Abstract
Urbanization displaces natural habitats with impervious surfaces and managed ornamental green infrastructure. This study compared the structure of lepidopteran community in an office campus cleared from forest to that in the remaining forest. For the comparison, we trapped 2,233 lepidopteran specimens of 56 species from an office campus and adjacent forest. The species richness of lepidopteran assemblage in the office campus was half of that in the forest and consisted primarily of the same species found in the forest. The abundance and biomass of Lepidoptera in the office campus was a quarter of that in the forest. The biomass and abundance of Lepidoptera decreased along with the impervious area within 100-meter radius around the traps and approached zero when impervious surfaces covered the area entirely. The specimens in the trapped lepidopteran assembly from the office campus were on average larger, indicating elevated mobility, than those caught from the forest. Our results support earlier studies concluding that fragmented urban landscape selects for large mobile species, which can feed on ornamental plants or can disperse between high-quality habitats within urban landscape. Green infrastructure with native plants, high-quality native habitats and their connectivity can maintain species-rich lepidopteran communities in urban landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization refers to a phenomenon where people move from rural areas to cities. This phenomenon causes cities to grow outward, displacing surrounding areas (Mahtta et al. 2019). Cities have often developed into biodiversity hotspots, and they can sometimes maintain high biodiversity despite urban development (Cincotta et al. 2000; Luck 2007). Cities, gardens, and ruderal areas can harbor hotspots e.g., for butterflies (Öckinger et al. 2009; Prudic et al. 2022). Densities of several bird species, including threatened ones, can be higher in urban than rural areas (Seress and Liker 2015; Jokimäki et al. 2018). On the other hand, an urbanization-related change in land use has often negative effects on many taxon groups (Piano et al. 2020; Fenoglio et al. 2020; Pignataro et al. 2020). Thus, the impact of urbanization can be positive or negative depending on species and the type of land use change involved (Seress and Liker 2015; Jokimäki et al. 2018; Prudic et al. 2022; Fenoglio et al. 2020; Piano et al. 2020; Pignataro et al. 2020).
Expanding cities turn their surrounding areas into built-up area and fragment the habitat of the surrounding areas (Liu et al. 2016; Mahtta et al. 2019). Urban built-up areas include many types of habitats such as gardens, parks, and lawns, but impervious surfaces are among the best characteristics of urban landscape seen as buildings, roads, and parking lots (Lu and Weng 2006). The displaced surrounding habitat can include natural habitats from deserts to rainforests in developing countries but often also agricultural land or other human-impacted habitats in old civilizations, which have already been urbanized for centuries (Williams 2008). In many new civilizations, the ongoing rapid urbanization takes place mainly at the expense of native forests. For example, the land displaced by built-up area was mostly (64–77%) forest at 34 Finnish city regions in 2000–2012 (Tiitu 2018). During 1990–2017 in Finland, 243,000 hectares of boreal forest has been cleared for built environment (Timonen 2020). Thus, Finland is a good site to examine the impact of urbanization on biodiversity at the expense of native boreal forest biome, which globally cover 1,100,000,000 hectares and ca. 30% of all forests in the world (Artaxo et al. 2022).
Urban environments differ from the surrounding areas in many ways, which shape biological communities. Heat island effect refers to elevated temperatures in cities (Frank and Backe 2023). For example, the city of Jyväskylä in Finland is 4.1 °C warmer than the surrounding rural area (OECD 2022). The heat island effect facilitates the poleward expansion of distribution ranges and prolongs the reproduction seasons of warm-adapted species (Murakami et al. 2007; Zuckerberg et al. 2011; Robinet et al. 2012; Greig et al. 2017).
The vegetation in urban areas differs from that in the surrounding landscape. The most common type of urban field vegetation is lawn, which can support only a limited herbivorous insect fauna. In urban landscapes, trees and shrubs are often cultivated non-native ornamental plants (Tallamy et al. 2021). For example, in Finland, non-native taxa comprised 92% of 1,200 tree and shrub taxa, which include all native and most of the cultivated species (ca. 500), hybrids (ca. 100), subspecies, varieties and important forms (ca. 100) and cultivars (ca. 500) occurring in the country (Hämet-Ahti et al. 1992). Non-native ornamental plants can serve as hosts for non-native insects and native generalists too (Kowarik et al. 2019; Kopačka et al. 2021; Tallamy et al. 2021). Often the abundance and species richness of herbivorous Lepidoptera is lower in non-native than in native plants, the difference being largest among specialists (Burghardt et al. 2010; Tallamy et al. 2021).
Urban fragmentation challenges animal species differently. Highly mobile species such as birds may cope with fragmentation. For example, a common urban bird, Western Jackdaw (Corvus monedula), finds suitable nest sites in cities but can seek for food several kilometers away from the nest site (Meyrier et al. 2017). Compared to birds, invertebrates have lower dispersal ability, which often span from tens to hundreds of meters in lepidopteran species (Ribeiro et al. 2012; Robinet et al. 2012; Kuussaari et al. 2014, 2021; Merckx and Van Dyck 2019). In 30% of global forests, the fragmentation has created patches, which have < 200 m distance to their edges (Haddad et al. 2015). We need to understand the changes in the species-rich invertebrate communities upon fragmentation at small (< 150 m) scale.
In this study, we examined changes in a Lepidoptera community related to the development of forest into an office campus, an example of typical urbanization-related land-use change displacing native habitat. We trapped Lepidoptera from the office campus and the surrounding forest with bait traps located within 150 m from the edge of habitat. The changes in the abundance, biomass and species richness were related to the land use change and explained by the vegetated area around the traps. We identified the winners and losers among the species related to the urbanization event.
Methods
Study area
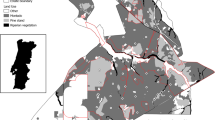
Our study area was the Ylistönmäki hill (62.227°N, 25.744°E) at a suburb of the seventh largest city in Finland, Jyväskylä (Fig. 1). The study area has been covered with forests at least for a century (Supporting information Text 1). Urbanization extended to the study area in early 1990s when 3.3 hectares of forest was cleared into an office campus as explained in detail in SI Text 1. During our study in 2022, the office campus had extended to an area of 6 hectares (Fig. 1).
An aerial photograph of the study area, an office campus surrounded by forest in May 2020. The dots c1–c5 show the locations of bait traps in the office campus delineated with a white border. The squares f1–f5 show the trap locations in the forest. The hashed area shows a forest patch, which was cleared before our sampling period for a padel hall.
Sampling design
To assess how the forest clearance into an office campus has affected the lepidopteran fauna, we placed ten bait traps in the study area, a set of five both in the office campus and in the surrounding forest. The lepidopteran catch between the two contrasting environments were compared and linked to the environments surrounding the traps.
Lepidoptera trapping
We used Jalas-type traps baited with wine (Pettersson and Franzén 2008). Traps with wine baits catch selectively nectar-feeding moths from few lepidopteran families, Noctuidae in particular. The traps were hung at 3 m elevation in deciduous trees at randomized locations but within 40–150 m from the forest edge. For the randomization, we selected 20 potential locations (excluding roofs and roads) for the traps on a map and used lottery to choose the final trap locations shown in Fig. 1. The catch of traps was collected daily between 28 June and 11 July 2022. The caught lepidopteran specimens were counted and identified to species according to external morphology. The main larval food plants and the habitat requirements for the trapped species were compiled from literature (Table S4).
The biomass of each species was determined as mean dry mass of 1–5 randomly selected specimens from the catch. The specimens were dried at + 60° for > 24 h and their mass was measured with an analytical balance at the accuracy of 0.01 mg. The biomass of lepidopteran assemblage caught in a trap was calculated by multiplying the number of counted specimens with their species-specific mean dry mass. The mean mass of specimens in the lepidopteran assemblage caught from a trap was calculated from the total biomass (described above) by dividing it with the number of individuals.
Description of environment
The environment around the traps was mapped at two scales: at 10 and 100 m radii. At 10-meter radius, the main type of vegetation, including the tree species, was determined during the field work. In the office campus, the sparse vegetation was recorded as lawns, planted ornamental trees and shrubs as well as a few non-planted herb species. The sparse vegetation in the office campus next to traps was compared to the larval foodplants of trapped Lepidoptera (Table S4). In the forest, the basal area of trees was determined with a relascope and the height of trees was measured with a hypsometer, and the total volumes of trees per hectare was calculated. The areal coverage of plant species in field layer were estimated with a quadrat. Based on the vegetation in field layer, the forest type was classified according to Cajander (1949).
At 100-meter radius, we separated landscape into impervious surfaces and green vegetated area from an aerial photograph. We determined the vegetated green area (lawns, planted vegetation, forest) using a manual measurement tool for area in an ArcGIS application (Karpalo) from an aerial photograph provided by National Land Survey of Finland. The selected 100-meter radius maximized the differences in vegetated areas around the traps and accounted for the connectivity between the forest and the office campus (i.e., edge effect Porensky and Young 2013).
Statistical analyses
The statistical analyses were made with the R Statistical Environment (version 4.1.2). The differences in the lepidopteran catch between the office campus and the forest were assessed with t-test, either as differences in the two-week total catches (two-tailed t-test with unequal variances) or species-specific daily catches (paired t-test). The latter test was applied only for the catches of abundant species following normal distribution. The dependence of lepidopteran catch on the vegetated area within 100-m radius of traps were assessed with regression analysis. The significance of dependence was assessed with F-parameter and the significance of regression coefficient and intercept with t-parameter.
Results
Description of the study area
The study area consisted of (i) Oxalis-Myrtillus type of Norway spruce forest and (ii) an office campus, which displaced a part of forest in early 1990s during a rapid phase of urbanization in the province of Central Finland (Fig. 1, SI Text 1). Within a 10 m radius, the five bait traps in forest were surrounded by Oxalis-Myrtillus type of Norway spruce forest, where the main species of herbs were Vaccinium myrtillus and Oxalis acetocella; and the main species of trees were Norway spruce (Picea abies), Scotch pine (Pinus sylvestris), Common aspen (Populus tremula), Silver birch (Betula pendula) and Rovan (Sorbus acuparia; Table S1, Figure S2).
The office campus was dominated by the impervious surfaces of large buildings, parking lots and roads (Fig. 1). The sparse vegetation consisted of lawns, planted ornamental trees and two shrub species. A tiny (< 10 m2) road-side meadow represented a bit higher diversity of herbs than the lawns. The about 60 planted trees > 10 m in height in a 6-hectare campus consisted partly of the same trees species as in the surrounding forest (Common aspen Populus tremula, Silver birch Betula pendula, Rovan Sorbus aucuparia) but also included Norway Maple (Acer platanoides) and non-native Common lime (Tilia x europea; Table S1, Figure S2). The traps were hung in these tree species and were surrounded by impervious surfaces (parking lots, roads, and buildings), urban green infrastructure (lawns, ornamental non-native Spiraea japonica and Aronia sp. shrubs), and in one case (trap c5) by a small road-side meadow (Table S1, Figure S2).
The vegetated green area within 100-meter radius from traps ranged from a minimum of 5,000 m2 (18% vegetated, 82% impervious surface) at Trap c2 in the office campus to a maximum of 31,416 m2 (100% vegetated, 0% impervious surface) at Trap f1 in the forest. Within 100-meter radius, the traps in the office campus were surrounded on average by 7,813 ± 1,767 m2 of vegetation, which was significantly less than for the traps in the forest (27,949 ± 2,351 m2; mean ± SD, n = 5; t7.4 = 15.1, p < 0.001; n = 5 traps; Table S2).
The abundance and biomass of Lepidoptera in the office campus and the forest
During a two-week period (28 June–11 July 2022), the ten traps caught altogether 2,233 specimens of 56 lepidopteran species (Table S2). The biomass of species ranged from 0.003 g specimen− 1 (n = 1, Schrankia costaestrigalis, Erebidae) to 0.270 ± 0.027 g specimen− 1 (mean ± SD, n = 2; Deilephila elpenor, Sphingidae); Table S3). The traps of the office campus caught 82 ± 60 specimens trap− 1 two weeks− 1, a quarter of 365 ± 148 specimens trap− 1 two weeks− 1 in the forest traps (mean ± SD, n = 5 traps; Table S2). Similarly, the biomass of caught lepidopteran assemblage was 3 ± 2 g trap− 1 two weeks− 1 in the office campus, a quarter of that in the forest (13 ± 5 g trap− 1 two weeks− 1; n = 5; t5.3 = 3.71, p = 0.001; Fig. 2A). The number of trapped species was 15 ± 8 trap− 1 two weeks− 1 in the office campus and 30 ± 3 trap− 1 two weeks− 1 in the forest with a significant difference (t5.6 = -3.37, p = 0.015; Fig. 2B).
The (A) biomass (grams trap− 1 two weeks− 1) and (B) species richness (number of species two weeks− 1 trap− 1) in the trapped lepidopteran assemblage in the forest and in the office campus. The whiskers show minimum and maximum, the box shows the first and third quartile, the cross refers to mean and horizontal line to median of 5 traps
Abundance and biomass of Lepidoptera in relation to the vegetated and impervious landscape
According to a regression analysis, the lepidopteran catch depended on the vegetated area within 100-meter radius of traps (for abundance F1,8 = 20.55, p = 0.002; for biomass F1,8 = 17.89, p = 0.003; Fig. 3). The vegetated area explained 72% and 69% of the number of specimens and biomass caught from traps, respectively (Fig. 3). In Fig. 3A, the regression coefficient (0.014 ± 0.003; estimate ± SE) is significantly different from zero (t = 4.53, p = 0.002), but the intercept (-34 ± 65) is not (t = -0.52, p = 0.61). In Fig. 3B, the regression coefficient (0.0005 ± 0.0001) is significantly different from zero (t = 4.23, p = 0.003), but the intercept (-0.84 ± 2.54) is not (t = -0.33, p = 0.75). The regression coefficients were significantly different from zero, but the intercepts were not (Fig. 3). The latter indicates an absence of Lepidoptera when the landscape lacks vegetated area and is entirely covered by impervious surface.
The dependence of (A) abundance (number of specimens two weeks− 1 trap− 1) and (B) biomass (grams two weeks− 1 trap− 1) of trapped Lepidoptera on the vegetated area within 100-meter radius of traps (m2). The dots show individual traps in the office campus (a group of five on left) and in the forest (a group of five on right). The lines illustrate the regression equations
Changes in the lepidopteran community structure related to urbanization
The most abundant moth was Acronicta rumicis (Noctuidae) both in the office campus and in the forest (Fig. 4; Table S2). Acronicta rumicis and 12 other species had significantly lower abundances in the office campus than in the forest when the daily catches (n = 14 days) were analyzed with paired t-test (Fig. 4). For example, Atolmis rubricollis (Erebidae) was absent from the office campus but abundant in the forest (Fig. 4). Agrotis exclamations (Noctuidae) had significantly higher abundance in the office campus than in the forest (Fig. 4). Among the abundant species, Oligia latruncula (Noctuidae), Acronicta megacephala (Noctuidae) and Thethea or (Drepanidae) had no significant differences in abundances between the office campus and the forest (Fig. 4, paired t-test, p > 0.05, df = 13). In the office campus, these moths were most abundant in the traps surrounded by their larval food plants (O. latruncula in trap c5 with grasses in a small meadow, A. megacephala and T. or in trap c2 with Populus, Tables S1, S2, Fig. S2). In the office campus, 99% of 410 specimens and 92% of 38 species belong to the same species found in the forest traps, when examining the total catch from five traps during the two-week study period (Table S2). Five out of six unique species in the office campus were singletons such as Acronicta aceris (Noctuidae), a new record for the province of Central Finland.
The number of specimens trapped from the forest and the office campus. The bars show the mean number of specimens trap− 1 two weeks− 1 at logarithmic scale arranged from the most abundant species (Acronicta rumicis) to least abundant species caught in singletons (Acronicta aceris – Moma alpinum). The whiskers show standard deviation of abundance in 5 traps. The asterisks show species with significant differences in abundance between the office campus and the forest (* = p < 0.05, ** = p < 0.01, *** = p < 0.001, paired t-test of daily catch, df = 13)
The biomass of specimens in the caught lepidopteran assemblage was 0.043 ± 0.002 g specimen− 1 in the office campus, 1.16-fold higher than that in the forest (0.037 ± 0.002 g specimen− 1; n = 5; t5.6 = -3.82; p = 0.001; Fig. 5).
Discussion
Our results clearly show that the biomass of a lepidopteran community decreased dramatically when native forest was cleared and turned into a conventional office campus dominated by impervious surfaces. The abundance and biomass of Lepidoptera decreased along with a decrease in vegetated area and approached zero in a landscape covered entirely with impervious surface. Displacement of forest with an office campus decreased species richness considerably and changed the species composition of the lepidopteran community. This type of urban development can cause local extinctions for some species such as Atolmis rubricollis and increase the mean size of specimens caught with bait traps.
A displacement of native forest with built-up area causes a dramatic loss in lepidopteran abundance
According to the catch of bait traps in this study, the biomass and abundance of lepidopteran community decreases to a quarter when native forest is replaced with an office campus dominated with impervious surfaces. A similar difference was found in the abundance of geometrid moths caught in light traps between the native forest and the urban area of Loja City in Ecuador (Gaona et al. 2021). In Britain, the biomass of trapped moths was about one third in urban area compared to woodland (Macgregor et al. 2019). In highly urbanized Belgium, the abundance of trapped macromoths did not differ between areas of low and high urbanization, but a significant decrease was found for butterflies in standard transects (Piano et al. 2020). In heavily urbanized western Europe, the biomass of flying insects has decreased during recent decades even in rural nature reserves and may have reduced the difference in the abundance of Lepidoptera between rural and urban areas (Hallmann et al. 2017). This study investigated Lepidoptera with bait traps, while other studies have used standard transects, nets and light traps (Macgregor et al. 2019; Piano et al. 2020; Fenoglio et al. 2020; Gaona et al. 2021). Independent of the method of investigation, the biomass of Lepidoptera generally decreases with urbanization and the decrease is the most dramatic when native forest is turned into urbanized area (Macgregor et al. 2019; Piano et al. 2020; Fenoglio et al. 2020; Gaona et al. 2021).
Impervious surfaces are unsuitable habitat for most Lepidoptera
The regression analyses of this study indicate that the abundance and biomass of Lepidoptera decrease along with a decrease of vegetated area and approach zero when the landscape is covered entirely with impervious surfaces such as parking lots, roads, and buildings. In similar manner, butterfly abundance approaches zero when the coverage of impervious surface in the landscape approaches 100% in Melbourne, Australia (Kurylo et al. 2020). Several studies have reported a decline in lepidopteran abundance or biomass with an increase of impervious surfaces (Melliger et al. 2017; Tzortzakaki et al. 2019; Merckx and Van Dyck 2019; Kuussaari et al. 2021). Our study and that of Kurylo et al. (2020) agree with the earlier findings and further indicate that a landscape without vegetation, e.g., in a highly urbanized city centers, is unsuitable habitat for most lepidopteran species. This is an obvious conclusion for the lepidopteran species and likely to other invertebrates feeding on green vegetation.
Changes in the community structure of Lepidoptera upon urbanization
According to this study, the species richness of lepidopteran community in an office campus is only half of that in an adjacent forest area. Our results agree with earlier general findings that urbanization decreases species richness of lepidopteran communities (Clark et al. 2007; Fenoglio et al. 2020; Gaona et al. 2021; Kurylo et al. 2020; Kuussaari et al. 2021; Piano et al. 2020; Pignataro et al. 2020).
In this study, the specimens were on average larger in the assemblage of Lepidoptera trapped in the office campus than in the forest. Similarly, the size of specimens in the trapped lepidopteran community increased with built-up area in Belgium (Merckx and Van Dyck 2019). Because mobility generally increases with size, these findings suggest an elevated mobility for the lepidopteran communities in fragmented urban environments, a general trend found also in other types of fragmented landscapes (Ewers and Didham 2006). Both this study and that of Merckx and Van Dyck (2019) used traps, which attract flying adult stages of Lepidoptera. Such traps can attract selectively mobile large-sized species from the surrounding good-quality habitats (e.g., a native forest in this study). If most Lepidoptera caught from the traps in the office campus arrived from forest, those moths flew across the 100–150 m wide poor habitat when seeking for nectar. Thus, the most lepidopteran species caught from the traps in office campus, primarily large noctuids or drepanids, can cope with habitat fragmentation when the connectivity between habitats is < 100–150 m. The mobility of lepidopteran species varies considerably, e.g., from 44 m of Siona lineata (Geometridae) to 985 m of Anthocharis cardamines (Pieridae) in a release experiment (Kuussaari et al. 2014). In another study, A. cardamines belongs to a group of butterfly species, which was able to exploit suitable habitat patches (urban gardens) within 500 m distance but did not coupe distances of ≥ 1,000 m like the most mobile group of butterflies in Barcelona, Spain (Pla-Narbona et al. 2022). The latter study shows that mobility and the connectivity between suitable habitats are key determinants of lepidopteran communities in urban environments.
The lepidopteran species with poor mobility e.g., limited to < 100 m, are not likely to occupy habitat patches beyond their dispersal ability (Pla-Narbona et al. 2022). In this study, Atolmis rubricollis was abundant in the forest but absent from the office campus. The larvae of A. rubricollis feed on lichens growing on branches of trees, spruce in particular (Hydén 2006) and obviously these moths avoided open urban landscape with sparse trees in our study area. The specialization on lichens and poor dispersal ability make A. rubricollis comparable to other food specialists with limited mobility, which tend to be the first to go locally extinct in urbanization-related habitat fragmentation (Clark et al. 2007; Kuussaari et al. 2021).
In this study, only one species, Agrotis exclamationis (Noctuidae), was significantly more abundant in the office campus than in the forest. According to Ahola and Silvonen (2011), A. exclamationis is abundant in manmade surroundings and may avoid woodland (Table S4). The larva is polyphagous on herbs (Table S4). Among abundant species, Oligia latruncula (Noctuidae), Acronicta megacephala (Noctuidae), and Tethea or (Drepanidae), were as abundant in the office campus as in the forest. The larvae of O. latruncula live inside stems or sheaths of grasses (Ahola and Silvonen 2011). Tethea or and A. megacephala are found in open forests and parks with Populus tremula, the host plant of their larvae (Mikkola et al. 1985; Ahola and Silvonen 2011). Populus tremula was abundant in the forest and planted in the office campus too. The urban traps caught one Acronicta aceris (Noctuidae) moth, a new record for the province of Central Finland and one of the northernmost records of this species (Figure S3). The larvae of Acronicta aceris feed on native Norway Maple (Acer platanoides) and non-native Common lime (Tilia x europea) planted in the office campus (Ahola and Silvonen 2011, Table S4). When examined together, A. exclamations, O. latruncula, A. megacephala, T. or, and A. aceris formed a group of species, which can find a suitable habitat and larval foodplants among the sparse vegetation of office campus. As this study concerned adult states (imago), a part of moths found in the traps of office campus may have emerged close to the traps or been attracted to copulate or lay eggs on larval foodplant. This study together with earlier findings indicates that an urban landscape selectively hosts herbivorous insects feeding on the planted native or non-native plant species (Kowarik et al. 2019; Kopačka et al. 2021).
Acronicta aceris found in the office campus belongs to a large group of warm-adapted lepidopteran species, which are expanding their ranges northward (Hällfors et al. 2020). The expansion history of A. aceris suggests that cities form a frontline for the species expansion (Figure S3). This study did not measure the temperature of office campus in the suburb of Jyväskylä, but according to satellite measurements the city of Jyväskylä is 4.1 °C warmer than the surrounding rural area (OECD 2022). The elevated temperature in the urban area can be expected to support the development and increase the survival of A. aceris, its hostplants Norway Maple (Acer platanoides) and Common lime (Tilia x europea) at Jyväskylä situated in the North edge of their distribution (Figure S3, Hämet-Ahti 1992). This study suggest that warm urban thermal niches may have facilitated the northward expansion of Acronicta aceris similarly as found earlier for plants and birds (Murakami et al. 2007; Zuckerberg et al. 2011).
How to mitigate biodiversity loss associated with urbanization?
Based on the findings of the present study, we propose some recommendations for maintaining species rich and abundant native lepidopteran communities. In city planning, one should (i) preserve hotspots of biodiversity such as native forests and (ii) maintain connectivity between forest patches within the dispersal ability of Lepidoptera (Hansen et al. 2017; Meerow and Newell 2017). Our study suggests that most but not all lepidopteran species can disperse between habitats separated by the distance of < 150 m. When urbanization displaces forests, instead of non-native plants as the ornamental green urban infrastructure, one should (iii) prefer native keystone trees, such as Populus spp., which can support larvae of several local lepidopteran species (Narango et al. 2020; Burghardt et al. 2010; Tallamy et al. 2021).
Data availability
Available upon request.
References
Ahola M, Silvonen K (2011) Larvae of northern European Noctuidae, volumes 1–3. KuvaSeppälä Yhtiöt Oy, Vaasa
Artaxo P, Hansson HC, Andreae MO et al (2022) Tropical and Boreal Forest - atmosphere interactions: a review. Tellus B Chem Phys Meteorol 74:24–163. https://doi.org/10.16993/tellusb.34
Burghardt KT, Tallamy DW, Philips C, Shropshire KJ (2010) Non-native plants reduce abundance, richness, and host specialization in lepidopteran communities. https://doi.org/10.1890/ES10-00032.1. Ecosphere 1:
Cajander AK (1949) Forest types and their significance. Acta Forestalia Fennica 56:1–36
Cincotta RP, Wisnewski J, Engelman R (2000) Human population in the biodiversity hotspots. Nature 404:990–992. https://doi.org/10.1038/35010105
Clark PJ, Reed JM, Chew FS (2007) Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosyst 10:321–337. https://doi.org/10.1007/s11252-007-0029-4
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc 81:117–142
Fenoglio MS, Rossetti MR, Videla M (2020) Negative effects of urbanization on terrestrial arthropod communities: a meta-analysis. Glob Ecol Biogeogr 29:1412–1429. https://doi.org/10.1111/geb.13107
Frank SD, Backe KM (2023) Effects of Urban Heat Islands on Temperate Forest Trees and Arthropods. Curr Forestry Rep 9:48–57
Gaona FP, Iñiguez-Armijos C, Brehm G et al (2021) Drastic loss of insects (Lepidoptera: Geometridae) in urban landscapes in a tropical biodiversity hotspot. J Insect Conserv 25:395–405. https://doi.org/10.1007/s10841-021-00308-9
Greig EI, Wood EM, Bonter DN (2017) Winter range expansion of a hummingbird is associated with urbanization and supplementary feeding. Proc Royal Soc B: Biol Sci 284. https://doi.org/10.1098/rspb.2017.0256
Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD, Cook WM (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1(2):e1500052. https://doi.org/10.1126/sciadv.1500052
Hällfors MH, Pöyry J, Heliölä J, Kohonen I, Kuussaari M, Leinonen R, Schmucki R, Sihvonen P, Saastamoinen M (2020) Combining range and phenology shifts offers a winning strategy for boreal Lepidoptera. Ecol Lett 24(8):1619–1632. https://doi.org/10.1111/ele.13774
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PloS One 12(10):e0185809. https://doi.org/10.1371/journal.pone.0185809.g001
Hämet-Ahti L (1992) In: Palmén A, Alanko P, Tigerstedt P (eds) Woody flora of Finland, 2nd edn. Finnish Dendrological Society, Helsinki
Hansen R, Rall EL, Rolf W, Pauleit S (2017) Urban green infrastructure planning. A Guide for Practitioners Nature-Based Solutions & Sustainable Urbanisation View project PHUSICOS
Hydén N (2006) Atolmis Rubricollis rödhalsad lavspinnare. Nationalnyckeln till Sveriges flora and fauna. Fjärilar: Ädelspinnare-tofsspinnare. Lasiocampidae-Lymantriidae. ArtDatabanken, Lepidoptera, pp 342–343
Jokimäki J, Jukka S, Marja-Liisa K-J (2018) Urban core areas are important for species conservation: a european-level analysis of breeding bird species. Landsc Urban Plan 178:73–81. https://doi.org/10.1016/j.landurbplan.2018.05.020
Kopačka M, Nachman G, Zemek R (2021) Seasonal changes and the interaction between the horse chestnut leaf miner Cameraria ohridella and horse chestnut leaf blotch Disease caused by Guignardia Aesculi. Forests 12. https://doi.org/10.3390/f12070952
Kowarik I, Hiller A, Planchuelo G et al (2019) Emerging urban forests: opportunities for promoting the wild side of the urban green infrastructure. Sustain (Switzerland) 11. https://doi.org/10.3390/su11226318
Kurylo JS, Threlfall CG, Parris KM et al (2020) Butterfly richness and abundance along a gradient of imperviousness and the importance of matrix quality. Ecol Appl 30. https://doi.org/10.1002/eap.2144
Kuussaari M, Saarinen M, Korpela EL et al (2014) Higher mobility of butterflies than moths connected to habitat suitability and body size in a release experiment. Ecol Evol 4:3800–3811. https://doi.org/10.1002/ece3.1187
Kuussaari M, Toivonen M, Heliölä J et al (2021) Butterfly species’ responses to urbanization: differing effects of human population density and built-up area. Urban Ecosyst 24:515–527. https://doi.org/10.1007/s11252-020-01055-6
Liu Z, He C, Wu J (2016) The relationship between habitat loss and fragmentation during urbanization: an empirical evaluation from 16 world cities. PLoS ONE 11. https://doi.org/10.1371/journal.pone.0154613
Lu D, Weng Q (2006) Use of impervious surface in urban land-use classification. Remote Sens Environ 102:146–160. https://doi.org/10.1016/j.rse.2006.02.010
Luck GW (2007) A review of the relationships between human population density and biodiversity. Biol Rev 82:607–645
Macgregor CJ, Williams JH, Bell JR, Thomas CD (2019) Moth biomass increases and decreases over 50 years in Britain. Nat Ecol Evol 3:1645–1649. https://doi.org/10.1038/s41559-019-1028-6
Mahtta R, Mahendra A, Seto KC (2019) Building up or spreading out? Typologies of urban growth across 478 cities of 1 million+. https://doi.org/10.1088/1748-9326/ab59bf. Environmental Research Letters 14:
Meerow S, Newell JP (2017) Spatial planning for multifunctional green infrastructure: growing resilience in Detroit. Landsc Urban Plan 159:62–75. https://doi.org/10.1016/j.landurbplan.2016.10.005
Melliger RL, Rusterholz HP, Baur B (2017) Habitat- and matrix-related differences in species diversity and trait richness of vascular plants, Orthoptera and Lepidoptera in an urban landscape. Urban Ecosyst 20:1095–1107. https://doi.org/10.1007/s11252-017-0662-5
Merckx T, Van Dyck H (2019) Urbanization-driven homogenization is more pronounced and happens at wider spatial scales in nocturnal and mobile flying insects. Glob Ecol Biogeogr 28:1440–1455. https://doi.org/10.1111/geb.12969
Meyrier E, Jenni L, Bötsch Y et al (2017) Happy to breed in the city? Urban food resources limit reproductive output in western Jackdaws. Ecol Evol 7:1363–1374. https://doi.org/10.1002/ece3.2733
Mikkola K, Jalas I, Peltonen O (1985) Mittarit 1. Suomen perhostutkijain seura, Helsinki
Murakami K, Matsui R, Morimoto Y (2007) Northward invasion and range expansion of the invasive fern Thelypteris dentata (Forssk.) St. John into the urban matrix of three prefectures in Kinki District, Japan. Am Fern J 97:186–198. https://doi.org/10.1640/0002-8444(2007)97[186:NIAREO]2.0.CO;2
Narango DL, Tallamy DW, Shropshire KJ (2020) Few keystone plant genera support the majority of Lepidoptera species. Nat Commun 11(1):1–8. https://doi.org/10.1038/s41467-020-19565-4
Öckinger E, Dannestam Ã, Smith HG (2009) The importance of fragmentation and habitat quality of urban grasslands for butterfly diversity. Landsc Urban Plan 93:31–37. https://doi.org/10.1016/j.landurbplan.2009.05.021
OECD (2022) Regions and cities at a glance - Finland. Paris
Pettersson LB, Franzén, M (2008) Comparing wine-based and beer-based baits for moth trapping: a field experiment. [Vin-baserade och öl-baserade beten för nattfjärilsfångst: ett fältexperiment.] Entomologisk Tidskrift 129(3):129–134
Piano E, Souffreau C, Merckx T et al (2020a) Urbanization drives cross-taxon declines in abundance and diversity at multiple spatial scales. Glob Chang Biol 26:1196–1211. https://doi.org/10.1111/gcb.14934
Pignataro T, Bressan P, Santos AL, Cornelissen T (2020) Urban gradients alter the diversity, specific composition and guild distribution in tropical butterfly communities. Urban Ecosyst 23:723–730. https://doi.org/10.1007/s11252-020-00975-7
Pla-Narbona C, Stefanescu C, Pino J et al (2022) Butterfly biodiversity in the city is driven by the interaction of the urban landscape and species traits: a call for contextualised management. Landsc Ecol 37:81–92. https://doi.org/10.1007/s10980-021-01347-y
Porensky LM, Young TP (2013) Edge-effect interactions in fragmented and patchy landscapes. Conserv Biol 27(3):509–519. https://doi.org/10.1111/cobi.12042
Prudic KL, Cruz TMP, Winzer JIB et al (2022) Botanical Gardens Are Local Hotspots for Urban Butterflies in arid environments. Insects 13. https://doi.org/10.3390/insects13100865
Ribeiro DB, Batista R, Prado PI et al (2012) The importance of small scales to the fruit-feeding butterfly assemblages in a fragmented landscape. Biodivers Conserv 21:811–827. https://doi.org/10.1007/s10531-011-0222-x
Robinet C, Imbert CE, Rousselet J et al (2012) Human-mediated long-distance jumps of the pine processionary moth in Europe. Biol Invasions 14:1557–1569. https://doi.org/10.1007/s10530-011-9979-9
Seress G, Liker A (2015) Habitat urbanization and its effects on birds. Acta Zool Academiae Scientiarum Hung 61:373–408. https://doi.org/10.17109/AZH.61.4.373.2015
Tallamy DW, Narango DL, Mitchell AB (2021) Do non-native plants contribute to insect declines? Ecol Entomol 46:729–742. https://doi.org/10.1111/een.12973
Tiitu M (2018) Expansion of the built-up areas in Finnish city regions – the approach of travel-related urban zones. Appl Geogr 101:1–13. https://doi.org/10.1016/j.apgeog.2018.10.001
Timonen R (2020) Study on land use change fees in construction. Publications of the Ministry of Environment 36–42
Tzortzakaki O, Kati V, Panitsa M et al (2019) Butterfly diversity along the urbanization gradient in a densely-built Mediterranean city: land cover is more decisive than resources in structuring communities. Landsc Urban Plan 183:79–87. https://doi.org/10.1016/j.landurbplan.2018.11.007
Williams M (2008) Deforesting the Earth. From Prehistory to Global Crisis. An abridgement. University of Chicago Press, Chicago
Zuckerberg B, Bonter DN, Hochachka WM et al (2011) Climatic constraints on wintering bird distributions are modified by urbanization and weather. J Anim Ecol 80:403–413. https://doi.org/10.1111/j.1365-2656.2010.01780.x
Acknowledgements
We thank Juha Ahonen, Mervi Koistinen and Emma Pajunen (University of Jyväskylä) for providing technical help in laboratory. Kirsi Mukkala (Regional Council of Central Finland) and Aino Melakari (Statistics Finland) elucidated the development of population in the province of Central Finland. City of Jyväskylä kindly gave a permit for trapping on their land. Two anonymous reviewers gave valuable advice for revision of the original manuscript.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). Department of Biological and Environmental Science, University of Jyväskylä, supported the study.
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Author information
Authors and Affiliations
Contributions
All authors designed the study. A Pulli, T Kulmala and R Marin performed field and laboratory work under supervision of J Haimi and A Vähätalo. A Pulli and T Kulmala executed statistical analysis. All authors contributed to manuscript preparation and agreed with final document submitted.
Corresponding author
Ethics declarations
Competing interests
We declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vähätalo, A.V., Pulli, A., Kulmala, T. et al. Urbanization related changes in lepidopteran community. Urban Ecosyst 27, 377–386 (2024). https://doi.org/10.1007/s11252-023-01456-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-023-01456-3