural gradient. Urban Ecosyst 1:21–36. https://doi.org/10.1023/A:1014359024275
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176. https://doi.org/10.1007/s11252-007-0045-4
McKinney ML, Lockwood JL (2001) Biotic homogenization: a sequential and selective process. In: Lockwood JL, McKinney ML (eds) Biotic homogenization. Kluwer Academic Publishers, Amsterdam, pp 1–17. https://doi.org/10.1007/978-1-4615-1261-5_1
Myšák J, Horáčková J (2011) Malakofauna údolí Tiché Orlice [Mollusc fauna of the Tichá Orlice River valley]. Malacol Bohemoslov 10:38–44. Available from http://mollusca.sav.sk (accessed August 2022)
Niemelä J (1999) Ecology and urban planning. Biodivers Conserv 8:119–131. https://doi.org/10.1023/A:1008817325994
Niemelä J (2000) Is there a need for a theory of urban ecology? Urban Ecosyst 3:57–65. https://doi.org/10.1023/A:1009595932440
Niemelä J, Kotze DJ, Venn S, Penev L, Stoyanov I, Spence J, Hartley D, De Oca EM (2002) Carabid beetle assemblages (Coleoptera, Carabidae) across urban-rural gradients: an international comparison. Landsc Ecol 17:387–401. https://doi.org/10.1023/A:1021270121630
Obrdlík P, Falkner G, Castella E (1995) Biodiversity of Gastropoda in European floodplains. Archiv Hydrobiol Suppl 101:339–356. https://doi.org/10.1127/lr/9/1996/339
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin RP, O’Hara RB, Simpson LG, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2020) Vegan: Community Ecology Package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan
Pauleit S, Duhme F (2000) Assessing the environmental performance of land cover types for urban planning. Landsc Urban Plann 52:1–20. https://doi.org/10.1016/S0169-2046(00)00109-2
Penev L, Stoyanov I, Dedov I, Antonova V (2008) Patterns of urbanisation in the City of Sofia as shown by carabid beetles (Coleoptera, Carabidae), ants (Hymenoptera, Formicidae), and terrestrial gastropods (Mollusca, Gastropoda Terrestria). Zookeys 18:483–509
Piano E, Souffreau C, Merckx T, Baardsen LF, Backeljau T, Bonte D, Brans KI, Cours M, Dahirel M, Debortoli N, Decaestecker E (2020) Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob Change Biol 26:1196–1211. https://doi.org/10.1111/gcb.14934
Pinheiro J, Bates D, DebRoy S, Sarkar D, Core Team R (2021) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–152. https://CRAN.R-project.org/package=nlme
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL. http://www.R-project.org
Rowson B, Turner J, Anderson R, Symondson B (2014) Slugs of Britain & Ireland: identification, understanding and control. Field Studies Council, Telford
Schlaepfer DR, Braschler B, Rusterholz HP, Baur B (2018) Genetic effects of anthropogenic habitat fragmentation on remnant animal and plant populations: a meta-analysis. Ecosphere 10:e02488. https://doi.org/10.1002/ecs2.2488
Shochat E, Lerman SB, Anderies JM, Warren PS, Faeth SH, Nilon CH (2010) Invasion, competition, and biodiversity loss in urban ecosystems. Bioscience 60:199–208. https://doi.org/10.1525/bio.2010.60.3.6
Ström L, Hylander K, Dynesius M (2009) Different long-term and short-term responses of land snails to clear-cutting of boreal stream-side forests. Biol Conserv 142:1580–1587. https://doi.org/10.1016/j.biocon.2009.02.028
Tajthi B, Horváth R, Mizser S, Nagy DD, Tóthmérész B (2017) Spider assemblages in floodplain forests along an urbanization gradient. Community Ecol 18:311–318. https://doi.org/10.1556/168.2017.18.3.10
Tappert A (1996) Die Molluscenfauna Von Koln. Dacheniana Beihefte 35:579–643
Tockner K, Stanford JA (2002) Riverine Flood plains: present state and future trends. Environ Conserv 29:308–330. https://doi.org/10.1017/S037689290200022X
Turnbull S, Scott G (2012) Urban Biodiversity: Successes and Challenges: Epigeal invertebrate abundance and diversity on Yorkshire allotments. The Glasgow Naturalist 25. Available from www.gnhs.org.uk (accessed August 2022)
United Nations (Department of Economic and Social Affairs, Population Division) (2018) World Urbanization Prospects: The 2018 Revision, Online Edition. Available from https://esa.un.org/unpd/wup/Publications (accessed August 2022)
Vakhlamova T, Rusterholz HP, Kanibolotskaya Y, Baur B (2014) Changes in plant diversity along an urban–rural gradient in an expanding city in Kazakhstan, Western Siberia. Landsc Urban Plann 132:111–120. https://doi.org/10.1016/j.landurbplan.2014.08.014
Vašátko J, Wohlgemuth E, Horsák M (2002) Nivní malakocenózy v povodí dolní Olšavy. Sborník Přírod Klubu v Uh Hradišti 7:77–88
Wada S, Kawakami K, Chiba S (2012) Snails can survive passage through a bird’s digestive system. J Biogeogr 39:69–73. https://doi.org/10.1111/j.1365-2699.2011.02559.x
Wang G, Jiang G, Zhou Y, Liu Q, Ji Y, Wang S, Chen S, Liu H (2007) Biodiversity conservation in a fast-growing metropolitan area in China: a case study of plant diversity in Beijing. Biodivers Conserv 16:4025–4038. https://doi.org/10.1007/s10531-007-9205-3
Ward JV, Tockner K, Schiemer F (1999) Biodiversity of floodplain river ecosystems: ecotones and connectivity1. River Res Appl 15:125–139. https://doi.org/10.1002/(SICI)1099-1646(199901/06)15:1/3%3C125::AID-RRR523%3E3.0.CO;2-E
Wiktor A (2004) Ślimaki lądowe Polski. Wydawnictwo MANTIS, Olsztyn
Yanes Y (2012) Anthropogenic effect recorded in the live-dead fidelity of land snail assemblages from San Salvador Island (Bahamas). Biodivers Conserv 21:3445–3466. https://doi.org/10.1007/s10531-012-0373-4
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This study was supported by projects of the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences (VEGA) project No. 2/0044/22 and No. 2/0108/21, by projects of Slovak Research and Development Agency (APVV) project No. APVV-19-0134 and No. APVV-21-0386, and by the Operational Programme Integrated Infrastructure (OPII) funded by the ERDF (project ENVIHEALTH, ITMS 313011T721).
Author information
Authors and Affiliations
Contributions
TČ, PM, BT and JO conceived the research idea; all authors collected field data; TČ and MČ identified land snails; MČ performed statistical analyses, retrieved GIS data and created figures and tables. All authors provided ecological interpretations of results, commented on previous versions of the manuscript, participated on writing and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Čiliak, M., Čejka, T., Tej, B. et al. Species richness patterns and community structure of land snail communities along an urban-rural gradient in river floodplains. Urban Ecosyst 27, 953–963 (2024). https://doi.org/10.1007/s11252-023-01501-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-023-01501-1