Abstract
Pollinating insects are essential for food production. Both bee and non-bee pollinators are undergoing dramatic declines due to land use intensification and its consequences on native ecosystems. While interactions between crops and bee pollinators are well studied, our understanding of the pollination service provided by non-bee flower visitors including flies, ants, beetles and others is still limited. Moreover, the effects of landscape urbanization and changes in floral and nesting resource availability on the network structure of pollinators with both cultivated and wild plants have been poorly studied. We assessed which common bee and non-bee flower visitor groups dominate the interactions with both wild (e.g. Trifolium pratense, Taraxacum officinales) and cultivated plants (e.g. Fragaria ananassa, Cucurbita pepo) in urban community gardens in Berlin and Munich and explored how these interactions between flower visitor groups and plants change over the growing season. We further investigated the effect of changes in urbanization surrounding community gardens, and the availability of floral and nesting resources within gardens on the complexity (i.e. nestedness, linkage density, connectance) of interaction networks. We observed 20 focal plant species and 13 common bee and non-bee flower visitor groups in 30 urban community gardens. We found that dominant plant visitors changed over the growing season, with non-bee flower visitors including ants and flies as dominant early season visitors, and bee pollinators as important visitors later in the season. Nestedness of the flower visitor network increased with increases in floral richness in community gardens, while neither floral abundance nor the impervious surface surrounding the community gardens, garden size or the availability of nesting resources in gardens strongly influenced the flower visitor networks. Our findings suggest that high floral richness in community gardens may ensure the complexity and, thus, the stability of flower visitor networks. Findings further suggest that the role of non-bee flower visitors should be considered for pollination service provision especially in the shoulder seasons. Finally, our results emphasize that urban gardeners play a key role in mediating flower visitor interactions through their gardening practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of vegetables, fruit and nut crops produced in agriculture for human consumption around the world are pollinated by arthropods (Klein et al. 2007). While both managed and wild pollinators are essential for food production in agricultural systems, insect pollinators are undergoing dramatic declines due to the intensification of land use, habitat loss, the lack of flowers, climate change, invasive species and disease (Panziera et al. 2022; Wood et al. 2020; Sánchez-Bayo and Wyckhuys 2019; Goulson et al. 2015; Smith et al. 2015; Winfree et al. 2009).
Diverse pollinator communities improve pollination services in agricultural and natural systems through resource use complementarity due to variations in morphology and behaviour among pollinator taxa (Dainese et al. 2019; Garibaldi et al. 2013; Albrecht et al. 2012; Winfree and Kremen 2009). For example, within bees (clade: Anthophila), species have different feeding specializations (e.g. generalist vs. specialist), socialities (e.g. solitary vs. social), as well as body sizes (e.g. bumble bees (large) vs. sweat bees (small)). In addition, the diversity of non-bee flower visitors (e.g. beetles, flies, butterflies, moths) can provide pollination services at different times of the day (i.e. moths pollinate at night), over larger temporal ranges, and in weather conditions unfavourable to some bee species (Rader et al. 2016, 2011; McCall and Primack 1992). Non-bee flower visitors may also differ in how they use floral resources by visiting different parts of the flower or visiting different flowers within a plant, which could complement pollination service provided by bees and therefore enhance the overall pollination service (Rader et al. 2016; Hoehn et al. 2008). How these diverse assemblages of bee and non-bee pollinators interact with both cultivated and wild plants in agricultural systems is therefore likely different, yet our understanding of the complexity of plant-pollinator interactions in agricultural systems is still limited. Furthermore, although non-bee pollinators may be as important as bee pollinators for the production of a variety of crops (Cusser et al. 2021; Howlett et al. 2021; Földesi et al. 2021; Rader et al. 2020, 2009; Jauker and Wolters 2008), our understanding of their interactions with plants is still limited compared to bees. Accordingly, understanding the role of both bee and non-bee pollinators and how they interact with cultivated and wild plants in agricultural systems may benefit sustainable food production.
Urban agricultural systems, or ‘urban agroecosystems’, are food-production oriented ecosystems within cities. The sustainable production of food and flowers in these agroecosystems is increasingly relevant to growing urban human populations (e.g., 15–20% of the global food production is already taking place in or close to urban areas) (Nicholls et al. 2020; Thornton 2008; Armar-Klemesu 2000). Urban agroecosystems may also have complex yet largely understudied plant-pollinator interactions, which underly food and flower production. In particular, urban community gardens often host species-rich communities of flowering plants that provide nectar and pollen resources throughout the season and thereby serve as important refuges for pollinators within cities (Cohen et al. 2021; Egerer et al. 2020; Olsson et al. 2021; Baldock et al. 2019; Levé et al. 2019; Hall et al. 2017; Lowenstein and Minor 2016; Hülsmann et al. 2015). Yet, it is unclear how a relatively high diversity of floral resources may influence how (e.g., visitation frequency) flower visitors interact with cultivated and wild plant species, to thereby influence pollination provision.
Increasing urbanisation through urban densification and expansion may reduce pollinator richness, lower flower visitation rates, and reduce the diversity of interactions between plants and flower visiting insects due to landscape fragmentation and habitat loss (Udy et al. 2020; Harrison et al. 2019; Deguines et al. 2016; Bates et al. 2011) – with potential cascading effects on pollination services (Theodorou et al. 2021; Aguilar et al. 2006; Cheptou and Avendaño 2006; but see also Verboven et al. 2014b), as urbanization often drives the loss of nesting and floral resources (Theodorou et al. 2020a, b; Harrison and Winfree 2015; Williams et al. 2010). However, at the local habitat scale, the availability of floral resources, namely floral richness and abundance can have a positive effect on plant-pollinator interactions (Udy et al. 2020), pollinator diversity (Tasker et al. 2020; Woodard and Jha 2017; Wojcik and McBride 2012; Potts et al. 2003), flower visitation frequency (Gilpin et al. 2022; Ebeling et al. 2008; Hegland and Boeke 2006) and pollen deposition (Monasterolo et al. 2022; Cohen et al. 2021). In fact, flower-rich urban habitats often host a higher diversity of pollinators than do rural habitats (Baldock et al. 2015; Verboven et al. 2014a). Garden size also plays an important role in predicting pollinator diversity. For example, Quistberg et al. (2016) found that urban gardens along the California central coast that were larger in size hosted a higher abundance and diversity of bee pollinators than smaller gardens. Along with many other studies, they also found that the availability of bare soil is an important habitat feature that promotes pollinator richness and abundance by providing nesting habitat for ground nesting pollinator species (Felderhoff et al. 2023; Ballare et al. 2019; Quistberg et al. 2016). Thus, floral management – in particular increases in floral diversity – garden size and nesting resource availability – in particular increases in bare ground availability – can mediate the impacts of landscape urbanization on flower visitor interactions. (McDougall et al. 2022; Baldock 2020; Bloom et al. 2019; but see also Udy et al. 2020).
Studies on urban pollinator communities in urban agroecosystems have predominantly investigated the effect of urbanization (e.g., landscape imperviousness) on bee pollinators (Hernandez et al. 2009; Winfree et al. 2009). Yet we are still missing information on how both bee and non-bee pollinators interact with cultivated and wild plants in urban community gardens, and how this may vary based on both garden floral management, nesting resource availability and landscape urbanization. Flower visitor networks can help to understand the complex interactions between cultivated or wild plant species and their potential pollinators. As species are added to the network, increasing species richness, the number of possible interactions between them increases. This results in higher network complexity, measured by e.g., modularity and nestedness (Bascompte et al. 2003; Olesen et al. 2007). The complexity of flower visitor networks reflects the diversity and interdependence of species within the ecosystem and has important implications for ecosystem functioning and stability (see e.g., the diversity-stability debate; (MacArthur 1955; Bersier and Banasek-Richter 2009; Dunne et al. 2002)). Because larger habitats can support more species (species-area relationship, Schoener 1976), and, thus, support more interactions between species, the complexity and stability of networks is expected to increase with habitat area (Galiana et al. 2018). Testing how local agroecosystem management factors in the context of landscape urbanization influence diversity and interactions between plants and flower visitors can provide important information to our understanding of flower visitor networks, and how this may relate to stable pollination services in cities.
Here we investigate the effect of landscape imperviousness, garden size and changes in floral resource availability and nesting resource availability on the network structures of both bee and non-bee flower visitors with cultivated and wild plants in urban community gardens. We also determine the most frequent interactions between flower visitors and common garden plants, explore changes in flower visitor diversity over the growing season, and investigate how flower visitation by bee and non-bee visitors changes over the course of a growing season. Specifically, we ask: (1) What are the dominant pollinator groups that interact with common cultivated and wild plants? Here we consider ‘dominance’ of a pollinator group as the frequency of flower visits by individuals of that group compared to other pollinator groups. (2) How do diversity indices such as richness and diversity of flower visitor communities change over the growing season (3) How does the number of flower visits by bee and non-bee flower visitors change over the growing season?, and (4) How do flower visitor interactions change with changes in floral diversity, nesting resource availability, garden size and landscape urbanization? We hypothesized that: (a) the flower visits in urban community gardens are dominated by bee pollinators (honeybees, bumblebees, wild bees); (b) richness and diversity of flower visitor communities increase over the growing season; (c) the proportion of flower visits by bee pollinators to flower visits by non-bee flower visitors increases over the growing season; and (d) flower visitor networks will simplify (less interactions between plant species and flower visitors, represented by common network indices) with increasing landscape urbanization and decreasing floral diversity (richness and abundance) and nesting resource availability (Prendergast et al. 2022; Theodorou et al. 2020a, b).
Materials and methods
Urban community gardens
We conducted our research in 30 urban community gardens in Berlin (52.5200° N, 13.4050° E) and Munich (48.1351° N, 11.5820° E), Germany. From May to August 2021, 15 gardens were studied in Berlin (Northeast of Germany; area 891.7 km2; population > 3.6 million) and 15 gardens in Munich (South of Germany; area 310.71 km2; population > 1.4 million). The gardens ranged from 0.04 and 0.97 ha in size.
At the center of each community garden, we established a 400 m2 (20 × 20-m or 40 × 10-m) sampling plot in which to concentrate our observations. We adjusted the size of the sampling plot where necessary (i.e., where gardens were more narrow than they were wide, we created a 40 × 10-m plot). We visited the gardens four times approximately every four weeks from May to August 2021 (henceforth ‘sampling round’): 13.-23. May 2021, 14.-24. June 2021, 20.-30. July 2021, 17.-26. August 2021. Between three and seven gardens were visited each day and the order in which they were visited was selected so that each garden was visited twice in the morning (08:30–11:30) and twice in the afternoon (12:00–17:30).
Focal plant species
Observation of focal plants is a simple and standardised method for monitoring flower-visiting insects (Roy et al. 2016). To compare flower visitor networks across community gardens, we selected both focal cultivated and wild plant species in each garden that were (a) in flower and (b) the main flower resource (species with the highest flower abundance) in the garden at that time period on which to conduct flower visitor observations. We define “cultivated” species as those that were intentionally sown or planted native or nonnative species in the gardens, classified either by taxonomic criteria (taxa only known as cultivated species or varieties) or traces of cultivation (i.e., cultivation in rows or beds, promotion by weeding). “Wild” growing species were species with exclusively spontaneous occurrences, that were not directly planted by humans. In some instances, a plant species was observed in both categories. The number of observed focal plants varied with flower availability in the different community gardens and sampling rounds. Between two and six different focal plant species were observed per sampling round and garden. Gardens were heterogenous in their vegetation composition, with only one plant species (Fragaria ananassa) occurring in all 30 community gardens (see Table 1). Within each garden, patches with the highest amount of focal plant individuals in flower were chosen for observations (one patch per focal plant species per sampling round). Only those observations were included in the analysis where more than two patches of one focal plant species were observed in all community gardens in one sampling round.
Flower visitor observations
To determine how flower visitor interactions across all 30 community gardens change in relation to changes with local floral and nesting resource factors and landscape imperviousness, we conducted visual flower visitor observations during the four sampling rounds. Observations were conducted by one observer to minimise observer bias and occurred between 09:00 and 17:00 on mostly sunny days with temperatures > 13 °C. During each five minute observation, within an observation area consisting of a semicircle with a radius of 70 cm (0.77 m2) placed in the centre of each focal plant patch (Fig. 1), we recorded the interactions between one focal plant species and 13 flower visitor groups selected because they are common in our system: honeybees, bumblebees, wild bees, butterflies, moths, flies, hoverflies, beetles, bugs, thrips, ants, social wasps, and solitary wasps. We observed all insect visitors that were in contact with the reproductive organs of a flower, identified them to a flower visitor group and counted the number of visits (i.e., one flower visitor individual can land on one plant individual and interact with multiple inflorescences) for each individual until it left the observation area. Where the number of flower visits per individual as well as the number of individuals was too high to count simultaneously, we calculated visitation rates for the specific plant-flower visitor pair (e.g. Apis mellifera and Origanum vulgare) by recording the number of flowers that one individual visits in 30 s three times and then averaging the three observations. Because pollination surveys and experiments were running at the time of the observations, we did not collect pollinating insects. Visual identifications of pollinator groups were conducted by a researcher trained in insect identification.
Floral and nesting resources
We simultaneously measured floral resource availability (flower richness and abundance) as well as nesting resource availability (ground cover composition) within the community gardens for each sampling round at the same time as flower visitor observations. To do so, we randomly placed eight 1 × 1 m quadrats within the 20 × 20 m plots along four parallel 5 × 20 m transects (two random plots per transect) (following Seitz et al. 2022; Felderhoff et al. 2023; see for full description of vegetation sampling methods).
For floral resource availability, within these quadrats, we identified all flowering herbaceous plants (except grasses) to species level using Jäger (2016) and the Pl@ntNet app (https://plantnet.org/en/; Affouard et al. 2023) (see Seitz et al. 2022). In addition, we counted the total number of flowers within each quadrat. Flower species richness and total number of flowers was determined for each sampling round and then averaged per garden and across all four sampling rounds. When counting the number of flowers, we counted flowers inside inflorescences as individual flowers regardless of size.
For nesting resource availability, within each quadrat, we recorded the percentage of bare soil cover, mulch cover, rock cover, grass cover, herbaceous vegetation cover, and wood cover as our local garden scale land cover variables. We averaged ground cover data from these quadrats per garden and across all four sampling rounds. Here we consider bare soil cover as a proxy for nesting resources for ground nesting species following others (Felderhoff et al. 2023; Quistberg et al. 2016; Cohen et al. 2021).
Landscape imperviousness
To classify community gardens according to the degree of landscape urbanization, we used the amount of landscape imperviousness (i.e. sealed surface) surrounding the gardens as a proxy (von der Lippe et al. 2020). We collected publicly available data on landscape imperviousness for Berlin and Munich. We used the Geographical Information Systems software ArcGIS 10.5.1 (Environmental Systems Research Institute (ESRI) 2011) and the Copernicus High Resolution Layer: Imperviousness Density (IMD) 2018 (European Union, Copernicus Land Monitoring Service, European Environment Agency (EEA)) to measure the impervious surface in a 1000 m buffer around each garden. We selected this scale to measure how larger-scale landscape urbanization (as opposed to local land use (100 m)) influences flower visitor interactions. Imperviousness ranged from 17 to 83%.
Data analysis
We: (1) determined the dominant pollinator groups for the common focal plants in urban community gardens; (2) compared pollinator group diversity indices (richness and Shannon index) for each sampling round (May, June, July, August); (3) compared the number of flower visits by bee and non-bee flower visitors for each sampling round; and (4) assessed the relationship between plant-flower visitor network indices, landscape imperviousness, floral richness and abundance, garden size, and the availability of nesting sites.
-
1.
What are the dominant flower visitor groups that interact with common cultivated and wild plants?
To assess the dominant flower visitor groups for focal plants in our study sites, we first determined five plant species that were most common in the surveyed community gardens across all four sampling rounds and second, summed up the number of visits they received from the 13 different flower visitor groups. To determine the number of visits realized by each flower visitor group, we averaged their number of visits per sampling round.
-
2.
How do diversity indices of flower visitor communities change over the growing season?
To assess changes in the flower visitor community over the growing season (i.e., between monthly sampling rounds), we calculated the Shannon index and species richness for flower visitor groups for each round using the vegan package for R (Oksanen et al. 2007; R Development Core Team 2021) and compared them between each sampling round, using a non-parametric Kruskal–Wallis test and the nonparametric Dunn´s test (p-values adjusted with the Bonferroni method) to determine significant differences between sampling round means.
-
3.
How does the number of flower visits by bee and non-bee flower visitors change over the growing season?
To test the contribution of bee and non-bee flower visitors to overall flower visitation, we summed flower visitor groups into bee pollinators (bumblebees, honeybees, wild bees) and non-bee flower visitors (butterflies, flies, hoverflies, moths, ants, bugs, beetles, thrips, social wasps, solitary wasps) and compared their average flower visits using the unpaired two-samples Wilcoxon rank sum test for non-parametric data. To assess the contribution of bee and non-bee flower visitors to overall flower visitation across the monthly sampling rounds, we compared the average flower visits of bee and non-bee flower visitors, respectively, using a non-parametric Kruskal–Wallis test and the non-parametric Dunn´s test (p-values adjusted with the Bonferroni method). We also tested for differences in the number of flower visits between bee and non-bee flower visitors across the monthly sampling rounds using non-parametric Wilcoxon Signed Rank tests.
-
4.
How do flower visitor networks change with changes in floral diversity, nesting resource availability and landscape imperviousness?
To explore how flower visitor networks change with changes in landscape imperviousness and with changes in floral richness and abundance, we created bipartite networks of flower visitor interactions using the “bipartite” package in R (v. 2.13, Dormann et al. 2008). Bipartite networks are used to visualize interactions within webs consisting of two levels, in our case plants and flower visitors, and calculate indices commonly used to describe ecological networks (see below) (Bascompte and Scheffer 2022; Daniels et al. 2020; Ballantyne et al. 2017). We created bipartite networks using the data of visual counts of visits between arthropods grouped into flower visitor groups and the flowering structure of a focal plant. The resulting networks allow for testing hypotheses about flower visitor diversity and network complexity across differences in amounts of landscape imperviousness, garden size, nesting resource availability and floral richness and abundance.
We used the networklevel function in “bipartite” (Campbell et al. 2011; Mariani et al. 2019) to calculate multiple indices that describe network structure and interactions within a network. For our study, three network-level indexes were chosen to represent a variety of commonly used indexes in community ecology: i) connectance, ii) linkage density, and iii) nestedness (NODF). Connectance (C) measures the fraction of interactions that occur in the network, out of all possible interactions. Linkage density (LD) is calculated as the average number of links per species in a network, but weighted by the average number of interactions across the species (Dormann et al. 2009; Bersier et al. 2002). Nestedness describes the extent to which specialists interact with a subset of species that also interact with generalists; this metric is based on overlap and decreasing fill. Nestedness can have important implications for network stability; a highly nested network is thought to be more robust to species extinctions, as species that interact with fewer partners are more likely to be lost due to environmental disturbance or stress (Almeida-Neto et al. 2007).
To examine which environmental factors drive changes in flower visitor networks, we used generalized linear models (GLMs) with the glm function in R (R Development Core Team 2021). We used connectance, linkage density, and nestedness (NODF) as dependent variables. For our predictor variables, we first identified variables that were significantly correlated with one another (using the cor function in “stats” (R Core Team)) and selected the one with the highest correlation coefficients with other variables to include in the analysis. Floral richness was not correlated with the level of urbanization within a 1000 m buffer around the community gardens (Spearman's rho = -0.068, P > 0.05). We selected a total of five explanatory variables: percent landscape imperviousness surface (1000 m buffer), garden size, floral richness (number of flowering plant species), floral abundance (number of flowers), percent bare soil cover. For all dependent variables, we tested for normal distribution using the Shapiro Wilk test and since they followed normal distribution, we used a Gaussian distribution as this provided the best fit. We used model selection and model averaging (Burnham and Anderson 2002). We fitted separate global models for the three response variables linkage density, nestedness and connectance. We derived a full set of models with all possible combinations of fixed effects from each global model. Next, we applied an information theoretic approach to determine a set of best models, including all models within a range of ΔAICc ≤ 2 (Akaike's information criterion for small sample sizes) relative to the best model. To gain weighted averages of parameter estimates from the respective set of best models, model averaging was used (Burnham and Anderson 2002). The stats (R Core Team) package was used to fit GLMs (Bates et al. 2012), and the “MuMIn” package was applied for model averaging (Barton 2016).
All analyses were performed in the R Statistical Environment (version 4.0.2; R Core Team 2021).
Results
In total, we conducted 692 visitor observations over 58 h of 22 focal plant species across all sampling rounds during which we observed a total of 3,503 flower visitor individuals and 76,638 flower visits.
The number of flower visits differed significantly between bee and non-bee flower visitor groups of focus (P = 0.0034), with bee-pollinators performing 22 times the number of flower visits compared to non-bee flower visitors. On average, across community gardens and sampling rounds, honeybee individuals visited 52.9 flowers (SD ± 583.3 flowers), wild bee individuals visited 4.7 flowers (SD ± 30.8 flowers), bumblebee individuals visited 48.6 flowers (SD ± 252.4 flowers), and hoverflie individuals visited 2.2 flowers (SD ± 5.3 flowers).
Dominant flower visitor groups for common garden plants
Interactions between the five most observed focal plant species and their flower visitor groups are presented in Table 2. Some focal plants were visited by either bee or non-bee flower visitors. For example, lavender (Lavendula angustifolia) was visited by bee pollinators, but not by non-bee flower visitors, while common yarrow (Alchemilla vulgaris) and daisies (Bellis perennis) were visited exclusively by non-bee pollinators.
Changes in diversity indices of flower visitors over the growing season
Average flower visitor diversity (Shannon index) varied significantly across sampling rounds (Kruskal–Wallis test, Chi = 12.442, p = 0.006) (Fig. 2). Flower visitor diversity was significantly lower in May than in August (Dunn´s test, z = -3.203, p = 0.004). Richness of flower visitors varied significantly across sampling rounds (Kruskal–Wallis test, Chi = 21.424, p < 0.001). Flower visitor richness was significantly lower in the earlier than in the later sampling rounds; it was lower in May than in July (Dunn´s test, z = -4.362, p < 0.001), in July than in August (Dunn´s test, z = -3.086, p = 0.005), and in June than in July (Dunn´s test, z = -2.812, p = 0.01).
Violin plots of flower visitor diversity (Shannon index) for each sampling round (May, June, July, August) where flower visitors were observed in 30 urban community gardens in Berlin and Munich, Germany. Violin plot outlines illustrate kernel probability density. Integrated boxplots indicate the median and quartiles with whiskers reaching up to 1.5 times the interquartile range
Changes in the number of flower visits by bee and non-bee flower visitors over the growing season
Across all focal plant species and all observations, there was a significant difference in flower visitation (Wilcoxon test, W = 260,499, p = 0.002) between bee pollinators (bumblebees, honeybees, wild bees) (mean flower visits = 106 ± 654.75) and non-bee flower visitors (butterflies, flies, hoverflies, moths, ants, bugs, beetles, thrips, social wasps, solitary wasps) (mean flower visits = 4.77 ± 23.67), with bee pollinators visiting significantly more flowers over the entire sampling than non-bee flower visitors. Flower visitation by both bee pollinators and non-bee flower visitors changed significantly across the growing season (Kruskal–Wallis test, Chi = 120.91, p < 0.001; Chi = 11.184, p = 0.011, respectively) (Fig. 2). Bee pollinators visited significantly less flowers in May than in June, July and August (Dunn´s test, z = -3.802, p < 0.001, z = -9.893, p < 0.001, z = -8.318, p < 0.001; respectively), and in June than in July and August (Dunn´s test, z = -6.204, p < 0.001; z = -4.523, p < 0.001, respectively). Non-bee flower visitors visited significantly more flowers in May than in June (Dunn´s test, z = 3.297, p < 0.001).
In May, non-bee flower visitors visited significantly more flowers than bee flower visitors (Wilcoxon test, W = 8618, p < 0.001) (Fig. 3), with bees providing 1.02 ± 2.55 mean flower visits and non-bees providing 2.56 ± 2.97 mean flower visits (number of visits averaged per season). There was no significant difference in the number of flowers visited by bee and non-bee visitors in June (p > 0.05). In July and August, bee pollinators visited significantly more flowers than non-bee flower visitors (Wilcoxon test, W = 18,552, p < 0.001, W = 20,981, p < 0.001, respectively).
Violin plots of mean number of flower visits for bee pollinator groups (honeybee, bumblebee, wild bee) and non-bee flower visitor groups (butterflies, moths, flies, hoverflies, beetles, bugs, thrips, ants, social wasps, solitary wasps) per sampling round (May, June, July, August). Axes of response variables (number of flower visits) are logtransformed.. Violin plot outlines illustrate kernel probability density. Integrated boxplots indicate the median and quartiles with whiskers reaching up to 1.5 times the interquartile range
The effect of changes in floral resources, nesting resources, garden size and landscape imperviousness on flower visitor networks
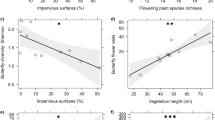
Flower visitor networks increased in nestedness (nestedness) with increasing floral richness in community gardens (Table 3 and Fig. 4). The best model explaining nestedness included floral richness abundance and garden size, however, only floral richness was siginificantly correlated. The availability of nesting resources (percent bare soil cover) and floral abundance and garden size was not significantly associated with changes in flower visitor networks (linkage density and connectance). Floral abundance was included in the best model explaining changes in likage density and floral abundance and richness, garden size and landscape urbanization were included in the best model explaining changes in connectance (Table 3).
Nestedness (NODF) of the networks in 30 urban community gardens (network indices calculated for each garden) was best explained by average floral richness. Closed circles denote mean values for each garden. Shaded area around fitted trend line indicates 95% confidence interval. Significance taken at alpha = 0.05
Discussion
The interactions between plants and their flower visitors underly a key ecosystem function in urban agroecosystems, namely pollination. Our study on flower visitor interactions in 30 urban community gardens in two of Germany’s most populated cities shows that both bee and non-bee flower visitors are important visitors of common wild (e.g. T. pratense, T. officinale) and cultivated (e.g. F. ananassa, C. pepo) garden plant species. Yet, who are the most frequent flower visitors changes over the growing season, with bee flower visitors dominating most of the season and non-bee flower visitors providing the majority of visits in the early season (May).. Neither landscape imperviousness nor garden size, floral abundance and nesting resource availability strongly influenced flower visitor networks, but floral richness increased network nestedness. Thus, city residents through their gardening practice, play a key role in mediating plant-pollinator interaction stability, and thereby, likely pollination provision.
Most frequent flower visitors of common garden plants
Not just bee, but non-bee flower visitors are common visitors of plants that are prevalent in urban community gardens. We found that wild bees, bumblebees and honeybees were the dominant flower visitors in the urban community gardens. However, non-bee flower visitors (butterflies, moths, flies, hoverflies, beetles, bugs, thrips, ants, social wasps, solitary wasps) are important flower visitors particularly early in the growing season when early blooming and economically important crops (i.e., strawberry) need insect pollination, but also when many wild plants including spring ephemerals are in bloom. Thus, for these plants, non-bee flower visitors may fill a temporal niche in pollination function for early flowering wild and cultivated plants, and potentially play an important role as ‘back-up’ pollinators in cooler conditions in the early season.
Our results support the importance of non-bee flower visitors belonging to the orders Diptera, Coleoptera and Hymenoptera for crop production. In a recent meta-analysis of 1,022 studies, Rader et al. (2020) show that 77% of 105 crops analyzed were visited by both bee and non-bee pollinators, and that the second most dominant flower-visiting order was Diptera (72% of crops), followed by Lepidoptera (54%) and Coleoptera (51%). In particular, hoverflies (order; Diptera; family Syrphidae) were the most dominant non-bee flower visitors, visiting over half of all crop species (Rader et al. 2020). In addition, our results are in line with a study from 33 farms in the UK, which found that non-syrphid Diptera comprise a majority of all flower-visiting Diptera (on average 82% abundance and 73% species richness), providing an estimated 84% of total pollen transport (Orford et al. 2015). We see similar dominance patterns in our system: ants and beetles and flies dominated overall flower visits early in May. The life histories of these common non-bee flower visitors may suggest resource use complementarity among non-bee visitors in the community gardens. For example, flies are often attracted to white flowers, ants to those flowers that offer high nectar rewards or extrafloral nectaries, while beetles to bowl-shaped flowers (Campbell et al. 2010; Fenster et al. 2004; Faegri and Van der Pijl 1979). In addition, these species all have large differences in their thermoregulation, a key factor in the foraging energetics of flower-visiting insects (Hegland et al. 2009). While ants are able to utilize flowers in spring and early summer when temperatures are still cool, many wild bees can only utilize flowers when average temperatures increase later in the summer. This may explain why we see shifts in pollinator floral visitation over the growing season.
Flower visitor communities, and their interactions with plants, change over the growing season
The shifts in flower visitation dominance over the growing season suggests that bee and non-bee flower visitors may provide complimentary pollination services over time in these community gardens. On average, bee pollinators deposit more pollen than non-bee pollinators (Rader et al. 2016; Orford et al. 2015) due to high efficiency, visitation rates and population numbers. However, even if non-bee flower visitors are less efficient at pollen deposition, these pollinators were the most frequent flower visitors in the early season here. This suggests that these flower visitors may be important pollinators for early spring flowering crops and ephemerals in community gardens. Although we include ants in our analysis as they are present in the system, we acknowledge that ants may be less efficient or even disruptive pollinators (Samra et al. 2014; Beattie et al. 1984; but see also Natsume et al. 2022).
Our work shows that we should consider the role of non-bee flower visitors for early season pollination service provision. These early season flower visitors may be increasingly important for the stability and maintenance of pollination in urban ecosystems, especially given bee pollinator declines and temporal variability that threatens bees. Future research should investigate the importance of non-bee flower visitors in the “shoulder” (early, late) seasons and explore potential synergies across multiple ecosystem services, such as natural pest control (Lundin et al. 2021). For example, some insect taxa are both efficient flower pollinators and pest predators in their adult stages (wasps and tachinid flies, Brock et al. 2021; Klein et al. 2002) or larval stages (hoverflies, Hodgkiss et al. 2018; Orford et al. 2015; Jauker and Wolters 2008). This may be especially important in the early season when some crops are flowering, but also young crops are most vulnerable to pests. Understanding how these complementary services can be utilized in an agricultural context may reduce pesticide applications and therefore lower the pressure on the beneficial insect communities (Egan et al. 2020). Our work emphasizes that urban gardeners play a key role in influencing interactions between their garden plants as well as wild plants and flower visitors, and thereby likely in pollination service provision.
In our study, we observed both wild and cultivated flowering plants in the community gardens, and some differences between plant type may explain the structure of networks. While the main pollinators of O. vulgare and C. officinalis were honeybees and wild bees, T. pratense and T. officinale) were mainly visited by non-bee flower visitors (ants and hoverflies, ants and beetles, respectively). The main flower visitors of F. ananassa were ants and wild bees. We note that focal plant species varied across community gardens due to garden heterogeneity, meaning that some flower visitors may be specialized on certain plant species that only occurred in some gardens (i.e. Thymus vulgare) and we could not control for this. Future research could expand to longer observations on e.g., experimental plants (Rivkin et al. 2020) to account for this variation. We further note that flowering woody species in the community gardens may have had an effect on network structure because they can offer prolific nectar and pollen resources, and can reduce focal plant visitation (‘dilution effect’, Yamamura 1999). Yet our work provides a realistic picture of common garden plants in situ, and their common flower visitors and interaction networks.
Floral diversity, but not landscape imperviousness nor nesting resource availability or garden size, modulates flower visitor interactions
In support of our hypothesis, network complexity (nestedness) increased with floral richness in community gardens. The influence of floral resources has been shown to support pollinators and influence their interactions over the season (Staab et al. 2020; Tasker et al. 2020). Yet, surprisingly, contrary to our hypothesis, neither landscape imperviousness nor nesting resource availability or garden size strongly influenced flower visitor interactions. We would expect that urban landscape imperviousness would alter flower visitor networks by reducing the number of flower visitor species (Bates et al. 2011) and changing the number of interactions between plant and flower visitor species (Geslin et al. 2013).
The lack of an effect of landscape could be explained by the local-scale effect of floral resource availability shown here and elsewhere (Wenzel et al. 2020), where floral resources mediate potential landscape-scale resource loss on network complexity. Flower visitor network complexity (nestedness) may increase or maintain network stability in urban habitats if floral resources are available and diverse, also if floral resources are low in the surrounding rural landscape (Theodorou et al. 2017). Yet studies show largely mixed results on these ‘mitigation effects’. Udy et al. (2020) show that although plant richness increased along an urbanization gradient (from city to village to farmland habitat), plant-pollinator network complexity decreased with increasing landscape imperviousness, concluding that cities hold rather poor pollinator communities and unstable networks. A study from Germany also found that although floral richness was higher in urban than in rural areas, bees visited a lower proportion of the available plants in cities than in rural habitats (Theodorou et al. 2017).
Of course, the differences in how network complexity responds to floral resources or landscape imperviousness largely depends on how the individual pollinators respond to these factors. For example, some pollinator groups, particularly bees, thrive in urban ecosystems and even exceed rural agricultural ecosystems in species richness and reproductive success (Casiker et al. 2021; Theodorou et al. 2020a, b; Theodorou et al. 2017; Baldock et al. 2015; Turrini and Knop 2015), while other pollinator groups such as hoverflies, are negatively affected by increases in landscape imperviousness (Persson et al. 2020; Bates et al. 2011; but see also Olsson et al. 2021). Geslin et al. (2013) conclude that hoverflies and solitary bees are more impacted by landscape imperviousness than bumblebees because they mostly visit open flowers while bumblebees visit flowers belonging to a different functional group.
The structure of flower visitor networks, and how it changes across land-use gradients, is relevant because network complexity can in turn influence pollination service provision (Tscharntke 2021), where increased pollinator richness and visitation can increase fruit and seed weight of crop plants (Lowenstein et al. 2015). Rivkin et al. (2020) have shown that negative effects of landscape imperviousness on flower visitor networks result in reduced plant reproductivity. What remains to be determined is how network complexity relates to pollination services of wild and cultivated plants in urban community gardens.
Conclusion
In sum, in our system, the main flower visitor communities change over the growing season, with bee and non-bee flower visitors differing in visitation dominance. Furthermore, the availability of flower resources, especially floral richness determines flower visitor network complexity, suggesting that local habitat scale factors and thus gardeners can influence flower visitor interactions in their gardens. Interestingly, and perhaps counterintuitively, this is regardless of the extent of urbanization surrounding the gardens and garden size. Here, gardeners can enhance floral diversity through both crop and non-crop plants that flower at different times of the season to their urban community gardens. Such efforts may provide benefits to gardeners through pollination provision, and may also help to mitigate the loss of insect habitats due to urban land use change and promote insect diversity and conservation at the local habitat scale.
Data availability
Summarised data will be uploaded on Dryad Digital Repository upon instruction.
References
Aguilar R, Ashworth L, Galetto L, Aizen MA (2006) Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol Lett 9(8):968–980
Affouard A, Joly A, Lombardo J, Champ J, Goeau H, Chouet M, Gresse H, Botella C, Bonnet P (2023) Pl@ntNet automatically identified occurrences. v1.8. Pl@ntNet. Dataset/Occurrence. https://ipt.plantnet.org/resource?r=queries&v=1.8. Accessed 25 Aug 2022
Albrecht M, Schmid B, Hautier Y, Müller CB (2012) Diverse pollinator communities enhance plant reproductive success. Proc Royal Soc b: Biol Sci 279(1748):4845–4852
Almeida‐Neto M R, Guimarães Jr P M, Lewinsohn T (2007) On nestedness analyses: Rethinking matrix temperature and anti‐nestedness. Oikos. 116(4):716–22
Armar-Klemesu M (2000) Urban agriculture and food security nutrition and health. Thematic Paper 4. In: N. Bakker, M. Dubbeling, S. Gündel, U. Sabel-Koschella, and H. Zeeuw (ed) Growing cities, growing food: urban agriculture on the policy agenda. Fefdafing, Germany: DSE, pp 99–118
Baldock KC (2020) Opportunities and threats for pollinator conservation in global towns and cities. Curr Opin Insect Sci 38:63–71
Baldock KC, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Osgathorpe LM, ... Memmott J (2015) Where is the UK's pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc Royal Soc B: Biol Sci 282(1803):20142849
Baldock KC, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Morse H, ... Memmott J (2019) A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat Ecol Evol 3(3):363–373
Ballantyne G, Baldock KC, Rendell L, Willmer PG (2017) Pollinator importance networks illustrate the crucial value of bees in a highly speciose plant community. Sci Rep 7(1):13
Ballare KM, Neff JL, Ruppel R, Jha S (2019) Multi-scalar drivers of biodiversity: Local management mediates wild bee community response to regional urbanization. Ecol Appl 29(3):e01869
Barton K (2016) MuMIn: Multi-Model Inference. R package version 1.15.6. http://CRAN.Rproject.org/package=MuMIn. Accessed 15 June 2022
Bascompte J, Scheffer M (2022) The Resilience of Plant-Pollinator Networks. Annu Rev Entomol 68:363–380
Bascompte J, Jordano P, Melián CJ, Olesen JM (2003) The nested assembly of plant-animal mutualistic networks. Proc Natl Acad Sci USA 100:9383–9387
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, … Grothendieck G (2012) Package ‘lme4’. CRAN. Vienna, Austria: R Foundation for Statistical Computing
Bates AJ, Sadler JP, Fairbrass AJ, Falk SJ, Hale JD, Matthews TJ (2011) Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS One 6(8):e23459
Beattie AJ, Turnbull C, Knox RB, Williams EG (1984) Ant inhibition of pollen function: a possible reason why ant pollination is rare. Am J Bot 71(3):421–426
Bersier LF, Banasek-Richter C (2009) The study of ecological networks. Ecological networks: linking structure to dynamics in food webs. Oxford University Press, Oxford, pp 1–11
Bersier LF, Banašek-Richter C, Cattin MF (2002) Quantitative descriptors of food‐web matrices. Ecology 83(9):2394–2407. https://www.R-project.org/. Accessed 19 Sept 2022
Bloom EH, Northfield TD, Crowder DW (2019) A novel application of the Price equation reveals that landscape diversity promotes the response of bees to regionally rare plant species. Ecol Lett 22(12):2103–2110
Brock RE, Cini A, Sumner S (2021) Ecosystem services provided by aculeate wasps. Biol Rev 96(4):1645–1675
Burnham K, Anderson D (2002) Model Selection and Multi-model Inference, 2nd edn. Springer, New York, NY
Campbell DR, Bischoff M, Lord JM, Robertson AW (2010) Flower color influences insect visitation in alpine New Zealand. Ecology 91(9):2638–2649
Campbell C, Yang S, Albert R, Shea K (2011) A network model for plant–pollinator community assembly. Proc Natl Acad Sci 108(1):197–202
Casiker CV, Jagadishakumara B, Sunil GM, Chaithra K, Devy MS (2021) Bee Diversity in the Rural-Urban Interface of Bengaluru and Scope for Pollinator-Integrated Urban Agriculture. The Rural-Urban Interface. Springer, Chambridge, pp 171–182
Cheptou PO, Avendaño VLG (2006) Pollination processes and the Allee effect in highly fragmented populations: consequences for the mating system in urban environments. New Phytol 172(4):774–783
Cohen H, Philpott SM, Liere H, Lin BB, Jha S (2021) The relationship between pollinator community and pollination services is mediated by floral abundance in urban landscapes. Urban Ecosyst 24(2):275–290
Cusser S, Haddad NM, Jha S (2021) Unexpected functional complementarity from non-bee pollinators enhances cotton yield. Agr Ecosyst Environ 314:107415
Dainese M, Martin EA, Aizen MA, Albrecht M, Bartomeus I, Bommarco R, ... Steffan-Dewenter I (2019) A global synthesis reveals biodiversity-mediated benefits for crop production. Sci Adv 5(10):eaax0121
Daniels B, Jedamski J, Ottermannn R, Ross Nickoll M (2020) A “plan bee” for cities: Pollinator diversity and plant-pollinator interactions in urban green spaces. PLoS One 15(7):e0235492
Deguines N, Julliard R, De Flores M, Fontaine C (2016) Functional homogenization of flower visitor communities with urbanization. Ecol Evol 6(7):1967–1976
Dormann CF, Fründ J, Blüthgen N, Gruber B (2009) Indices, graphs and null models: analyzing bipartite ecological networks. Open J Ecol 2(1)
Dormann CF, Gruber B, Fründ J (2008) Introducing the bipartite Package: Analysing ecological networks. R News 8(2):8–11
Dunne JA, Williams RJ, Martinez ND (2002) Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol Lett 5(4):558–567
Ebeling A, Klein AM, Schumacher J, Weisser WW, Tscharntke T (2008) How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 117(12):1808–1815
Egan PA, Dicks LV, Hokkanen HM, Stenberg JA (2020) Delivering integrated pest and pollinator management (IPPM). Trends Plant Sci 25(6):577–589
Egerer M, Cecala JM, Cohen H (2020) Wild bee conservation within urban gardens and nurseries: effects of local and landscape management. Sustainability 12(1):293
Faegri K, Van Der Pijl L (1979) Principles of Pollination Ecology. Pergamon Press, Oxford
Felderhoff J, Gathof AK, Buchholz S, Egerer M (2023) Vegetation complexity and nesting resource availability predict bee diversity and functional traits in community gardens Ecol Appl 33(2):e2759
Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD (2004) Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst 35:375–403
Földesi R, Howlett BG, Grass I, Batáry P (2021) Larger pollinators deposit more pollen on stigmas across multiple plant species—a meta-analysis. J Appl Ecol 58(4):699–707
Galiana N, Lurgi M, Claramunt-López B, Fortin MJ, Leroux S, Cazelles K, ... Montoya JM (2018) The spatial scaling of species interaction networks. Nat Ecol Evol 2(5):782–790
Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, ... Klein AM (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339(6127):1608–1611
Geslin B, Gauzens B, Thebault E, Dajoz I (2013) Plant pollinator networks along a gradient of urbanisation. PLoS One 8(5):e63421
Gilpin AM, O’Brien C, Kobel C, Brettell LE, Cook JM, Power SA (2022) Co-flowering plants support diverse pollinator populations and facilitate pollinator visitation to sweet cherry crops. Basic Appl Ecol 63:36–48
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229):1255957
Hall DM, Camilo GR, Tonietto RK, Ollerton J, Ahrné K, Arduser M, ... Threlfall CG (2017) The city as a refuge for insect pollinators. Conserv Biol 31(1):24–29
Harrison T, Winfree R (2015) Urban drivers of plant-pollinator interactions. Funct Ecol 29(7):879–888
Harrison T, Gibbs J, Winfree R (2019) Anthropogenic landscapes support fewer rare bee species. Landscape Ecol 34(5):967–978
Hegland SJ, Boeke L (2006) Relationships between the density and diversity of floral resources and flower visitor activity in a temperate grassland community. Ecol Entomol 31(5):532–538
Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø (2009) How does climate warming affect plant-pollinator interactions? Ecol Lett 12(2):184–195
Hernandez JL, Frankie GW, Thorp RW (2009) Ecology of urban bees: a review of current knowledge and directions for future study. Cities Environ (CATE) 2(1):3
Hodgkiss D, Brown MJ, Fountain MT (2018) Syrphine hoverflies are effective pollinators of commercial strawberry. J Pollination Ecol 22:55–66
Hoehn P, Tscharntke T, Tylianakis JM, Steffan-Dewenter I (2008) Functional group diversity of bee pollinators increases crop yield. Proc Royal Soc b: Biol Sci 275(1648):2283–2291
Howlett BG, Todd JH, Willcox BK, Rader R, Nelson WR, Gee M, ... Davidson MM (2021) Using non-bee and bee pollinator-plant species interactions to design diverse plantings benefiting crop pollination services. In Advances in Ecological Research 64, Academic Press, Cambridge, United States, pp. 45–103
Hülsmann M, von Wehrden H, Klein AM, Leonhardt SD (2015) Plant diversity and composition compensate for negative effects of urbanization on foraging bumble bees. Apidologie 46(6):760–770
Jäger EJ, Ebel F, Hanelt P, Müller GK (2016) Rothmaler-Exkursionsflora von Deutschland: Krautige Zier-und Nutzpflanzen. Berlin: Springer-Verlag
Jauker F, Wolters V (2008) Hover flies are efficient pollinators of oilseed rape. Oecologia 156(4):819–823
Klein AM, Steffan-Dewenter I, Buchori D, Tscharntke T (2002) Effects of land-use intensity in tropical agroforestry systems on coffee flower-visiting and trap-nesting bees and wasps. Conserv Biol 16(4):1003–1014
Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc Royal Soc b: Biol Sci 274(1608):303–313
Levé M, Baudry E, Bessa-Gomes C (2019) Domestic gardens as favorable pollinator habitats in impervious landscapes. Sci Total Environ 647:420–430
Lowenstein DM, Minor ES (2016) Diversity in flowering plants and their characteristics: integrating humans as a driver of urban floral resources. Urban Ecosyst 19(4):1735–1748
Lowenstein DM, Matteson KC, Minor ES (2015) Diversity of wild bees supports pollination services in an urbanized landscape. Oecologia 179(3):811–821
Lundin O, Rundlöf M, Jonsson M, Bommarco R, Williams NM (2021) Integrated pest and pollinator management–expanding the concept. Front Ecol Environ 19(5):283–291
MacArthur R (1955) Fluctuations of animal populations and a measure of community stability. Ecology 36(5):533–536
Mariani MS, Ren ZM, Bascompte J, Tessone CJ (2019) Nestedness in complex networks: observation, emergence, and implications. Phys Rep 813:1–90
McCall C, Primack RB (1992) Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am J Bot 79(4):434–442
McDougall R, Kristiansen P, Latty T, Jones J, Rader R (2022) Pollination service delivery is complex: Urban garden crop yields are best explained by local canopy cover and garden-scale plant species richness. J Appl Ecol 59(5):1248–1257
Monasterolo M, Poggio SL, Medan D, Devoto M (2022) High flower richness and abundance decrease pollen transfer on individual plants in road verges but increase it in adjacent fields in intensively managed agroecosystems. Agr Ecosyst Environ 333:107952
Natsume K, Hayashi S, Miyashita T (2022) Ants are effective pollinators of common buckwheat Fagopyrum esculentum. Agric Entomol 24(3):446–452
Nicholls E, Ely A, Birkin L, Basu P, Goulson D (2020) The contribution of small-scale food production in urban areas to the sustainable development goals: A review and case study. Sustain Sci 15(6):1585–1599
Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, Suggests MASS (2007) The vegan package. Community Ecol Package 10(631–637):719
Olesen JM, Bascompte J, Dupont YL, Jordano P (2007) The modularity of pollination networks. Proc Natl Acad Sci USA 104(50):19891–19896
Olsson RL, Brousil MR, Clark RE, Baine Q, Crowder DW (2021) Interactions between plants and pollinators across urban and rural farming landscapes. Food Webs 27:e00194
Orford KA, Vaughan IP, Memmott J (2015) The forgotten flies: the importance of non-syrphid Diptera as pollinators. Proc Royal Soc b: Biol Sci 282(1805):20142934
Panziera D, Requier F, Chantawannakul P, Pirk CW, Blacquière T (2022) The Diversity Decline in Wild and Managed Honey Bee Populations Urges for an Integrated Conservation Approach. Front Ecol Evol 10:767950
Persson AS, Ekroos J, Olsson P, Smith HG (2020) Wild bees and hoverflies respond differently to urbanisation, human population density and urban form. Landsc Urban Plan 204:103901
Potts SG, Vulliamy B, Dafni A, Ne’eman G, Willmer P (2003) Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84(10):2628–2642
Prendergast KS, Dixon KW, Bateman PW (2022) A global review of determinants of native bee assemblages in urbanised landscapes. Insect Conserv Divers 15:385–405
Quistberg RD, Bichier P, Philpott SM (2016) Landscape and local correlates of bee abundance and species richness in urban gardens. Environ Entomol 45(3):592–601
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rader R, Edwards W, Westcott DA, Cunningham SA, Howlett BG (2011) Pollen transport differs among bees and flies in a human-modified landscape. Divers Distrib 17(3):519–529
Rader R, Cunningham SA, Howlett BG, Inouye DW (2020) Non-bee insects as visitors and pollinators of crops: Biology, ecology, and management. Annu Rev Entomol 65:391–407
Rader R, Howlett BG, Cunningham SA, Westcott DA, Newstrom‐Lloyd LE, Walker MK, ... Edwards W (2009) Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. J Appl Ecol 46(5):1080–1087
Rader R., Bartomeus I, Garibaldi LA, Garratt MP, Howlett BG, Winfree R, ... Woyciechowski M (2016) Non-bee insects are important contributors to global crop pollination. Proc National Acad Sci 113(1):146–151
Redlands, C. E. S. R. I. (2011). ArcGIS Desktop: Release 10.
Rivkin LR, Nhan VJ, Weis AE, Johnson MT (2020) Variation in pollinator-mediated plant reproduction across an urbanization gradient. Oecologia 192(4):1073–1083
Roy HE, Baxter E, Saunders A, Pocock MJ (2016) Focal plant observations as a standardised method for pollinator monitoring: Opportunities and limitations for mass participation citizen science. PLoS One 11(3):e0150794
Samra S, Samocha Y, Eisikowitch D, Vaknin Y (2014) Can ants equal honeybees as effective pollinators of the energy crop Jatropha curcas L. under Mediterranean conditions? GCB Bioenergy 6(6):756–767
Sánchez-Bayo F, Wyckhuys KA (2019) Worldwide decline of the entomofauna: A review of its drivers. Biol Cons 232:8–27
Schoener TW (1976) The species–area relationship within archipelagoes: models and evidence from island birds. Proc XVI Int Ornithol Congr 6:629–642
Seitz B, Buchholz S, Kowarik I, Herrmann J, Neuerburg L, Wendler J, ... Egerer M (2022) Land sharing between cultivated and wild plants: urban gardens as hotspots for plant diversity in cities. Urban Ecosyst 25(3):927–939
Smith MR, Singh GM, Mozaffarian D, Myers SS (2015) Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis. The Lancet 386(10007):1964–1972
Staab M, Pereira-Peixoto MH, Klein AM (2020) Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 194(3):465–480
Tasker P, Reid C, Young AD, Threlfall CG, Latty T (2020) If you plant it, they will come: quantifying attractiveness of exotic plants for winter-active flower visitors in community gardens. Urban Ecosyst 23(2):345–354
Theodorou P, Albig K, Radzevičiūtė R, Settele J, Schweiger O, Murray TE, Paxton RJ (2017) The structure of flower visitor networks in relation to pollination across an agricultural to urban gradient. Funct Ecol 31(4):838–847
Theodorou P, Baltz LM, Paxton RJ, Soro A (2021) Urbanization is associated with shifts in bumblebee body size, with cascading effects on pollination. Evol Appl 14(1):53–68
Theodorou P, Herbst SC, Kahnt B, Landaverde-González P, Baltz LM, Osterman J, Paxton RJ (2020) Urban fragmentation leads to lower floral diversity, with knock-on impacts on bee biodiversity. Sci Rep 10(1):1–11
Theodorou P, Radzevičiūtė R, Lentendu G, Kahnt B, Husemann M, Bleidorn C, ... Paxton RJ (2020a) Urban areas as hotspots for bees and pollination but not a panacea for all insects. Nat Commun 11(1):1–13
Thornton A (2008) Beyond the metropolis: Small town case studies of urban and peri-urban agriculture in South Africa. In Urban Forum, Springer, Netherlands 19(3):243–262
Tscharntke T (2021) Disrupting plant-pollinator systems endangers food security. One Earth 4(9):1217–1219
Turrini T, Knop E (2015) A landscape ecology approach identifies important drivers of urban biodiversity. Glob Change Biol 21(4):1652–1667
Udy KL, Reininghaus H, Scherber C, Tscharntke T (2020) Plant–pollinator interactions along an urbanization gradient from cities and villages to farmland landscapes. Ecosphere 11(2):e03020
Verboven HA, Aertsen W, Brys R, Hermy M (2014) Pollination and seed set of an obligatory outcrossing plant in an urban–peri-urban gradient. Perspect Plant Ecol, Evol Syst 16(3):121–131
Verboven HA, Uyttenbroeck R, Brys R, Hermy M (2014) Different responses of bees and hoverflies to land use in an urban–rural gradient show the importance of the nature of the rural land use. Landsc Urban Plan 126:31–41
von der Lippe M, Buchholz S, Hiller A, Seitz B, Kowarik I (2020) CityScapeLab Berlin: A research platform for untangling urbanization effects on biodiversity. Sustainability 12(6):2565
Wenzel A, Grass I, Belavadi VV, Tscharntke T (2020) How urbanization is driving pollinator diversity and pollination–A systematic review. Biol Cons 241:108321
Williams NM, Crone EE, T’ai HR, Minckley RL, Packer L, Potts SG (2010) Ecological and life-history traits predict bee species responses to environmental disturbances. Biol Cons 143(10):2280–2291
Winfree R, Kremen C (2009) Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc Royal Soc b: Biol Sci 276(1655):229–237
Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA (2009) A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90(8):2068–2076
Wojcik VA, McBride JR (2012) Common factors influence bee foraging in urban and wildland landscapes. Urban Ecosyst 15(3):581–598
Wood TJ, Michez D, Paxton RJ, Drossart M, Neumann P, Gerard M, ... Vereecken NJ (2020) Managed honey bees as a radar for wild bee decline? Apidologie 51(6):1100–1116
Woodard SH, Jha S (2017) Wild bee nutritional ecology: predicting pollinator population dynamics, movement, and services from floral resources. Curr Opin Insect Sci 21:83–90
Yamamura K (1999) Relation between plant density and arthropod density in cabbage fields. Popul Ecol 41:177–182
Acknowledgements
We thank the following people for their help with data collection: Felix Conitz, Susan Karlebowski, Christian Lanfranchi, Michael Miesl, Astrid Neumann, Hien Nguyen, Nina Schäle, Veronica Sebald, Dominik Sperling, Isabel Sperling, Ulrike Sturm. We are grateful to all community gardens and their gardeners for granting us permission to carry out this research. We thank the Heidehof Stiftung (grant numbers #57358011, #57358022) and the Technical University of Munich for funding and supporting this research.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Heidehof Stiftung (Grant numbers #57358011 and #57358022).
Author information
Authors and Affiliations
Contributions
JMS and ME designed the study; JMS collected the data, analysed the data, prepared the figures and led the writing; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmack, J.M., Egerer, M. Floral richness and seasonality influences bee and non-bee flower interactions in urban community gardens. Urban Ecosyst 26, 1099–1112 (2023). https://doi.org/10.1007/s11252-023-01353-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-023-01353-9