Abstract
Understanding phenological responses of plants to changing temperatures is important because of multiple associated ecological consequences. Cities with their urban heat island can be used as laboratories to study phenological adaptation to climate change. However, previous phenology studies focused on trees and did not disentangle the role of micro-climate and urban structures.
We studied reproductive phenology of dry grassland species in response to micro-climate and urbanization in Berlin, Germany. Phenological stages were recorded weekly at the individual plant level for five native grassland species across 30 dry grassland sites along an urbanization and temperature gradient. We estimated 50% onset probabilities for flowering and seed maturation of populations, and analysed variation in onset dates using regression models.
Early flowering species significantly advanced flowering phenology with increasing mean air temperature but were little influenced by urbanization. By contrast, late-flowering species showed significant phenological responses to both air temperature and urbanization, possibly because micro-climate was most affected by urbanization in late summer. Surprisingly, not all grassland species showed an advanced phenology with increasing intensity of urbanization.
This contradicts observed patterns for urban trees, indicating that phenological shifts in urban areas cannot be generalized from the observation of one growth form or taxonomic group. Growth form appears as a possible determinant of phenological responses. Results suggest that the phenology of dry grassland species may directly respond to the urban heat island, albeit with variable direction and magnitude. This has implications for ecosystem services, shifted allergy seasons, changes of biogeochemical cycles and potential ecological mismatches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In an era of accelerating urbanization, understanding the environmental factors that control urban biodiversity and ecosystem functioning is crucial for biodiversity conservation and the continued provision of ecosystem services in cities (Knapp et al. 2021; Swan et al. 2021). Natural or near-natural grassland is an ecosystem type of conservation concern in cities globally (Fischer et al. 2013; van der Walt et al. 2015; Williams et al. 2005). Urbanization has been shown to modulate the taxonomical and functional composition (Buchholz et al. 2020; Gathof et al. 2022; Williams et al. 2006; Zeeman et al. 2017) and ecosystem functioning (Onandia et al. 2019; Schittko et al., 2022) of urban grasslands and their associated species. While these studies demonstrate a high sensitivity of grassland to changing urban environments, responses of grassland species to the urban climate are clearly understudied. This is a significant gap of knowledge given that the ‘Urban Heat Island’ (UHI) is a key feature of urban environments resulting from a high proportion of impervious surfaces and dense urban structures (Stewart & Oke, 2012, Deilami et al. 2018).
Previous studies showed that microclimatic variability related to urban structures directly affects plant phenology (Chmielewski and Rötzer 2002; Dallimer et al. 2016; Jochner and Menzel 2015; Zipper et al. 2016). Yet these studies have largely focused on tree species (Lu et al. 2006; Mimet et al. 2009; Roetzer et al. 2000) or tree-dominated ecosystems (Li et al. 2017; Liang et al. 2016, but see Cheptou et al. 2008; König et al. 2018; Lambrecht et al. 2016; Neil et al. 2010). In response to the UHI phenological changes such as advanced flowering phenology of urban trees (Lu et al. 2006; Mimet et al. 2009) and an advanced start and delayed end of growing season have been observed (Li et al. 2017).
Besides micro-climatic drivers, urbanization variables can have a direct role in influencing phenology, too (Li et al. 2020; Wohlfahrt et al. 2019) showed that flowering, fruiting and leaf development advance with greater urbanization represented by higher impervious cover, while leaf senescence is delayed. Further, higher human population densities (as a proxy of urbanization) coupled with higher annual precipitation caused delayed flowering offset and extended flowering duration of urban trees (Li et al. 2020).
The effect of urbanization on microclimate strongly varies with seasons, with a stronger effect of urban structures on urban temperatures in summer (Schatz and Kucharik 2014). Hence, it is likely that this relationship generates a knock-on effect on the phenological response of species to urbanization. For instance, phenological response has been shown to varies among species which exhibit key phenological stages at different times of the year (Li et al. 2020).
Phenology of herbaceous plants has been shown to be sensitive to changes in micro-climate (König et al. 2018). So far, phenological studies largely covered non-urban systems such as alpine grassland and prairies (Cornelius et al. 2011; Dunne et al. 2012; Bennie et al. 2018; Wilsey et al. 2018, but see Cheptou et al. 2008; König et al. 2018; Lambrecht et al. 2016; Neil et al. 2010 ), as well as Californian annual grasslands (Bart et al. 2017; Chiariello 1989; Yang and Leigh 2020). For instance, alpine meadow species advance their timing of flowering in response to experimental warming and snow-removal treatment in conjunction with warmer soil temperatures (Dunne et al. 2012). Such studies of grassland phenology at the species level are still lacking in urban habitats.
In order to elucidate on phenology response to climate and urbanization two approaches are commonly used: in-situ and remote sensing assessments. In-situ fieldwork studies assess phenological responses at a species and individual level and give information on distinct phenophases (Fotiou et al. 2011; Lambrecht et al. 2016; Lu et al. 2006; Mimet et al. 2009; Yakub and Tiffin 2017). On the other hand, satellite based phenology studies use remotely sensed vegetation indices on a coarse spatial resolution on the habitat/ecosystem level (Coseo and Larsen 2014; Dallimer et al. 2016; Liang et al. 2016; Melaas et al. 2016; Xiao et al. 2006; Zhang et al. 2004; Zipper et al. 2016) .
Urban phenological studies relying on urban-rural comparisons (Bernard-Verdier et al. 2022; Dallimer et al. 2016; Zhang et al. 2004) or spatial gradients from city center to rural areas (Li et al. 2020; Ohashi et al. 2012) found that flowering phenology advances in urban compared to rural areas, as well as towards the urban centres. Studies using spatial gradients can be particularly suitable to model climate change effects on ecosystems via space-for-time substitution, allowing conclusion on responses to future climate change (Jochner and Menzel 2015; Lahr et al. 2018; Zhang et al. 2004) show that vegetation phenology in both urban core areas and surrounding regions is significantly influenced by urban heat island regimes. For temperature and vegetation phenology, the ecological footprint of urban land cover extends about 10 km beyond the perimeter of urban areas. In their study, the footprint of urban climates on vegetation phenology was 2.4 times the size of the actual urban land cover (Zhang et al. 2004). Similarly, tree remote sensing studies analyzing influence of urban structure on phenology, show consistent trends of phenological advances in urban areas at the ecosystem level (Lambrecht et al. 2016), and differences in phenological response among tree species and growth forms (Li et al. 2020).
Although grassland is an important component of urban vegetation with multiple social and ecological functions (Ignatieva et al. 2020; Onandia et al. 2019; Southon et al. 2017), phenological responses of grassland species to the urban climate are critically understudied. A recent study based on trait data found that the allergy season, corresponding to the flowering season, of urban grassland species in Berlin ended later with higher degree of urbanization (Bernard-Verdier et al. 2022).
While there is some urban phenological research on the herb growth forms, such as a for the annual herb Crepis sancta (Cheptou et al. 2008; Lambrecht et al. 2016) or herbarium record studies on ephemeral plants (Neil et al. 2010), so far studies of phenology of multiple grassland species in response to urbanization and urban micro-climate are scarce.
Urban grasslands are suitable model habitats for urban phenological studies since they are a frequent ecosystem type in cites globally, with self-assembled and often diverse plant communities present along a broad urbanization gradient (Fischer et al. 2016; van der Walt et al. 2015; Williams et al. 2005). With many diverse grassland under pressure from land-use changes in rural areas (Poschlod et al. 2005), cities can contribute to grassland conservation and restoration to ensure their ecological functions (Klaus 2013). However, phenological changes of grassland species may affect ecological functions of grassland (Jochner and Menzel 2015). An enhanced understanding of urban grassland phenology is important to predict and evaluate vegetation feedback on micro-climate (Penuelas et al. 2010), to understand the timing of ecosystem services to urban inhabitants (reviewed in Jochner and Menzel 2015) and potential ecological mismatches (especially with birds and pollinators) (Primack et al. 2009), as well as effects on human health in relation to allergies (Ziska et al. 2003). However, the seasonally varying role of the urban heat island on microclimate and thus indirectly on the phenology of different grassland species has not been studied before.
This study thus aimed to disentangle the role of multiple environmental variables related either to the local micro-climate or the adjacent level of urbanization for the phenology of dry grassland species along an urbanization gradient. We used a network of 30 extensively managed dry permanent grasslands in the Berlin metropolitan area to monitor phenology of three herb and two grass species over an entire growing season. We assessed the influence of different urban structure and urban micro-climate variables on dry grassland phenology in order to answer the following questions:
-
1)
How do local air temperature variables like mean day and night temperatures, mean monthly temperatures relate to the flowering phenology and seed maturation of urban dry grassland species?
-
2)
What is the effect of urban structure variables such as impervious surface, floor area ratio and road density on dry grassland species phenology when controlling for micro-climatic variables?
We hypothesized that:
-
1)
Flowering and seed maturation phenology of urban dry grassland species advances in response to local air temperature variables.
-
2)
Urbanization (the effect of urban structures) results in advances in urban dry grassland phenology. We further hypothesize a stronger urbanization effect for summer species compared to spring species, due to a stronger urban heat island effect in summer.
Materials and methods
Study area and study system
The study was conducted in Berlin, Germany’s capital and largest city, which in 2020 had 3.8 million inhabitants within a total area of 892 km². Berlin has a temperate climate, laying within the Köppen−Geiger oceanic Cfb climate zone which is characterized by a seasonal environment with an unimodal distribution of the vegetation period from spring to autumn (Kottek et al. 2006). The yearly average air temperature between 1970 and 2000 was 9.2 °C and the average precipitation 589.2 mm/year in Berlin Dahlem (FU Berlin, n.d.) Local temperatures are modulated by the urban structure, leading to increasing mean temperature values with the impervious surface (i.e. buildings, roads) close to weather stations (Quanz et al. 2018).
About 5% of Berlin’s surface is covered by grassland of which 43% can be assigned to dry grassland or other grassland types with a legal protection status according to the Berlin Nature Conservation Act (Fischer et al. 2013). Accordingly, grassland in Berlin harbors a range of endangered plant species (Planchuelo et al. 2019). We chose dry grassland as study system because this vegetation type spans over a range of near-natural to strongly human-influenced sites throughout the city including parks, roadside greenery, airports, and vacant land. Dry grassland represents an anthropogenic vegetation type that is usually managed by mowing once or twice a year - without fertilization, irrigation or herbicide application and intensive trampling (von der Lippe et al. 2020). Due to the low level of management, dry grassland is generally of conservation concern in Berlin (Fischer et al. 2013). Further, dry grassland sites share a suite of common plant species that allow for testing for phenological changes along a double gradient of temperature and urbanization.
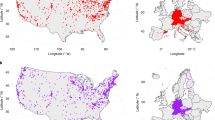
We performed phenological analyses in dry grassland sites that have been selected as a model ecosystem within the CityScapeLab Berlin, an experimental research platform established for the evaluation of biodiversity patterns and ecological processes in urban environments (von der Lippe et al. 2020). All plots belong to the same biotope type (biotope code 05120, i.e., dry grasslands; Senate Department for Urban Development and Housing, 2014) and the same vegetation type following the phytosociological classification (i.e., Sedo-Scleranthetea). We selected a subset of 30 dry grassland plots which contained the highest number of target species for phenological analysis, which evenly covered the area of Berlin city and were subject to different levels of urbanization in their surroundings, as indicated by percentage of impervious surface (Fig. 1). Average temperature during the study period (week 12–50 of the year) ranged between 12.7 and 14.32 C in the study sites, sky view factor between 0.64 and 0.99 and Sealing in a 500 m radius between 0.23 and 62.78 (Supplementary 5).
Our target species included two annual herbs (Cerastium semidecandrum, Trifolium arvense), one perennial herb (Potentilla argentea) and two perennial grasses (Agrostis capillaris, Festuca brevipila). While C. semidecandrum and F. brevipila were most frequent (28 and 26 sites, respectively), the other species occurred on at least 11 sites (Table 1). Despite the lower occurrence of the later phenology species in the monitoring network, the distribution pattern of P. argentea, A. capillaris and P. argentea evenly covered an urban-rural gradient (Fig. 1).
Location of study sites of all species (A) and each individual grassland species (B-F) in Berlin, with city boundaries in blue (basemap: OpenStreetMap, 2017)
Sampling of phenological phases
Phenological observations took place every week between March 14th and August 25th in 2017. We monitored six randomly chosen individuals per species in each plot where the species was present. Individuals were marked with colored plastic skewers and geo-located on a fine scale map. The BBCH-Code classification (Hack et al. 1992) was used for phenological monitoring and slightly adapted to the different growth forms (Supplementary 1). The BBCH-Code assigns numbered codes to morphological stages of plant development and we used the key stages 5 (heading, closed bud), 6 (flowering, open flower), 7 (fruit development, closed seed capsule) and 8 (seed maturation/ripening, open seed capsule) to describe phenological stages (Supplementary 1). All BBCH-stages present on any given individual were recorded each week. We chose this sampling interval because a more frequent sampling did not strongly affect the estimates of onset dates in another study (Cornelius et al. 2011).
We used onset of flowering, onset of seed maturation and length of flowering as phenological response variables, since they mark important stages in the plant life cycle, with flowering as the beginning of pollination and seed maturation as a prerequisite for dispersal.
To obtain population wide aggregates of onset dates for flowering and seed maturation of each species, the BBCH-stages for individual plants were converted to ordinal codes so that an occurred event (e.g. flowering and seed maturation) was assigned a 1 and the lack of occurrence a 0. Then, to calculate the population-response variable for a phenological stage and retrieve a date as phenological response variable, the day of 50% probability of flowering onset and seed maturation onset for each site was calculated for each species according to Cornelius et al. (2011) who suggested modelling progression curves of plant development with Ordinal Logistic Regression Models.
A generalized linear model with a binomial distribution was fitted with the ordinal phenology variable (0 or 1) as response and the date as predictor (Supplementary 2). The date for each the probability of the phenological event (in our case 50% flowering or seed maturation) was calculated as following:
Equation 1: Calculation of statistical probabilities for flowering/ seed maturation.
where P is the probability of the event and Y is the ordinal response variable (0 and 1 for phenological events), α is the intercept parameter, bi the slope parameters and Xi the explanatory variable, in this case day of the year. This way we calculated days of the year (DOY) for 50% flowering and 50% seed maturation probability for each species in each population according to Cornelius et al. (2011) (Supplementary 2).
Lastly, the length of flowering period was determined as the difference between modeled 50% probabilities of seed maturation and flowering onset:
Equation 2: Calculation of length of flowering period.
Sampling of micro-climate and urbanization variables
On each site, air temperature and relative humidity were recorded at 2 m height by micro-climate loggers (EasyLog EL-USB-2+, Lascar Electronics) with a case that protected them from rain and direct sunlight (Protective Cover for Outdoor Transmitter, TFA Dostmann). Microclimatic measurements were taken with a resolution of 10 min.
Environmental variables related to urbanization included percentage of impervious surface (e.g. buildings, roads), floor area ratio (i.e. ratio of a building’s floor area in relation to the size of the parcel its located on) and road density in a 500 m radius around sites and sky view factor at the site (Table 2) and were obtained from the CityScapeLab Berlin (see von der Lippe et al. 2020, for further details).
Climate data was measured in 10 min intervals and aggregated to weekly and monthly resolution of day and night average, overall average, minima, maxima, and sums of air temperatures. For climate data gaps due to vandalism of loggers or technical problems at four plots, temporal interpolation of missing data was executed for each climate variable via linear regression analysis. To that end, a linear model was created that predicted each missing climate variable of the site based on the linear relation between the on-site measurements and the equivalent climate variable at the long-term weather station at Tempelhofer Feld of the German Weather Service (DWD, n.d.) during a two-week period prior to the data gap. The linear equation was then applied to the period of data gaps to interpolate the missing period via linear regression.
As climate predictor variables for phenology, we extracted the air temperature values of different climate variables per month/week prior to the median of 50% flowering/seed maturation (DOY) of each species across the study sites. Hence, different time periods were used to characterize the microclimate for each of our five study species, ranging from March climate values for C. semidecandrum to July climate values for P. argentea.
Statistical analysis
We quantified changes in species phenology in response to both urbanization and micro-climate using linear regression models for each of the five species.
To test how phenology of a given species relates to micro-climate, we ran a suite of linear regression models for effects of various monthly and weekly micro-climate variables onto phenology (Supplementary 3). We chose weekly average daytime temperature (Tavg Day) and average nighttime temperatures (Tavg Night) as representative micro-climate variables for further analysis (Table 2).
To elucidate on temporal patterns of the effect of urban structure and micro-climate throughout the different months of the observation period, we used single linear models. We used Seal500 as a representative response variable and average monthly minimum (T Mon avg Min) temperatures as representative predictor variable, since the effect of the UHI in summer is mostly pronounced at night, and hence influences mainly the temperature minima (Heaviside et al. 2016; Lopes et al. 2013; van Hove et al. 2015).
To test how phenology of a given species responds to urbanization, we used single regression models with Seal500 as a predictor and modelled its effect on phenological variables. We further used backward selected multiple linear regression models with multiple urban structure variables (impervious surface, floor area ratio, road density and sky view factor) and micro-climate (mean day and night temperatures) as predictors. We chose urban structure variables in a 500 m radius, as it has been previously observed that local climate variability is best explained by impervious surface in a radius of 500 m around study areas (Schatz and Kucharik 2014). For each species and each phenological variable, a full linear model was fitted including all pre-selected urban structure and micro-climate predictors: Seal500, FAR500, RdDen500 and SVF, Tavg Night and Tavg Day (Table 2). From this full model backward selection was performed to choose the best urban predictor combination for flowering and seed maturation of each grassland species based on Akaike’s Information Criterion (AIC).
Linear models were deemed appropriate for all parts of the analysis as all phenological and climate response variables appeared normally distributed according to a Shapiro Wilk test. All statistical analyses were carried out in R (version 3.6.0).
Results
Micro-climate, flowering phenology and seed maturation
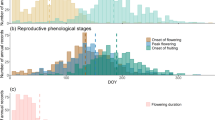
The five monitored species had different staggered flowering and seed maturation phenology (Fig. 2). The earliest flowering species was C. semidecandrum, followed by F. brevipila, P. argentea, A. capillaris and T. arvense. While the early-flowering species C. semidecandrum and F. brevipila showed low variation in the onset of flowering and seed maturation, the late-flowering species P. argentea and A. capillaris showed an amplitude of 40–60 days for all phenological events across the monitored sites. Amplitude of flowering length was largest for A. capillaris (52.1 days) followed by P. argentea (39.8 days), C. semidecandrum (13 days), T. arvense (10.5 days) and F. brevipila (9.7 days).
Phenological stages in five dry grassland species in the vegetation period in 2017 (see Table 1 for abbreviations of species names) during days of the year (DOY), species ordered by the median of 50% flowering onset. (A) for flowering onset, (B) for seed maturation onset, (C) for flowering length. Cer = Cerastium semidecandrum, Fes = Festuca brevipila, Pot = Potentilla argentea, Agr = Agrostis capillaris, Tri = Trifolium arvense
The response of 50% flowering and seed maturation onset to preceding weekly temperatures differed greatly among species in both significance, magnitude and direction (Table 3, and see Supplementary 4). Flowering of the early species C. semidecandrum was significantly advanced by 0.85 days/°C in response to night-time temperatures (Table 3). In contrast, flowering of F. brevipila was delayed by 1.9 days/°C in response to day-time temperatures. The three later flowering species P. argentea, A. capillaris and T. arvense were not significantly affected by either Tavg Night or Tavg Day. Overall, the two early flowering species (C. semidecandrum and F. brevipila) responded stronger to micro-climate of the preceding week than the later flowering species.
For seed maturation, no significant influence of micro-climate was found across species. P. argentea showed a trend of advanced seed maturation by 7.7 days/°C in response to Tavg Day and (p = 0.052) and A. capillaris a trend of delay by 5.4 days /°C in response to T avg Night (p = 0.069).
Urbanization and grassland phenology
Urbanization, represented here by the percentage of sealed surfaces in 500 m buffer around grasslands (Seal500), was associated with an increase in mean minimum temperatures in spring and summer (Fig. 3). This relationship became stronger as the season progressed, from a non-significant trend in March (p = 0.098) to an increasingly strong positive linear relationship towards July (p < 0.001) (Fig. 3). With progressing growing season, the variance in temperature explained by urbanization (adjusted R2) increased steadily from 6% in March to 66% in July, showing a tighter relationship between sealed surface and monthly averaged minima in summer.
Single regression models of effect of urbanization on phenology showed that flowering and seed maturation of A. capillaris, as well as seed maturation of P. argentea, were delayed with increased Seal500 (Supplementary 4). By contrast, F. brevipila flowered earlier and longer as sealing increased. All other species showed no significant or a slightly negative response, i.e. earlier onset dates. Overall, Seal500 explained a larger share of seed maturation phenology for the later species P. argentea, A. capillaris and T. arvense, than for C. semidecandrum and F. brevipila (Fig. 4b).
Multiple regression models obtained through backwards selection showed a similar trend to the single regression models, as urban structure and the micro-climate variables showed the high explanatory power for the later species (Fig. 4). Large explanatory power was found for the flowering stage, especially for A. capillaris and T. arvense (Fig. 4; Table 4) where a combination of urban micro-climate and urban structure significantly explained 80% and 70% of variation (adj. R2). Flowering of C. semidecandrum and F. brevipila was significantly influenced by the micro-climate variables TAVGday and by RdDen, albeit in opposite directions and the multiple models significantly explained 20.1% and 43.2% of variation, respectively.
Explanatory power (adjusted R2) of multiple regression models (see Table 3) for the joint effect of micro-climate and urban structure on flowering, seed maturation and length of flowering of the five species. Species ordered by the median of 50% flowering onset
In the seed maturation models, a combination of TAVG day and Seal significantly explained 61.9% of P. argentea seed maturation, while FAR explained 44.1% for A. capillaris. Seed maturation of all other species remained entirely unaffected by all urban structure and micro-climate variables.
Length of flowering models showed a similar pattern with high R2 for the later species P. argentea, A capillaris and T. arvense and an inclusion of both urban structure and micro-climate variables in the selected models.
By contrast, F. brevipila flowered earlier and longer as sealing increased. All other species showed no significant or a slightly negative response, i.e. earlier onset dates. Overall, Seal500 explained a larger share of seed maturation phenology for the later species P. argentea, A. capillaris and T. arvense, than for C. semidecandrum and F. brevipila (Fig. 4b).
Discussion
Cities can harbour biodiverse grasslands, and the phenology of such urban ecosystems is particularly important for a range of ecological processes (Jochner and Menzel 2015). Yet grassland phenology at a single species level has not been assessed in an urban context so far. Here we show that dry grassland species phenology responds to urbanization in a species-specific manner. Along with an increasing effect of urban structures on micro-climate from spring to summer, phenology of later species tended to respond stronger to a combination of both micro-climate and urbanization, while phenology of early species responds predominantly to micro-climate.
Grassland phenology and urban micro-climate
In this study we observed clear differences among species in phenological response to micro-climate. Our hypothesis that overall phenology advances with increasing air temperature of the previous week proved to be only valid for the early spring herb C. semidecandrum, while other species, especially the later ones, such as P. argentea and A. capillaris showed no response to preceding air temperatures, and F. brevipila exhibited phenological delays. This is contrary to the multitude of tree phenological studies in urban areas, which have shown consistent advances in flowering phenology (Li et al. 2020; Lu et al. 2006; Mimet et al. 2009; Neil et al. 2010).
The early flowering species C. semidecandrum was significantly influenced by mean night and day-time temperatures, which is in line with findings on the high sensitivity of early phenology species to microclimatic cues and the winter UHI (Jochner and Menzel 2015). This is further strengthened by long-term phenological findings revealing that flowering of spring-species responded the strongest to temperature of preceding months (Fitter and Fitter 2002). Studies on urban tree phenology have found an influence of temperatures on a monthly scale on for instance flowering (Lu et al. 2006). In this study weekly micro-climate was more predictive for phenological onsets than monthly micro-climate. This could possibly be due to fast-growing plants like grasses being faster and/or more plastic in timing their internal physiological and chemical dynamics (König et al. 2018).
Many studies find that phenological response varies due to functional traits and growth forms of the plant species (Fitter and Fitter 2002; Iversen et al. 2009; König et al. 2018; Li et al. 2020; Lu et al. 2006). A. capillaris and P. argentea (both perennial) responded positively to the urban environment (delayed onset), while C. semidecandrum (annual) and T. arvense (annual or biennial) responded negatively (advanced onset). Due to our low number of repetitions per growth form, we cannot generalize on a growth-form specific effect of phenology response to the UHI, however we observed a trend that is consistent with previous literature: König et al. (2018) found that early flowering grasses and annual herbs exhibited more intense advances in flowering onset than late-flowering grasses and perennial herbs – as in our study. While in König et al. (2018) climate zone related to advance in onset of herbs, for grasses micro-climate site conditions had a negligible effect and plant specific traits explained phenological responses, such that high specific leaf area was related to advances in onsets, while high leaf dry matter content was related to phenological delays (König et al. 2018).
Contrary to our hypothesis on advanced urban grassland phenology, we found a difference in direction of response among species, such as unexpected delays in flowering or seed maturation. Delays in flowering dates are not uncommon among different growth forms (König et al. 2018; Munson and Long 2017). Flowering time of C3 grass species (perennial and annual) advanced with increasing mean annual air temperature, while flowering time of all but two C4 grass species (perennial and annual) was either delayed or unaffected (Munson and Long 2017). This was explained by higher optimal air temperatures for development of late-growing species resulting in heterogeneous patterns of phenological shifts, and shows a dependency of phenological response on life history and photosynthetic pathway. Moreover, flowering delays due to increased temperature have previously been explained by an eco-region effect, with grass species that grow in relatively cold eco-regions (high elevation or northern latitude) advancing phenology with warming, while those in relatively warm eco-regions (low elevation or southern latitude) delaying phenology with warming (Munson and Long 2017). At the much smaller local scale of our study, we found large variation of micro-climatic zones along the urban-rural gradient. This could be analogous the “ecoregion-effect” and explain the delay of A. capillaris flowering due to higher air temperatures at the strongly urban and hence warmer sites.
Lambrecht et al. (2016) provide another explanation for delayed phenological responses: their study on urban and rural plants of the Mediterranean species Crepis sancta suggests that selection favors later phenology when air temperatures, moisture regimes and nitrogen-availability are strongly modified, and CO2 is elevated. Especially moisture regimes might be a potential critical factor causing the phenological delays of grasses in our study, as observed in Californian annual grasslands where soil water potential thresholds were a reliable predictor for grassland green up dates (Bart et al. 2017). Without connection to groundwater and limited root depths particularly in annual species, dry grassland species must rely on soil moisture. We thus hypothesize that grasses with late summer flowering phenology which coincide with pronounced drought periods might delay their onset dates until after the maximum drought peak to relocate reproductive phenology to periods when site conditions, especially moisture, are favorable. This hypothesis is strengthened by the opposite response of early flowering herbs, which grow in favorable moisture conditions in spring with almost no water restrictions and therefore match the usual pattern of phenological advance in response to temperature.
Grassland phenology and urbanization
Our findings show an increase in explanatory power of urbanization in predicting micro-climate from March to July. Cities have consistently shown to be hotter than adjacent rural areas, leading to the well-known Urban Heat Island (UHI) effect (Stewart, 2011). In Berlin, like in many mid-latitude climates, the UHI was found to be most pronounced in summer and during the night in response to surface cover (Fenner et al. 2014). This results in higher urban-rural difference for sites with a larger percentage of urban fabric during the summer months.
Accordingly, species exhibiting crucial phenological phases in summer such as A. capillaris and T. arvense responded strongly to a combination of micro-climatic cues and urban structure variables, while early species such as C. semidecandrum mostly responded to micro-climate. This trend is in line with the temporal pattern of the UHI and the exacerbated link between urban structure and micro-climate in summer, as shown in our results. Similarly, Li et al. (2020) found that flowering onset of spring species in urban environments was advanced and more sensitive to increased temperatures than summer species. Further studies are needed to test this hypothesis, since we had a lower sample size of the later phenology species due to lower occurrence in our monitoring network compared to the earlier flowering species and hence reducing the statistical power of the relationships. Future research could extend the urban-rural gradient beyond the boundaries of Berlin, or use a similar research design in cities with a similarly high proportion of grassland habitat.
We found varying effects of urbanization on the flowering and seed maturation phenology of the five species, both in terms of direction and magnitude (Table 3). For instance, F. brevipila flowering was explained by road density and temperature averages, while a combination of the urban structure variables road density, floor area ratio and degree of soil sealing in combination with temperature averages explained a large share of the phenology of the later flowering species A. capillaris and T. arvense. While Loheide (2016) estimated that degree of soil sealing explained ~ 50–70% of remotely sensed start/end of the growing season, we also observed this urban structure variable to be a prominent predictor of grassland phenology, explaining up to 35–38% of seed maturation and flowering timing for A. capillaris. Loheide (2016) also found that the magnitude of urban-structure driven changes in phenology was determined by the prevailing weather conditions during seasonal transitions. This matches our findings; as under hot air temperatures in summer when urban structure influences micro-climate the most and creates local intra-urban weather patterns with associated changes in other climate variables, phenology shows the strongest reaction.
While our multiple regression models combining urban structure and micro-climate succeed in explaining up to 80% of phenological variability for some grassland species, for other species explanatory power was low, suggesting that a suite of other non-measured factors affect urban grassland phenology. In urban areas photoperiod, chilling time and nutrients, hydrological modifications (precipitation, air humidity), diseases, pests, competition, pollutants, individual genes and age have been found to impact plant phenology (reviewed in Jochner and Menzel 2015). For instance, warm or cold spells could influence phenological behavior at the local scale: High air temperatures shortly before the phenological onset of a specific event are likely to result in a simultaneous onset while low air temperatures rather contribute to a larger delay between urban and rural onset dates (Jochner et al. 2011). In general, extreme weather conditions might have greater effects on phenology than changes in mean air temperatures, by disturbing the synchronization between organisms due to less predictability (Jentsch et al., 2007). Moreover, flowering onset and flowering time has been shown to be extended by 8–15% on south aspects compared to north aspects in a Californian annual grassland (Yang and Leigh 2020).
Along the same lines, precipitation changes could play an important role for phenology and even drive flowering onset stronger than increasing air temperatures (König et al. 2018). Despite lack of measurement in this study, precipitation could represent a limiting factor for water supply and could be especially crucial in dry grasslands in the summer months. The effect of water availability and drought on phenology in the summer months as a consequence of the UHI could be a driver causing controversial shifts in phenological onset dates of grasses and would need to be included in future urban phenological studies. This is especially crucial because of trends of increased drought intensity in Germany (NASA, 2020) and particularly in cities (Lahr et al. 2018).
Additionally, plant diversity could be a determinant for phenology as it has been shown to affect coordination of phenology among species with earlier flowering in response to reduced diversity in a serpentine grassland (Wolf et al. 2017). These differences in phenology were caused by the effects of plant diversity on soil surface temperature, available soil nitrogen, and soil moisture which in turn affected timing of flowering (Wolf et al. 2017). While in our dry grassland sites network no differences in plant species richness along the urbanization gradient were found (Speaman’s R2=-0.0258; P = 0.85), species richness increased slightly with maximum daily summer temperatures (R2 = 0.30, P = 0.02) (data not shown), making plant species richness an interesting driver to include in future urban phenology studies.
While there is a large body of literature on the phenological triggers of Mediterranean grasslands, such as in California (Bart et al. 2017; Chiariello 1989; Hufstader 1978; Yang and Leigh 2020), comparatively little is known on the multitude of potential triggering factors for flowering and seed dispersal in urban habitats. Urban grassland phenology studies including precipitation, soil temperatures and plant traits, as well as with more species from each growth form, growing at different times of the spring and summer and additional phenophases would be useful to better understand the triggers of urban grassland phenology responses to the urban heat island. More research is needed on the predictors of flowering and seed dispersal in urban habitats, as these phenophases are likely to be regulated by different environmental and physiological triggers (Segrestin et al. 2018). Dispersal timing has been shown to be more variable and less responsive to climate constraints such as water deficits, than flowering. Moreover timing of phenological events can be influenced by a coordination between phenophases, like among timing of seed dispersal, seed maturation and flowering timing (Segrestin et al. 2018). Such a phenophase-coordination remains to be tested in an urban grassland context.
Finally, since the urban-rural gradient resembles an air temperature gradient, urban environments can be used as laboratories to study the future impacts of climate change (Ziska et al. 2003), for instance through space-for-time substitution (Lahr et al. 2018). The advantage of this in a phenology context is that such intra-urban studies eliminate the confounding effects of varying day length and reducing sample effort and costs compared to phenological studies over latitudinal gradients (Jochner and Menzel 2015). This has been done successfully using community science photographs of flowering across US cities and with space-for-time substitution approach to show climate-change induced increases in flowering length (Li et al. 2020). It remains to be tested for the grassland species we assessed whether phenological response to micro-climate change equals climate variability over space, and whether a space for time substitution could reliably elucidate on the future and consequences of dry grasslands in a warmer world.
Conclusion
Cities can be used as laboratories for plant adaptation to climate change because of the urban heat island established here. This study, probably the first on the phenology of grassland species in field populations along a double gradient of temperature and urbanization, showed that individual species respond significantly - but differently - to increased temperatures in cities. This points to the importance of different life forms or seasonal development of plant species for their response to the urban heat island. Future studies should illuminate the underlying physiological mechanisms and ecological consequences, especially for biotic interactions.
Grasslands are particularly useful for urban phenological studies due to their spontaneous occurrence along the whole urban-rural gradient, covering a huge variety of micro-climates, stress-levels and environmental conditions. The tendency of early flowering species responding mainly to air temperature of the precedent weeks, while late species seem to be influenced by both micro-climate and urban structure variables, points towards a pattern of dry grassland response the urban heat island effect, which is most pronounced in summer. It remains to be tested whether the mechanism behind this response is due to species plasticity or due to evolutionary adaptations of the grassland species and to what extend growth form and functional traits mediate phenological response.
Future research on urban grassland phenology in response to urban micro-climate could be useful, because of the high sensitivity of grasslands to urban environmental conditions. A possible research focus could be on quantifying the magnitude and direction of shifts of more representative species to create a catalogue of species phenological response. Along with that, predictors like drought and soil moisture conditions, which are crucial for grassland species and might be strong phenological triggers, could be included in future grassland phenology studies to untangle the effects of different climatological and meteorological drivers.
The potential of dry grassland species in describing urban warming patterns, provides promising avenues for urban environmental research and towards an increased understanding of the impacts of on urban ecosystems.
Data Availability
Data will be uploaded to Mendeley data upon acceptance.
Code Availability
All R codes will be uploaded to Mendeley data upon acceptance.
References
Bart RR, Tague CL, Dennison PE (2017) Modeling annual grassland phenology along the central coast of California. Ecosphere 8(7). https://doi.org/10.1002/ecs2.1875
Bennie J, Davies TW, Cruse D, Bell F, Gaston KJ (2018) Artificial light at night alters grassland vegetation species composition and phenology. J Appl Ecol 55(1):442–450. https://doi.org/10.1111/1365-2664.12927
Bernard-Verdier M, Seitz B, Buchholz S, Kowarik I, Lasunción Mejía S, Jeschke JM (2022) Grassland allergenicity increases with urbanisation and plant invasions. Ambio 2261–2277. https://doi.org/10.1007/s13280-022-01741-z
Buchholz S, Gathof AK, Grossmann AJ, Kowarik I, Fischer LK (2020) Wild bees in urban grasslands: Urbanisation, functional diversity and species traits. Landscape and Urban Planning, 196(October 2019), 103731. https://doi.org/10.1016/j.landurbplan.2019.103731
Cheptou P-O, Carrue O, Rouifed S, Cantarel A (2008) Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proceedings of the National Academy of Sciences, 105(10), 3796–3799. https://doi.org/10.1073/pnas.0708446105
Chiariello NR (1989) PHENOLOGY OF CALIFORNIA GRASSLANDS. 47–48
Chmielewski FM, Rötzer T (2002) Annual and spatial variability of the beginning of growing season in Europe in relation to air temperature changes. Climate Res 19(3):257–264. https://doi.org/10.3354/cr019257
Cornelius C, Petermeier H, Estrella N, Menzel A (2011) A comparison of methods to estimate seasonal phenological development from BBCH scale recording. Int J Biometeorol 55(6):867–877. https://doi.org/10.1007/s00484-011-0421-x
Coseo P, Larsen L (2014) How factors of land use/land cover, building configuration, and adjacent heat sources and sinks explain Urban Heat Islands in Chicago. Landsc Urban Plann 125:117–129. https://doi.org/10.1016/j.landurbplan.2014.02.019
Dallimer M, Tang Z, Gaston KJ, Davies ZG (2016) The extent of shifts in vegetation phenology between rural and urban areas within a human-dominated region. Ecol Evol 6(7):1942–1953. https://doi.org/10.1002/ece3.1990
Deilami K, Kamruzzaman M, Liu Y (2018) Urban heat island effect: A systematic review of spatio-temporal factors, data, methods, and mitigation measures. International Journal of Applied Earth Observation and Geoinformation, 67(September 2017), 30–42. https://doi.org/10.1016/j.jag.2017.12.009
Dunne JA, Harte J, Taylor KJ, Monographs SE, Feb N (2012) Subalpine Meadow Flowering Phenology Responses to Climate Change: Integrating Experimental and Gradient Methods. Ecol Soc Am 73(1):69–86. https://doi.org/10.1890/0012-9615(2003)073
DWD. (n.d.). Deutscher Wetter Dienst. Climate Data Center. Retrieved February 20 (2018) from https://www.ashrae.org/resources--publications/bookstore/climate-data-center
Fenner D, Meier F, Scherer D, Polze A (2014) Spatial and temporal air temperature variability in Berlin, Germany, during the years 2001–2010. Urban Clim 10(P2):308–331. https://doi.org/10.1016/j.uclim.2014.02.004
Fischer LK, Rodorff V, von der Lippe M, Kowarik I (2016) Drivers of biodiversity patterns in parks of a growing South American megacity. Urban Ecosyst 19(3):1231–1249. https://doi.org/10.1007/s11252-016-0537-1
Fischer LK, von der Lippe M, Kowarik I (2013) Urban land use types contribute to grassland conservation: The example of Berlin. Urban Forestry and Urban Greening 12(3):263–272. https://doi.org/10.1016/j.ufug.2013.03.009
Fitter AH, Fitter RSR (2002) Rapid Changes in Flowering Time in British Plants. Science 296(5573):1689–1691. https://doi.org/10.1126/science.1071617
Fotiou C, Damialis A, Krigas N, Halley JM, Vokou D (2011) Parietaria judaica flowering phenology, pollen production, viability and atmospheric circulation, and expansive ability in the urban environment: Impacts of environmental factors. Int J Biometeorol 55(1):35–50. https://doi.org/10.1007/s00484-010-0307-3
FU Berlin. (n.d.). Meteorologische Mittel- und Extremwerte von Berlin-Dahlem. Institut für Meteorologie der Freien Universität Berlin. Retrieved June 14 (2018) from http://www.met.fu-berlin.de/~stefan/extrema.htm
Gathof AK, Grossmann AJ, Herrmann J, Buchholz S (2022) Who can pass the urban filter? A multi-taxon approach to disentangle pollinator trait–environmental relationships. Oecologia 199(1):165–179. https://doi.org/10.1007/s00442-022-05174-z
Hack et al,(1992) H. Die erweiterte BBCH-Skala
Heaviside C, Vardoulakis S, Cai XM (2016) Attribution of mortality to the urban heat island during heatwaves in the West Midlands, UK. Environ Health: Global Access Sci Source 15(Suppl 1). https://doi.org/10.1186/s12940-016-0100-9
Hufstader RW (1978) Growth Rates and Phenology of Some Southern California Grassland Species. J Range Manag 31(6):465. https://doi.org/10.2307/3897208
Ignatieva M, Haase D, Dushkova D, Haase A (2020) Lawns in cities: From a globalised urban green space phenomenon to sustainable nature-based solutions. Land 9(3). https://doi.org/10.3390/land9030073
Iversen M, Brathen KA, Yoccoz NG, Ims RA (2009) Predictors of plant phenology in a diverse high-latitude alpine landscape: Growth forms and topography. J Veg Sci 20(5):903–915. https://doi.org/10.1111/j.1654-1103.2009.01088.x
Jochner S, Beck I, Behrendt H, Traidl-Hoffmann C, Menzel A (2011) Effects of extreme spring temperatures on urban phenology and pollen production: a case study in Munich and Ingolstadt. 49:101–1122http://www.int-res.com/abstracts/cr/v49/n2/p101-112/
Jochner S, Menzel A (2015) Urban phenological studies - Past, present, future. Environ Pollut 203:250–261. https://doi.org/10.1016/j.envpol.2015.01.003
Klaus VH (2013) Urban grassland restoration: A neglected opportunity for biodiversity conservation. Restor Ecol 21(6):665–669. https://doi.org/10.1111/rec.12051
Knapp S, Aronson MFJ, Carpenter E, Herrera-Montes A, Jung K, Kotze DJ, La Sorte FA, Lepczyk CA, Macgregor-Fors I, Macivor JS, Moretti M, Nilon CH, Piana MR, Rega-Brodsky CC, Salisbury A, Threlfall CG, Trisos C, Williams NSG, Hahs AK (2021) A Research Agenda for Urban Biodiversity in the Global Extinction Crisis. Bioscience 71(3):268–279. https://doi.org/10.1093/biosci/biaa141
König P, Tautenhahn S, Cornelissen JHC, Kattge J, Bönisch G, Römermann C (2018) Advances in flowering phenology across the Northern Hemisphere are explained by functional traits. Glob Ecol Biogeogr 27(3):310–321. https://doi.org/10.1111/geb.12696
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F(2006) World Map of Köppen – Geiger Climate Classification Main climates. Meteorol
Lahr EC, Dunn RD, Frank SD(2018) Getting ahead of the curve: cities as surrogates for global change. Proceedings of the Royal Society B: Biological Sciences, 285(1882), 20180643. https://doi.org/10.1098/rspb.2018.0643
Lambrecht SC, Mahieu S, Cheptou PO (2016) Natural selection on plant physiological traits in an urban environment. Acta Oecol 77:67–74. https://doi.org/10.1016/j.actao.2016.09.002
Li D, Barve N, Brenskelle L, Earl K, Barve V, Belitz MW, Doby J, Hantak MM, Oswald JA, Stucky BJ, Walters M, Guralnick RP (2020) Climate, urbanization, and species traits interactively drive flowering duration. Glob Change Biol 1–12. https://doi.org/10.1111/gcb.15461
Li X, Zhou Y, Asrar GR, Mao J, Li X, Li W (2017) Response of vegetation phenology to urbanization in the conterminous United States. Glob Change Biol 23(7):2818–2830. https://doi.org/10.1111/gcb.13562
Liang S, Shi P, Li H (2016) Urban spring phenology in the middle temperate zone of China: dynamics and influence factors. Int J Biometeorol 60(4):531–544. https://doi.org/10.1007/s00484-015-1049-z
Lopes A, Alves E, Alcoforado MJ, Machete R(2013) Lisbon urban heat island updated: New highlights about the relationships between thermal patterns and wind regimes. Advances in Meteorology, 2013(December). https://doi.org/10.1155/2013/487695
Lu P, Yu Q, Liu J, Lee X (2006) Advance of tree-flowering dates in response to urban climate change. Agric For Meteorol 138(1–4):120–131. https://doi.org/10.1016/j.agrformet.2006.04.002
Melaas EK, Wang JA, Miller DL, Friedl MA(2016) Interactions between urban vegetation and surface urban heat islands: a case study in the Boston metropolitan region Interactions between urban vegetation and surface urban heat islands : a case study in the Boston metropolitan region
Mimet A, Pellissier V, Quénol H, Aguejdad R, Dubreuil V, Rozé F (2009) Urbanisation induces early flowering: Evidence from Platanus acerifolia and Prunus cerasus. Int J Biometeorol 53(3):287–298. https://doi.org/10.1007/s00484-009-0214-7
Munson SM, Long AL (2017) Climate drives shifts in grass reproductive phenology across the western USA. New Phytol 213(4):1945–1955. https://doi.org/10.1111/nph.14327
NASA (2020) Parched Conditions in Germany Again. https://earthobservatory.nasa.gov/images/146647/parched-conditions-in-germany-again
Neil KL, Landrum L, Wu J (2010) Effects of urbanization on flowering phenology in the metropolitan phoenix region of USA: Findings from herbarium records. J Arid Environ 74(4):440–444. https://doi.org/10.1016/j.jaridenv.2009.10.010
Ohashi Y, Kawakami H, Shigeta Y, Ikeda H, Yamamoto N (2012) The phenology of cherry blossom (Prunus yedoensis “Somei-yoshino”) and the geographic features contributing to its flowering. Int J Biometeorol 56(5):903–914. https://doi.org/10.1007/s00484-011-0496-4
Onandia G, Schittko C, Ryo M, Bernard-Verdier M, Heger T, Joshi J, Kowarik I, Gessler A (2019) Ecosystem functioning in urban grasslands: The role of biodiversity, plant invasions and urbanization. PLoS ONE (Vol 14(11). https://doi.org/10.1371/journal.pone.0225438
OpenStreetMap (2017) Researcher Information - OpenStreetMap Wiki. https://wiki.openstreetmap.org/wiki/Researcher_Information
Penuelas J, Rutishauser T, Filella I(2010) Phenology Feedbacks on Climate Change. Science, 324(May 2009), 887–888
Planchuelo G, von der Lippe M, Kowarik I (2019) Untangling the role of urban ecosystems as habitats for endangered plant species. Landsc Urban Plann 189:320–334. https://doi.org/10.1016/j.landurbplan.2019.05.007
Poschlod P, Bakker JP, Kahmen S (2005) Changing land use and its impact on biodiversity. Basic Appl Ecol 6(2):93–98. https://doi.org/10.1016/j.baae.2004.12.001
Primack RB, Higuchi H, Miller-Rushing AJ (2009) The impact of climate change on cherry trees and other species in Japan. Biol Conserv 142(9):1943–1949. https://doi.org/10.1016/j.biocon.2009.03.016
Quanz JA, Ulrich S, Fenner D, Holtmann A, Eimermacher J (2018) Micro-Scale Variability of Air Temperature within a Local Climate Zone in Berlin, Germany, during Summer. Climate 6(1):5. https://doi.org/10.3390/cli6010005
Roetzer T, Wittenzeller M, Haeckel H, Nekovar J (2000) Phenology in central Europe - Differences and trends of spring phenophases in urban and rural areas. Int J Biometeorol 44(2):60–66. https://doi.org/10.1007/s004840000062
Schatz J, Kucharik CJ (2014) Seasonality of the urban heat island effect in Madison, Wisconsin. J Appl Meteorol Climatology 53(10):2371–2386. https://doi.org/10.1175/JAMC-D-14-0107.1
Schittko, C. et al. Biodiversity maintains soil multifunctionality and soil organic carbon in novel urban ecosystems. J.Ecol. 110, 916–934 (2022).
Segrestin J, Bernard-Verdier M, Violle C, Richarte J, Navas ML, Garnier E (2018) When is the best time to flower and disperse? A comparative analysis of plant reproductive phenology in the Mediterranean. Funct Ecol 32(7):1770–1783. https://doi.org/10.1111/1365-2435.13098
Southon GE, Jorgensen A, Dunnett N, Hoyle H, Evans KL (2017) Biodiverse perennial meadows have aesthetic value and increase residents’ perceptions of site quality in urban green-space. Landsc Urban Plann 158:105–118. https://doi.org/10.1016/j.landurbplan.2016.08.003
Stewart, I. D. & Oke, T. R. Local climate zones for urban temperature studies. Bull. Am. Meteorol. Soc. 93, 1879–1900 (2012).
Swan CM, Brown B, Borowy D, Cavender-Bares J, Jeliazkov A, Knapp S, Lososová Z, Padullés Cubino J, Pavoine S, Ricotta C, Sol D (2021) A framework for understanding how biodiversity patterns unfold across multiple spatial scales in urban ecosystems. Ecosphere 12(7). https://doi.org/10.1002/ecs2.3650
van der Walt L, Cilliers SS, Kellner K, Du Toit MJ, Tongway D (2015) To what extent does urbanisation affect fragmented grassland functioning? J Environ Manage 151:517–530. https://doi.org/10.1016/j.jenvman.2014.11.034
van Hove LWA, Jacobs CMJ, Heusinkveld BG, Elbers JA, Van Driel BL, Holtslag AAM (2015) Temporal and spatial variability of urban heat island and thermal comfort within the Rotterdam agglomeration. Build Environ 83:91–103. https://doi.org/10.1016/j.buildenv.2014.08.029
von der Lippe M, Buchholz S, Hiller A, Seitz B, Kowarik I (2020) CityScapeLab Berlin: A research platform for untangling urbanization effects on biodiversity. Sustain (Switzerland) 12(6):1–30. https://doi.org/10.3390/su12062565
Williams NSG, McDonnell MJ, Seager EJ (2005) Factors influencing the loss of an endangered ecosystem in an urbanising landscape: A case study of native grasslands from Melbourne, Australia. Landsc Urban Plann 71(1):35–49. https://doi.org/10.1016/j.landurbplan.2004.01.006
Williams NSG, Morgan JW, McCarthy MA, McDonnell MJ (2006) Local extinction of grassland plants: The landscape matrix is more important than patch attributes. Ecology 87(12):3000–3006. https://doi.org/10.1890/0012-9658(2006)87[3000:LEOGPT]2.0.CO;2
Wilsey BJ, Martin LM, Kaul AD (2018) Phenology differences between native and novel exotic-dominated grasslands rival the effects of climate change. J Appl Ecol 55(2):863–873. https://doi.org/10.1111/1365-2664.12971
Wohlfahrt G, Tomelleri E, Hammerle A (2019) The urban imprint on plant phenology. Nat Ecol Evol 3(12):1668–1674. https://doi.org/10.1038/s41559-019-1017-9
Wolf A, Zavaleta E, Selmants P(2017) Flowering phenology shifts in response to biodiversity loss. Proceedings of the National Academy of Sciences, 114, 201608357. https://doi.org/10.1073/pnas.1608357114
Xiao J, Shen Y, Ge J, Tateishi R, Tang C, Liang Y, Huang Z (2006) Evaluating urban expansion and land use change in Shijiazhuang, China, by using GIS and remote sensing. Landsc Urban Plann 75(1–2):69–80. https://doi.org/10.1016/j.landurbplan.2004.12.005
Yakub M, Tiffin P (2017) Living in the city: urban environments shape the evolution of a native annual plant. Glob Change Biol 23(5):2082–2089. https://doi.org/10.1111/gcb.13528
Yang O, Leigh R(2020) Phenology dynamics in California grasslands: Abiotic and biotic influences on the duration of flowering. UC Berkeley Electronic Theses and Dissertations Title, 67. https://escholarship.org/uc/item/98384265
Zeeman BJ, McDonnell MJ, Kendal D, Morgan JW (2017) Biotic homogenization in an increasingly urbanized temperate grassland ecosystem. J Veg Sci 28(3):550–561. https://doi.org/10.1111/jvs.12507
Zhang X, Friedl MA, Schaaf CB, Strahler AH, Schneider A (2004) The footprint of urban climates on vegetation phenology. Geophys Res Lett 31(12):10–13. https://doi.org/10.1029/2004GL020137
Zipper SC, Schatz J, Singh A, Kucharik CJ, Townsend PA, Loheide SP (2016) Urban heat island impacts on plant phenology: intra-urban variability and response to land cover. Environ Res Lett 11(5):054023. https://doi.org/10.1088/1748-9326/11/5/054023
Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG (2003) Cities as harbingers of climate change: Common ragweed, urbanization, and public health. J Allergy Clin Immunol 111(2):290–295. https://doi.org/10.1067/mai.2003.53
Acknowledgements
We thank Julia Bartsch, Anjes Bloch and Johann Hermann for their help during the data collection.
Funding
This research was funded by the German Federal Ministry of Education and Research BMBF within the collaborative project ‘Bridging in Biodiversity Science – BIBS’ (funding number 01LC1501A-H).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
TC, MvdL and MBV conceived ideas and designed methodology. IK and MvdL supervised the study. MvdL, AH, BS, SB and TC set up the plot network and collected the data. TC performed the statistical analysis supervised by MvdL. TC led the writing of the manuscript. All authors critically contributed to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christmann, T., Kowarik, I., Bernard-Verdier, M. et al. Phenology of grassland plants responds to urbanization. Urban Ecosyst 26, 261–275 (2023). https://doi.org/10.1007/s11252-022-01302-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-022-01302-y