Abstract
In an increasingly urbanized world, there is a need to study urban areas as their own class of ecosystems as well as assess the impacts of anthropogenic impacts on biodiversity. However, collecting a sufficient number of species observations to estimate patterns of biodiversity in a city can be costly. Here we investigated the use of community science-based data on species occurrences, combined with species distribution models (SDMs), built using MaxEnt and remotely-sensed measures of the environment, to predict the distribution of a number of species across the urban environment of Los Angeles. By selecting species with the most accurate SDMs, and then summarizing these by class, we were able to produce two species richness models (SRMs) to predict biodiversity patterns for species in the class Aves and Magnoliopsida and how they respond to a variety of natural and anthropogenic environmental gradients.
We found that species considered native to Los Angeles tend to have significantly more accurate SDMs than their non-native counterparts. For all species considered in this study we found environmental variables describing anthropogenic activities, such as housing density and alterations to land cover, tend to be more influential than natural factors, such as terrain and proximity to freshwater, in shaping SDMs. Using a random forest model we found our SRMs could account for approximately 54% and 62% of the predicted variation in species richness for species in the classes Aves and Magnoliopsida respectively. Using community science-based species occurrences, SRMs can be used to model patterns of urban biodiversity and assess the roles of environmental factors in shaping them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The expansion of cities, both in their geographic scope as well as their use of resources, can result in ecological degradation (Elmqvist et al. 2016; Johnson and Munshi-South 2017). However, urban environments are not necessarily just degraded forms of pre-existing ecosystems but may be more accurately considered as a class of anthropogenic biome (Pincetl 2015; Fleming and Bateman 2018; Teixeira and Fernandes 2020). Urban environments are shaped by a combination of variables both natural, such as bioclimate and topography (Qian et al. 2020; Kendal et al. 2018;), and anthropogenic, such as land use and artificial illumination (Johnson et al. 2018; Pauwels et al. 2019). Through a variety of human activities, ranging from transport to habitat modification, urban environments contain a mix of native and non-native species (Helden et al. 2012; Gaertner et al. 2017; Godefroid and Ricotta 2018; Gruver and CaraDonna 2021). While heavily modified, these environments can contain diverse and functioning ecosystems (Baldock et al. 2019; Beller et al. 2019; Wenzel et al. 2020; Casanelles-Abella et al. 2021). This in turn has motivated interest in the study and management of urban ecosystems in their own right, and how they are shaped by environmental gradients (Grêt-Regamey et al. 2017; Shaffer 2018; Montero 2020; Uchida et al. 2021).
Research has been carried out in the potential of various measures of biodiversity to be used as environmental indicators in urban environments (Godefroid 2001; Llop et al. 2012; Guilland et al. 2018; Alquezar et al. 2020). Though the presence or abundance of various species have been used as environmental indicators, there is still the ill-defined problem of selecting such species for a given set of environmental assessment criteria (Siddig et al. 2016). Furthermore, while some urban ecosystems have been systematically assessed (Baldock et al. 2019; Planillo et al. 2021; Casanelles-Abella et al. 2021), there have often been significant limitations in obtaining a sufficient number of observations with which to build and test assessment models for most cities (Cappa et al., 2021).
To address these limitations we propose using machine learning, in combination with community science-based species observations, to select and evaluate both the accuracy and behavior of species for use as indicators of environmental quality. Community science, the collection or analysis of data by non-professional scientists, has shown promise in enabling the collection of sets of species observations on a far larger scale than from individual research projects (McCaffrey 2005; Silvertown 2009; Kobori et al. 2016; Ballard et al. 2017; Spear et al. 2017), especially on private lands which are typically undersampled in urban environments (Ballard et al. 2017). Machine learning, in particular species distribution models (SDMs), can then further extend the geographic extent of our understanding of species distributions by generating predictions on how the presence of species will vary in response to environmental conditions, even from a relatively small set of observations (Elith and Leathwick 2009). The accuracy of SDMs has been used to investigate the potential of various species, both native and non-native, to act as environmental indicators (Sergio and Newton 2003; Growns et al., 2013; Vallecillo et al. 2016). In urban environments, SDMs have been investigated as a means for assessing patterns of biodiversity at spatial resolutions not typically possible with point-based sampling (Milanovich et al. 2012; Stas et al. 2020; Wellmann et al. 2020; Casanelles-Abella et al. 2021; Planillo et al. 2021). Urban SDMs have also enabled comparisons of the effects of socio-ecological factors, driven by anthropogenic activity, versus natural variations in the environment (Rhodes et al. 2006; Le Louarn et al. 2018; Liu et al. 2019). Additionally, models predicting the richness of various groups of species, species richness models (SRMs), have also been studied in a similar fashion as SDMs for investigating the impacts of environmental conditions on urban biodiversity (Gavier-Pizarro et al. 2010; Perillo et al. 2017; Fröhlich and Ciach 2019).
In order to investigate the potential of combining the use of SDMs and SRMs with community science-based observations of species, we used the city of Los Angeles as our test case. We selected Los Angeles as there is current interest in assessing it ecologically (Jenerette et al. 2016; Spear et al. 2017; McGlynn et al. 2019; Avolio et al. 2020; Rauser 2021), covers significant variations in both elevation and microclimates (Tayyebi and Jenerette, 2016), lies within one of the 36 most biodiverse terrestrial ecosystems in the world (Myers et al. 2000), and contains a large number of observations from community scientists (Vendetti et al. 2018; Leong and Trautwein 2019; Callaghan et al., 2020). As Los Angeles is a heavily urbanized area within a designated biodiversity ‘hotspot’ (Gillespie et al. 2018), it can also serve as a model city for assessing the potential of various species to act as environmental indicators in an urban context.
Within Los Angeles we then propose to investigate the following in an urban environment:
-
(1)
Compare the accuracy of SDMs constructed from native and non-native species.
-
(2)
Assess the importance of anthropogenic and natural environmental variables in shaping urban biodiversity patterns as described by SDMs.
-
(3)
Identify individual species, given the accuracy of their SDMs, which may act as accurate environmental indicators.
-
(4)
Construct and assess the reliability of SRMs, assembled from species with the most accurate SDMs, to predict species richness across an urban landscape.
Methods
Software and workflow
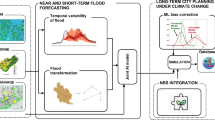
Our analysis was conducted in R v4.1.2 using RStudio 2021.09.1 + 372 “Ghost Orchid” Release (RStudio Team, 2021). Processing of geospatial data involving the use of Geospatial Data Abstraction Library (GDAL) was done using version 3.4.1 of that software. A diagram of our analysis workflow is illustrated in Fig. 1.
Study area
Our study area covered the city of Los Angeles, an urban environment centered at 34.05° N, 118.24° W with approximately 4 million inhabitants covering over 1200 km2 (Kawabata and Shen 2006). The city is part of the greater Los Angeles area, an urban agglomeration containing over 18 million people covering approximately 88,000 km2 of southern California biomes ranging from chaparral to coastal oak forests (Tayyebi and Jenerette, 2016). This region is within a Mediterranean climatic zone, receiving the bulk of its annual rainfall (40 cm) during the winter season (Hill et al. 2016). While the environment of this region is heavily modified through the expansion of impervious surfaces, irrigation, river channelization and transportation networks, it has been found to contain a diverse, albeit heavily altered, set of species (Pataki et al. 2013; Li et al. 2019; Adams et al. 2020; Rogers et al. 2020).
Occurrence data
We sought to obtain as diverse an array of species as possible from public databases in order to assess their behavior as potential environmental indicators. Our initial set of species observational data was obtained from the Global Biodiversity Information Facility (GBIF) (GBIF.org, 2021) with the following query: (1) observations made within the period 2010–2020, (2) within the spatial extent of Los Angeles County, and (3) with a spatial uncertainty of less than 30 m. We selected GBIF data within the decade 2010–2020 as it both provided for species observations covered by the range of time when our environmental data sets were collected (SI: File 4). This initial set of 120,713 observations was then filtered using the function st_intersection from the R package sf v1.0-5 (Pebesma 2018) to contain only observations within the political boundaries of the city of Los Angeles and with a spatial uncertainty of less than 10 m.
To reduce the effects of spatial clustering of our occurrence data we performed spatial thinning, using the function thin within the R package spThin v0.2.0 (Aiello-Lammens et al. 2015), with a minimum separation distance of 500 m. Species were retained if at least 25 presence points remained after spatial thinning, as this is a conservative minimum number for generating accurate SDMs using MaxEnt (van Proosdij et al. 2016), leaving 20,050 observations covering 122 species for analysis. These species were assigned a native or non-native status using a CalFlora list of plants and fungi native to Los Angeles County (SI: File 1), and a corresponding list of animal species curated by the Los Angeles Sanitation & Environment (LASAN, 2021) (SI: File 2), producing a split of 96 native and 26 non-native species across 10 classes (Table 1).
The number of remaining presences used to estimate sampling bias for each species ranged from 25 to 242. This method allows for the number of sampling of background points for each species to grow or shrink in proportion to its sampling effort. This method of sampling is often used to account for spatial bias in non-systematic sampling efforts (Phillips et al. 2009; Syfert et al. 2013; Molloy et al. 2017).
Environmental data
We obtained our initial set of 39 environmental layers, 25 natural and 14 anthropogenic, from a variety of sources (https://doi.org/10.5068/D1W988). We selected these initial layers (SI: File 4), as they cover both variations in bioclimate and topography as well as anthropogenic modifications to the environment such as housing density and exposure to light pollution, which have been found to influence the spatial distributions of a wide variety of species (Davies et al. 2008; Santorufo et al. 2012; Chong et al., 2014; Norton et al. 2016; Lin et al. 2021; Simons et al. 2021). We also selected a number of composite measures of anthropogenic disturbance, specifically habitat quality (Brown, 2019), the global human modification index (gHM) (Kennedy et al. 2019), the Calenviroscreen pollution exposure score (PollutionS) and its composite with a human population vulnerability index (CIscore) (Faust et al. 2017). These measures integrate data on land cover, habitat connectivity, and anthropogenic disturbance, impacts to human health, and interactions between human population characteristics and environmental contamination, all of which have been found to influence urban biodiversity (Table 2).
This set of environmental layers was then clipped and aligned to the city boundaries of Los Angeles using GDAL (Warmerdam 2008) with the project coordinate reference system (EPSG:2229) and resolution (30 ft / 9.1 m). From this initial set of environmental layers we retained 21 after removing those with a Pearson’s correlation greater than 0.7 to other layers (Barber et al., 2021). This was done using the function removeCollinearity within the R package virtualspecies v1.5.1 (Leroy et al. 2016) with 100,000 randomly selected points. Of the layers we retained, 12 described anthropogenic variables and 9 natural environmental variables (Table 2). We calculated the variability in these remaining environmental layers by calculating their coefficients of variation using the function cellStats in raster (SI: Table 5).
Accounting for spatial bias in species observations
To visualize geographic biases in observational data we converted our spatially thinned occurrences into a heatmap using the function stat_density_2d in the R package ggplot2 v3.3.5 (Wickham et al. 2016). Prior to running species distribution models, spatial biases in species observations were accounted for by modifying the sampling of background points using a probability function corresponding to sampling effort for each species (Filazzola et al. 2018; von Takach et al. 2020). This probability density function was generated for each species using a two-dimensional kernel density estimate, with the function kde2d in the R package MASS v7.3-54 (Ripley et al. 2013), using the density of spatially thinned observations for each species. The spatial bias raster of each species was then generated, along with the spatially thinned data set as a whole (SI: Fig. 4), in the project coordinate reference system and resolution, from this kernel density estimate using the function raster in the R package raster v2.5-2 (Hijmans and van Etten., 2015). Each of these spatial bias rasters were then clipped and aligned to the boundaries of Los Angeles using the function gdalwarp within the R package gdalUtils v2.0.3.2 (Greenberg and Mattiuzzi 2015).
Building SDMs
In order to construct SDMs we used the machine learning technique of Maximum Entropy (MaxEnt). We selected MaxEnt for constructing our SDMs as it can work with presence-only data for species observations (Elith et al., 2011), which are commonly found in databases such as the GBIF (Edwards 2004). The accuracy of MaxEnt-based SDMs have also been used in assessing the potential of species to act as environmental indicators (Jose V, 2020), that is, species whose likelihood of presence can be accurately predicted by a set of environmental conditions.
For each species a set of 10 MaxEnt models were run using the function maxent, within the R package dismo v1.3-5 (Hijmans et al. 2017), with its default settings. Species input data consisted of both presence points, as well as 10,000 background points sampled using the function xyFromCell within the package raster and a sampling probability determined using a species specific spatial bias raster. To enable an assessment of accuracy, each model utilized a randomly sampled set of 80% of presence and background points for model training, while the remaining 20% were used for testing.
Evaluating SDMs
Comparing native to non-native SDM accuracy: To assess the accuracy of these models rates of true and false positives and negatives were found using the function evaluate within the dismo package. These rates were then used to calculate the True Skill Statistic (TSS) for each of the 10 models run per species. The TSS was used as it has been found to be a metric with little dependence on species prevalence (Allouche et al. 2006). The value of the TSS ranges from − 1 to 1, with a value of 1 corresponding to a perfect agreement between predicted and actual distributions, and negative values indicating a model’s predictions are no better than random (Allouche et al. 2006; Liu et al. 2009). For each species we calculated the mean and standard on the TSS scores for its maxent models (SI: File 3).
To visualize the distribution of mean TSS scores for each species, and how they differ between native and non-native species, the functions geom_violin and facet_grid were used from ggplot2. These functions were also used to visualize the distributions of the percent relative importances of both anthropogenic and natural environmental variables in our SDMs. To test if the distribution of TSS scores differed significantly between SDMs made for native and non-native species, we used a Kruskal-Wallis (K-W) test implemented with the function kruskal.wallis in the R package stats where α was set to 0.05.
Comparing the importance of natural and anthropogenic environmental variables: The percent relative importance of the 21 environmental variables used to generate each SDM were calculated, as a percent contribution to each model, using the function var.importance within the R package ENMeval v2.0.2 (Muscarella et al. 2014). The mean and standard deviation on these relative importance values were calculated for each species (SI: File 3).
To visualize the rank mean relative importance of environmental variables for each SDM the function geom_tile, within the R package ggplot2, was used to visualize a heatmap. To test if the distribution of the percent relative importance for natural and anthropogenic environmental variables differed significantly, for both native and non-native SDMs, we again used a K-W where α was set to 0.05.
Selecting environmental indicator species
Species were selected for constructing our SRMs following a cutoff on the mean TSS scores for their SDMs (≥ 0.3). While a TSS cutoff of 0.4 has been used to classify SDMs as accurate (Thuiller et al. 2019), only one of our species exceeded this threshold (Ardea herodias, mean TSS of 0.43), and so we used a more relaxed threshold of 0.3 to select SDMs with a fair level of accuracy (Landis and Koch 1977). This selection criterion produced a list of 9 native species in the class Aves, and 13 in the class Magnoliopsida (Table 1). For species in both lists, SDM maps were generated using the function predict within the R package raster. Each SDM map was generated using the maxent function with all available spatially thinned presence points, 10,000 background points, and a presence threshold set as the maximum sum of the specificity and sensitivity.
Constructing and evaluating SRMs
The SDMs from these two classes were then summed, using the calc function in raster, to construct a SRM for species in each class (Fig. 2). These two SRMs represent the richness of nine species within the class Aves and another representing the richness of thirteen species within the class Magnoliopsida (SI: Table 6). Visualization of these SRM maps, which illustrate the predicted species richness per 900 ft2 / 83.6 m2 map call, was done using the function leaflet in the R package leaflet v2.0.4.1 (Cheng et al. 2018).
To evaluate the accuracy of our SRMs to respond to our environmental variables (Table 2) we used a random forest model, which has been shown to be an accurate method for predicting the spatial distribution of other ecological data sets (Rodriguez-Galiano et al. 2012). For each SRM we constructed a set of 100 random forest models using training data sets and the function tuneRF, within the R package randomForest v4.6-14 (Liaw and Wiener 2002), with stepFactor set to a value of 1 and doBest set as ‘true’. Data for each random forest model of a SRM was extracted from 300 randomly selected locations within our study area using the function extract within raster. We chose 300 locations as this was found to be the minimum number needed to consistently model significant (Pearson correlation, p < 10− 4) predictions of both SRMs using random forest. A training set was generated by randomly sampling 80% of these extracted data using the function kfold within dismo. Predicted SRM values were calculated using the function predict within randomForest, which were then compared against their actual counterparts with a Pearson correlation coefficient. The root mean square error (RMSE) for each random forest model was calculated using the function rmse in the R package Metrics v0.1.4 (Hamner et al. 2018).
The mean and standard deviation on the 100 Pearson correlation coefficients, generated in evaluating each random forest model of a SRM, were then calculated for each class’ SRM. This was done by first performing a Fisher transformation, using the FisherZ function within the R package DescTools v0.99.44 (Signorelli, 2020), on the set of Pearson correlation coefficients. We used a Fisher transformation as it has been found to produce less biased summary statistics for a set of Pearson correlation coefficients (Corey et al., 1998). The average and standard deviation were then inverse Fisher transformed, using the function FisherZInv within the DescTools package, to produce a single statistic per SRM. For each SRM the median and variance on the RMSE values was also calculated. To account for the percent variance explained by our SRMs we used the following formula for each SRM:
To compare the relative importance of environmental variables within our models we used the function importance within randomForest. The relative importance of our environmental variables was calculated as their mean decrease in node impurity as quantified by their Gini indices, with a larger value denoting greater relative importance. To generate individual partial dependence plots we used the function partialPlot within randomForest.
To visualize a heat map of the 100 iterations of the partial dependence plots for our environmental variables for each SRM, we then used the function geom_bin2d within ggplot2. For all our partial dependence heat maps, we divided our axes into 20 bins and generated a best-fit curve using the function stat_smooth within ggplot2.
Results
Evaluating SDMs
Comparing native to non-native SDM accuracy
We found native species had SDMs which tended to be more accurate, as assessed by their mean TSS scores (Fig. 3a) (K-W test, χ2 = 7.57, p < 10−2), than their non-native counterparts. For all of our SDMs, the relative importance of anthropogenic environmental variables tended to exceed their natural counterparts (K-W test, χ2 = 16.54, p < 10−4). Even within native and non-native species groups (Fig. 3B), anthropogenic environmental variables tended to have a greater relative importance for both native (K-W test, χ2 = 11.58, p < 10−3) and non-native species (K-W test, χ2 = 5.23, p < 0.05).
Comparing the importance of natural and anthropogenic environmental variables: For a majority of species we found their spatial distributions to be strongly influenced by the density of housing units and slope (SI: Fig. 5). Both habitat quality and proximity to streams were found to be of high relative importance in influencing the SDMs of some plants, birds, and insects (SI: Fig. 5a-c). For members of Magnoliopsida and Liliopsida the aspect of the local terrain also tended to be influential on the spatial distribution of most species (SI: Fig. 5a and h).
Selecting environmental indicator species
Of the 122 species in this study, 28 were found to have SDMs considered accurate (Table 1), that is those with a TSS score of at least 0.3 (Landis and Koch 1977). Of these species 26 were native to Los Angeles, and were predominantly in the class Aves (9 species) or Magnoliopsida (13 species) (Table 1 and SI: Table 6).
Constructing and evaluating SRMs
SRMs were assembled by summing SDMs for Los Angeles native species in the class Aves and Magnoliopsida (Fig. 2), which were the only two sets of species in this study to provide for much variation in richness (Table 1 and SI: Table 6). The richness of species in both classes could be reliably predicted across Los Angeles, from the environmental variables used in this study, albeit with a moderate level of uncertainty as described by the RMSEs of SRMs from each class (Table 3).
For both SRMs the density of housing units was the most influential variable considered (Table 4), which was found to generally have a negative relationship with species richness in both classes (SI: Figures 6a and 7a). Both slope and the CIscore, which is a composite of various measures of environmental contamination along with both human health and socioeconomic factors, tended to be highly important in predicting the local richness of species in the class Aves or Magnoliopsida (Table 4). For both SRMs a decline in species richness tended to be associated with an increase in slope or CIscore (SI: Figures 6d-e and 7c and e). The richness of bird species, more than species in the class Magnoliopsida, tended to be more strongly influenced by proximity to either streams or lakes (Table 4), with species richness tending to decline with distance to either type of freshwater (SI: Fig. 6b-c). The richness of species in Magnoliopsida were found to be strongly influenced by both habitat quality and the gHM (Table 4 ), a measure of anthropogenic modification to the landscape, with a positive trend associated with habitat quality and a negative one with the gHM (SI: Fig. 7b and d).
While the relative importance of environmental variables declines quickly with rank order for both SRMs (Table 4 ), we do note the predicted behavior of both models to be somewhat counterintuitive with a variety of the environmental variables considered. For our avian SRM we predict a general increase in species richness with various measures of anthropogenic disturbance ranging from nearby vehicular traffic density, to soil and water contamination, pesticide use, and light pollution (SI: Fig. 6f-i, o-p, s-t). For our Magnoliopsida SRM, species richness was also predicted to increase with various measures of soil and water contamination and pesticide use (SI: Fig. 7n-o, q-s). However, both SRMs tend to increase with variables which are composite metrics of anthropogenic disturbance. In particular, we modeled species richness declining with the gHM and CIScore and increasing with habitat quality for both SRMs (SI: Figures 6e, j and m and 7 b, d-e).
Discussion
Evaluating SDMs
Comparing native to non-native SDM accuracy
For the species we studied, SDMs derived from native species tended to be significantly more accurate than SDMs derived from non-native species (Fig. 3a). This may reflect the tendency of non-native species, especially in urban environments, to be able to occupy a broader set of environmental conditions than native species (Le Viol et al. 2012; Cervelli et al., 2013; Concepción et al., 2016; Callaghan et al., 2019; Colléony and Shwartz, 2020). The result of this ecological tendency being less expected variation in the likelihood of a species being present in response to variations in the environment.
We found that membership in our list of potential environmental indicator species (Table 1), particularly those native to Los Angeles, were biased towards members of the classes Aves and Magnoliopsida. This reflects a bias in the community science data sets found in GBIF towards observations of both classes (Troudet et al. 2017; Petersen et al. 2021). Despite this bias, species from both classes have been used as environmental indicators in a variety of studies of urban areas. For example, avian diversity has been found to respond to human modification of landscapes (Callaghan et al., 2021), the extent and density of the built environment (Pinho et al. 2016), as well as habitat quality and groundwater contamination (Mekonen 2017), while the diversity of flowering plants has been found to respond to the impact of road traffic on air and soil quality (Philips et al., 2021) as well as landscape modifications (Ioja and Breuste 2020).
Comparing the importance of natural and anthropogenic environmental variables: For both native and non-native species, we tend to find the relative importance of anthropogenic environmental variables to be greater in our SDMs than natural variables (Fig. 3b and SI: Fig. 5). This pattern has been observed in other cities (Aronson et al. 2014; Liu et al. 2017), and the greater importance of anthropogenic factors in shaping urban biodiversity (Faeth et al. 2011; Li et al. 2019) may simply reflect the fact of cities being some of the most anthropogenically modified habitats on Earth (Chase and Chase, 2016).
Many of the species we selected as potential environmental indicators also have large geographic ranges, often extending well beyond California (Table 1). A large geographic extent for a species can be associated with a wide climatic tolerance (Slatyer et al. 2013), although this may only weakly hold for various plant species (Bocsi et al. 2016). With many of our potential environmental indicator species having ranges which cover multiple climatic zones, this may partly explain the low relative importance of bioclimatic variables in their SDMs (SI: Fig. 5), which would reduce the overall relative importance of natural environmental variables in our overall set of SDMs. The lower relative importance for natural environmental variables, may in part also result from their lower levels of variation across the landscape of Los Angeles (SI: Table 5).
Selecting environmental indicator species
We find our species with the most environmentally responsive SDMs to be strongly biased toward native species in the class Aves or Magnoliopsida (Table 1). While this in part reflects taxonomic biases in our source data, the presence of species responsive enough to environmental variations to be reliable as indicators in an urban environment may stem from traits conducive to tolerance of anthropogenic environments such as small size and high dispersal (McKinney and Lockwood 1999; Lizée et al. 2011).
Constructing and evaluating SRMs
Our SRMs were able to capture a number of ecological relationships involving simple environmental variables, such as the distance to freshwater or housing density, indicating their potential utility in assessing environmental conditions in an urban environment such as Los Angeles. For example, housing density has been associated with a decline in avian species richness (Gagné and Fahrig 2011), which has also been more broadly observed in relation to the overall fraction of an urban area dedicated to buildings (Aronson et al. 2014). Both this decline in urban species richness, as well as the importance of the role of building density, has also been observed in a variety of plant species (Godefroid and Koedam 2007). Our Avian SRM also appears to capture, at least on the scale of approximately 10 km (SI: Fig. 6b-c), the expected decline in species richness with distance to freshwater (Chin and Kupfer 2020; de Camargo Barbosa et al. 2020). At a similar geographic scale our Magnoliopsida SRM predicts a decline with the distance to freshwater (SI: Fig. 7f and j), in line with the observed decline of plant species richness with distance to freshwater observed in Mediterranean climates such as Los Angeles (Hawkins et al. 2003).
Less intuitive relationships between species richness and individual anthropogenic variables we also observed. For example, variables describing the number of unique pollutants within nearby impaired water bodies (iwb) and the areal density of pesticide use (pesticides), were both positively associated with a rise in Avian and Magnoliopsidan species richness (SI: Figures 6f and i and 7 o and s). While pesticide use or impaired water bodies would be expected to reduce biodiversity, in urban environments they are often associated with irrigated and fertilized landscapes, which are often more species rich as a result (Clarke et al., 2013; Avolio et al. 2020). Another measure of anthropogenic disturbance, the density of road traffic (traffic), was also associated with a positive response in species richness with our Aves SRM (SI: Fig. 6 h). Nonetheless, this response may be a result of bias in community scientists tending to take bird observations in proximity to roads (Keller and Scallan 1999; Mair and Reute, 2016).
We find additional potential in our method for constructing SRMs for urban environmental assessments given the responses of our two SRMs to more integrated measures of anthropogenic disturbance: an index of the quality of habitat for species native to Los Angeles (HabitatQuality), a measure of anthropogenic modification (gHM), an integrated measure of exposure to pollution (PollutionS), and an integrated measure of exposure to pollution weighted by local measures of socioeconomic vulnerability and health outcomes (CIscore). The value of HabitatQuality integrates data on land cover and vegetation found to support native biodiversity in Los Angeles, along with a measure of geographic connectivity between habitats supportive of native biodiversity (Brown, 2019). That our two SRMs both predict a positive relationship between HabitatQuality and species richness (SI: Figures 6j and 7b) is not surprising, with urban species richness generally found to increase with related metrics such as the area or connectivity of vegetated habitats (Aronson et al. 2014; Beninde et al. 2015; Callaghan et al. 2018). In a similar fashion the value of the gHM, in integrating the areal fraction dedicated to human activities such as impervious surfaces and electricity transmission (Kennedy et al. 2019), would also correspond to habitat fragmentation and decline in species richness (SI: Fig. 6 m and 7d).
Another unexpected result were discrepancies between the predicted species richness of our SRMs and the values of PollutionS and the CIscore. The value of PollutionS incorporates exposure to measures of air pollution, such as ground-level ozone concentration, human health effects, such as asthma hospitalization rates, and additional measures of environmental degradation, such as the density of hazardous waste sites (Meehan August et al. 2012). However, the value of PollutionS is not associated with a predicted decline in species richness in either of our SRMs (SI: Fig. 6 g and 7n). When the pollution metrics summarized in PollutionS are multiplied against demographic and socioeconomic characteristics, such as the fraction of the population under 5 or over 65 or people over 25 without a secondary education, to produce the CIscore it is associated with a predicted decline in species richness (SI: Figures 6e and 7e). This difference in predicted responses of species richness between exposure to pollution and a composite measure of it with characteristics of the human population may reflect a relationship between the interactions of the physical environment, socioeconomic factors, and biodiversity in an urban context (Schell et al. 2020).
Limitations
Beyond the overrepresentation of species from a few classes in GBIF (Troudet et al. 2017; Petersen et al. 2021), there remain issues with geographic biases in the observational data used to construct SDMs. We found geographic biases in the locations of all the species used in this study (SI: Fig. 4a), as well those used to construct our SRMs (SI: Fig. 4b-c), and this type of observational bias has been observed in prior studies using community science data (Mair and Ruete 2016; Petersen et al. 2021). While we were able to account for these spatial biases in generating our SDMs, we found our data to be clustered in large urban parks (SI: Fig. 4). This geographic bias toward large urban parks may in part be a result of their accessibility, as well as the expectation of community scientists on finding more diversity to capture in park spaces versus more developed land (Bonney et al. 2009; Callaghan et al., 2020). In urban environments biodiversity tends to be relatively high in large park spaces (Matthies et al. 2013), with the richness of species in parks following a species-area relationship (Nielsen et al. 2014). Additionally, cities are often built in biodiverse areas and many of these park spaces may simply reflect this relic biodiversity (Kühn et al. 2004; Luck 2007).
Geographic biases in species observations may also stem from the skewed demographics of people collecting community science data, which is biased towards those who are under 65 years of age and have a post-secondary degree (Ganzevoort et al. 2017; Lopez et al. 2020). Such a bias may affect the number of species observations in areas with a higher proportion of the population below 5 or over 65 years of age, which would bias predicted species richness downwards. This may in part explain our negative relationship between pollution exposure, which factors in exposure of a variety of environmental pollutants to more vulnerable young and elderly populations, and species richness as predicted by our SRMs. Such biases in observer demographics may then tend to skew observed biodiversity downwards, enhancing any modeled negative response between species richness and various socio-ecological variables.
Beyond the potential confounding of observer biases with socioeconomic and anthropogenic environmental variables, is the issue of temporal gaps between the collection of species occurrences and environmental variables used to generate SDMs. First, our species observations are recent and only cover a decade, mirroring the rapid but recent growth in community science-based platforms for recording species observations (Di Cecco et al. 2021). We aggregated our observations across this window of time to provide for sufficient data density to generate SDMs and SRMs, although this coarse temporal resolution may obscure a number of ecological patterns. A similar issue exists with many of our environmental layers, such as those derived from the Calenviroscreen or bioclimate, which are updated only once every few years and may therefore reduce the predictive power of any SDM or SRM which incorporates them.
We also note that the spatial resolutions of a number of the remotely sensed environmental layers used in this study (SI: File 4) may not be fine enough to sufficiently capture their influence in a highly heterogeneous urban environment. For example, while urban areas tend to produce highly localized microclimates which influence local patterns of biodiversity (Fournier et al. 2020; Casanelles-Abella et al. 2021), the resolution the bioclimatic variables we used are near a kilometer in scale and may effectively obscure the signal any potential relationship. Exposure to artificial light at night is also mapped at the same spatial resolution (Falchi et al. 2016), although its illuminance has been found to vary by orders of magnitude at the scale of only hundreds of meters (Simons et al. 2020). Our species observation data then, while large in scale thanks to the efforts of numerous community scientists, may be mapped against a level of environmental variation artificially lowered by spatially coarse remote sensing data.
Future prospects
The volume of data captured through community science has grown rapidly in recent years. Although there are biases in the type of species observed, and where they’re observed, these may be compensated for through the use of environmental DNA (eDNA). Using eDNA from soil, sediment, water, or air sample inventories, we can complement existing species monitoring efforts through the identification of thousands of species at once, including plants, animals, and microbes (Stat et al. 2019; Lin et al. 2021; Nørgaard et al. 2021). Use of eDNA can greatly complement traditional species monitoring by enabling greater taxonomic resolution (Deiner et al. 2017; Ruppert et al. 2019), the detection of species which tend to avoid the presence of humans (Yonezawa et al. 2020; Mas-Carrió et al. 2021), or organisms such as bacteria and fungi which can be difficult to monitor using traditional observations (Frøslev et al. 2019; Liddicoat et al. 2022). Comparisons of eDNA with observational methods have also indicated their potential to help capture additional elements of ecologically relevant information, such as the functional diversity of various groups of species (Aglieri et al., 2021; Donald et al. 2021; Sigsgaard et al. 2021), particularly with regards to identifying ecological indicators (Yan et al. 2018; Blattner et al. 2021; Seymour et al. 2021).
With the recent developments in routine low-cost hyperspectral imaging there is the potential to overcome a number of these limitations in being able to acquire frequent, and high resolution, environmental data to improve models of urban biodiversity (Mozgeris et al. 2018; Zhang et al. 2020; Hartling et al. 2021). Such remotely sensed environmental data may be captured using cubesats (Kimm et al. 2020; Grøtte et al. 2021), small and low-cost satellites, as well as airborne drones (Räsänen et al. 2020; Dierssen et al. 2021). Of particular use to monitoring highly dynamic and heterogeneous urban environments, such data can be collected at resolutions under 3 m in scale (Salgado-Hernanz et al. 2021) and daily in time (Rhodes et al. 2022).
Underlying many SDMs there is the assumption that the likely geographic distribution of species is purely a function of the environmental gradients present. While environmental variations may be an important driver in the geographic distribution of species, a variety of ecological factors ranging from interspecies competition to dispersal will also have some degree of influence (Soberón 2007). One potential method for inferring the components of variation in SDMs which may be attributed to interspecies interactions, or unknown environmental factors, is the use of joint-SDMs (jSDMs) (Pollock et al. 2014). Although, as with our study, there is still a large degree of uncertainty in disentangling the contributions to variations in SDMs with a large number of potentially interacting species (Zurell et al. 2018).
Conclusions
As a predominantly urban species there is a need for humans to better understand the ecology, and condition, of their most common habitat. This presents particular difficulties in developing ecological assessments for urban areas as they are both highly heterogeneous, and in a global environment rapidly responding to anthropogenic activity, ever more dynamic. Despite potential biases, both in the spatial distribution of sampling efforts and towards particular taxonomic groups, we demonstrate the potential use of species distribution modeling and community science-based observations to both identify potential environmental indicators and assess the response of biodiversity to environmental conditions in an urban environment. We found evidence that native SDMs tend to be more accurate than their non-native counterparts, and that biodiversity patterns in urban environments are driven more by anthropogenic activities than variations in the natural environment. In constructing SRMs from the most accurate SDMs, we were able to detect a number of plausible responses of urban biodiversity to environmental conditions. Of particular interest is the potential for our SRMs to detect declines in biodiversity associated with measures which integrate both exposure to various pollutants, as well socioeconomic characteristics, on a local basis. However, because of various biases associated with the backgrounds of community scientists, some of the environmental responses of our SRMs may be confounded with socioeconomic variables. We therefore recommend future development of this methodology to incorporate a broader initial set of environmental and socioeconomic variables in order to better correct for potential observer biases, and subsequently improve the accuracy of result SDMs and SRMs to capture meaningful environmental responses in urban environments.
Data Availability
All map layers used in this analysis are available at https://doi.org/10.5068/D1W988. All species observations were obtained from https://www.gbif.org .
Code Availability
All scripts used in this analysis are available here: https://github.com/LASanitation/LASAN.
Change history
16 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11252-022-01275-y
References
Adams BJ, Li E, Bahlai CA et al (2020) Local- and landscape-scale variables shape insect diversity in an urban biodiversity hot spot. Ecol Appl 30. https://doi.org/10.1002/eap.2089
Aiello-Lammens ME, Boria RA, Radosavljevic A et al (2015) spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography (Cop) 38:541–545. https://doi.org/10.1111/ecog.01132
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43:1223–1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x
Alquezar RD, Tolesano-Pascoli G, Gil D, Macedo RH (2020) Avian biotic homogenization driven by airport-affected environments. Urban Ecosyst 23:507–517. https://doi.org/10.1007/s11252-020-00936-0
Aronson MFJ, Lepczyk CA, Evans KL et al (2017) Biodiversity in the city: key challenges for urban green space management. Front Ecol Environ 15:189–196
Aronson MFJ, La Sorte FA, Nilon CH et al (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc B Biol Sci 281. https://doi.org/10.1098/rspb.2013.3330
Avolio M, Pataki DE, Jenerette GD et al (2020) Urban plant diversity in Los Angeles, California: Species and functional type turnover in cultivated landscapes. Plants People Planet 2:144–156. https://doi.org/10.1002/ppp3.10067
Baldock KCR, Goddard MA, Hicks DM et al (2019) A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat Ecol Evol 3:363–373. https://doi.org/10.1038/s41559-018-0769-y
Ballard HL, Robinson LD, Young AN et al (2017) Contributions to conservation outcomes by natural history museum-led citizen science: Examining evidence and next steps. Biol Conserv 208:87–97. https://doi.org/10.1016/j.biocon.2016.08.040
Barber RA, Ball SG, Morris RKA, Gilbert F (2022) Target-group backgrounds prove effective at correcting sampling bias in Maxent models. Divers Distrib 28:128–141. https://doi.org/10.1111/ddi.13442
Beller EE, Spotswood EN, Robinson AH et al (2019) Building Ecological Resilience in Highly Modified Landscapes. Bioscience 69:80–92. https://doi.org/10.1093/biosci/biy117
Beninde J, Veith M, Hochkirch A (2015) Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol Lett 18:581–592
Bertoncini AP, Machon N, Pavoine S, Muratet A (2012) Local gardening practices shape urban lawn floristic communities. Landsc Urban Plan 105:53–61
Blattner L, Ebner JN, Zopfi J, von Fumetti S (2021) Targeted non-invasive bioindicator species detection in eDNA water samples to assess and monitor the integrity of vulnerable alpine freshwater environments. Ecol Indic 129:107916
Bocsi T, Allen JM, Bellemare J et al (2016) Plants’ native distributions do not reflect climatic tolerance. Divers Distrib 22:615–624. https://doi.org/10.1111/ddi.12432
Bonney R, Cooper CB, Dickinson J et al (2009) Citizen science: A developing tool for expanding science knowledge and scientific literacy. Bioscience 59:977–984
Bornstein C, Fross D et al (1953) (2005)California native plants for the garden
Brown IT, Gillespie TW, Gold MA (2019) Managing Cities as Urban Ecosystems: Analysis Tools for Biodiversity Stewardship in Los Angeles. University of California, Los Angeles
Bullock SH, Ceseña JMS, Rebman JP, Riemann H (2008) Flora and vegetation of an isolated mountain range in the desert of Baja California. Southwest Nat 53:61–73. https://doi.org/10.1894/0038-4909(2008)53[61:FAVOAI]2.0.CO;2
Callaghan CT, Major RE, Lyons MB et al (2018) The effects of local and landscape habitat attributes on bird diversity in urban greenspaces. Ecosphere 9. https://doi.org/10.1002/ecs2.2347
Casanelles-Abella J, Chauvier Y, Zellweger F et al (2021) Applying predictive models to study the ecological properties of urban ecosystems: A case study in Zürich, Switzerland. Landsc Urban Plan 214:104137
Cheng J, Karambelkar B, Xie Y(2018) Leaflet: Create interactive web maps with the javascript’leaflet’library.R Packag version2
Chin EY, Kupfer JA (2020) Identification of environmental drivers in urban greenway communities. Urban For Urban Green 47:126549
Ciach M, Fröhlich A (2017) Habitat type, food resources, noise and light pollution explain the species composition, abundance and stability of a winter bird assemblage in an urban environment. Urban Ecosyst 20:547–559
Cronquist A, Holmgren AH, Holmgren NH et al (1984) Intermountain Flora: Vascular Plants of the Intermountain West, USA The New York Botanical Garden
Cushing L, Faust J, August LM et al (2015) Racial/ethnic disparities in cumulative environmental health impacts in California: evidence from a statewide environmental justice screening tool (CalEnviroScreen 1.1). Am J Public Health 105:2341–2348
Datry T, Malard F, Gibert J (2006) Effects of artificial stormwater infiltration on urban groundwater ecosystems. Urban groundwater management and sustainability. Springer, pp 331–345
Davies RG, Barbosa O, Fuller RA et al (2008) City-wide relationships between green spaces, urban land use and topography. Urban Ecosyst 11:269–287
de Camargo Barbosa KV, Rodewald AD, Ribeiro MC, Jahn AE (2020) Noise level and water distance drive resident and migratory bird species richness within a Neotropical megacity. Landsc Urban Plan 197:103769
Deiner K, Bik HM, Mächler E et al (2017) Environmental DNA metabarcoding: Transforming how we survey animal and plant communities. Mol Ecol 26:5872–5895
Del Hoyo J, Elliot A, Sargatal J (1992) Handbook of the Birds of the World. Vol. 1.,(Lynx Edicions: Barcelona, Spain)
Di Cecco GJ, Barve V, Belitz MW et al (2021) Observing the Observers: How Participants Contribute Data to iNaturalist and Implications for Biodiversity Science. Bioscience 71:1179–1188. https://doi.org/10.1093/biosci/biab093
Dierssen HM, Ackleson SG, Joyce KE et al(2021) Living up to the hype of hyperspectral aquatic remote sensing: science, resources and outlook.Front Environ Sci9
Donald J, Murienne J, Chave J et al (2021) Multi-taxa environmental DNA inventories reveal distinct taxonomic and functional diversity in urban tropical forest fragments. Glob Ecol Conserv 29:e01724
Drilling N (2002) Mallard (Anas platyrhynchos). Birds North Am
Edwards JL (2004) Research and societal benefits of the Global Biodiversity Information Facility. Bioscience 54:485–486
Elith J, Leathwick JR (2009) Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu Rev Ecol Evol Syst 40:677–697. https://doi.org/10.1146/annurev.ecolsys.110308.120159
Elmqvist T, Zipperer W, Güneralp B (2016) Urbanization, habitat loss, biodiversity decline: solution pathways to break the cycle. In, Seta, Karen; Solecki, William D; Griffith, Corrie A(eds) Routledge Handb Urban Glob Environ Chang London New York Routledge 2016:139–151
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: patterns and mechanisms. Ann N Y Acad Sci 1223:69–81. https://doi.org/10.1111/j.1749-6632.2010.05925.x
Falchi F, Cinzano P, Duriscoe D et al (2016) The new world atlas of artificial night sky brightness. Sci Adv 2. https://doi.org/10.1126/sciadv.1600377
Faust J, August L, Bangia K et al (2017) CalEnviroScreen 3.0 Report. Calif Environ Prot Agency, Off Environ Heal Hazard Assess Sacramento, CA
Ferreira CSS, Walsh RPD, Ferreira AJD (2018) Degradation in urban areas. Curr Opin Environ Sci Heal 5:19–25
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Filazzola A, Sotomayor DA, Lortie CJ (2018) Modelling the niche space of desert annuals needs to include positive interactions. Oikos 127:264–273. https://doi.org/10.1111/oik.04688
Fleming PA, Bateman PW (2018) Novel predation opportunities in anthropogenic landscapes. Anim Behav 138:145–155. https://doi.org/10.1016/j.anbehav.2018.02.011
Fournier B, Vázquez-Rivera H, Clappe S et al (2020) The spatial frequency of climatic conditions affects niche composition and functional diversity of species assemblages: the case of Angiosperms. Ecol Lett 23:254–264
Fröhlich A, Ciach M (2019) Nocturnal noise and habitat homogeneity limit species richness of owls in an urban environment. Environ Sci Pollut Res 26:17284–17291. https://doi.org/10.1007/s11356-019-05063-8
Frøslev TG, Kjøller R, Bruun HH et al (2019) Man against machine: Do fungal fruitbodies and eDNA give similar biodiversity assessments across broad environmental gradients? Biol Conserv 233:201–212
Gaertner M, Wilson JRU, Cadotte MW et al (2017) Non-native species in urban environments: patterns, processes, impacts and challenges. Biol Invasions 19:3461–3469. https://doi.org/10.1007/s10530-017-1598-7
Gagné SA, Fahrig L (2011) Do birds and beetles show similar responses to urbanization? Ecol Appl 21:2297–2312. https://doi.org/10.1890/09-1905.1
Ganzevoort W, van den Born RJG, Halffman W, Turnhout S (2017) Sharing biodiversity data: citizen scientists’ concerns and motivations. Biodivers Conserv 26:2821–2837. https://doi.org/10.1007/s10531-017-1391-z
Gavier-Pizarro GI, Radeloff VC, Stewart SI et al (2010) Housing is positively associated with invasive exotic plant species richness in New England, USA. Ecol Appl 20:1913–1925. https://doi.org/10.1890/09-2168.1
GBIF.org (2021) GBIF.org. https://doi.org/10.15468/dl.vs5s6n. Accessed 29 Jan 2021
Gillespie TW, Ostermann-Kelm S, Dong C et al (2018) Monitoring changes of NDVI in protected areas of southern California. Ecol Indic 88:485–494. https://doi.org/10.1016/j.ecolind.2018.01.031
Godefroid S (2001) Temporal analysis of the Brussels flora as indicator for changing environmental quality. Landsc Urban Plan 52:203–224. https://doi.org/10.1016/S0169-2046(00)00117-1
Godefroid S, Koedam N (2007) Urban plant species patterns are highly driven by density and function of built-up areas. Landsc Ecol 22:1227–1239. https://doi.org/10.1007/s10980-007-9102-x
Godefroid S, Ricotta C (2018) Alien plant species do have a clear preference for different land uses within urban environments. Urban Ecosyst 21:1189–1198. https://doi.org/10.1007/s11252-018-0792-4
Greenberg JA, Mattiuzzi M(2015) gdalUtils: wrappers for the geospatial data abstraction library (GDAL) utilities. R Packag version 2
Greenwell BM (2017) pdp: An R Package for Constructing Partial Dependence Plots. R J 9:421. https://doi.org/10.32614/RJ-2017-016
Grêt-Regamey A, Altwegg J, Sirén EA et al (2017) Integrating ecosystem services into spatial planning—A spatial decision support tool. Landsc Urban Plan 165:206–219. https://doi.org/10.1016/j.landurbplan.2016.05.003
Grøtte ME, Birkeland R, Honoré-Livermore E et al (2021) Ocean Color Hyperspectral Remote Sensing With High Resolution and Low Latency—The HYPSO-1 CubeSat Mission. IEEE Trans Geosci Remote Sens 60:1–19
Gruver A, CaraDonna P (2021) Chicago Bees: Urban Areas Support Diverse Bee Communities but With More Non-Native Bee Species Compared to Suburban Areas. Environ Entomol 50:982–994
Guilland C, Maron PA, Damas O, Ranjard L (2018) Biodiversity of urban soils for sustainable cities. Environ Chem Lett 16:1267–1282. https://doi.org/10.1007/s10311-018-0751-6
Guin JD, Gillis WT, Beaman JH (1981) Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol 4:99–114. https://doi.org/10.1016/S0190-9622(81)70014-8
Guinan JA, Gowaty PA, Eltzroth EK(2000) Western Bluebird(Sialia mexicana). birds North Am 32
Hamner B, Frasco M, LeDell E (2018) Metrics: Evaluation metrics for machine learning. R Packag version 01 4:2018
Hancock J, Kushlan JA(2010) The herons handbook. A&C Black
Hapke CJ, Reid D, Richmond BM et al (2006) National assessment of shoreline change Part 3: Historical shoreline change and associated coastal land loss along sandy shorelines of the California Coast. US Geol Surv Open File Rep 1219:79
Harrison T, Winfree R (2015) Urban drivers of plant-pollinator interactions. Funct Ecol 29:879–888
Hartling S, Sagan V, Maimaitijiang M (2021) Urban tree species classification using UAV-based multi-sensor data fusion and machine learning. GIScience Remote Sens 58:1250–1275. https://doi.org/10.1080/15481603.2021.1974275
Hassall C (2014) The ecology and biodiversity of urban ponds. Wiley Interdiscip Rev Water 1:187–206
Hawkins BA, Field R, Cornell HV et al (2003) ENERGY, WATER, AND BROAD-SCALE GEOGRAPHIC PATTERNS OF SPECIES RICHNESS. Ecology 84:3105–3117. https://doi.org/10.1890/03-8006
Helden AJ, Stamp GC, Leather SR (2012) Urban biodiversity: comparison of insect assemblages on native and non-native trees. Urban Ecosyst 15:611–624. https://doi.org/10.1007/s11252-012-0231-x
Heringer G, Del Bianco Faria L, Villa PM et al(2021) Urbanization affects the richness of invasive alien trees but has limited influence on species composition.Urban Ecosyst1–11
Hijmans RJ(2015) Raster:Geographic Data Analysis and Modeling
Hijmans RJ, Phillips S, Leathwick J et al (2017) Package ‘dismo ’ Circles 9:1–68
Hill RA, Weber MH, Leibowitz SG et al (2016) The Stream-Catchment (StreamCat) Dataset: A Database of Watershed Metrics for the Conterminous United States. JAWRA J Am Water Resour Assoc 52:120–128. https://doi.org/10.1111/1752-1688.12372
Hölker F, Wolter C, Perkin EK, Tockner K (2010) Light pollution as a biodiversity threat. Trends Ecol Evol 25:681–682
Houlahan JE, Findlay CS (2003) The effects of adjacent land use on wetland amphibian species richness and community composition. Can J Fish Aquat Sci 60:1078–1094
Ioja C, Breuste J (2020) Urban Protected Areas and Urban Biodiversity. Making Green Cities. Springer, pp 289–398
Jenerette GD, Clarke LW, Avolio ML et al (2016) Climate tolerances and trait choices shape continental patterns of urban tree biodiversity. Glob Ecol Biogeogr 25:1367–1376. https://doi.org/10.1111/geb.12499
Johnson AL, Borowy D, Swan CM (2018) Land use history and seed dispersal drive divergent plant community assembly patterns in urban vacant lots. J Appl Ecol 55:451–460. https://doi.org/10.1111/1365-2664.12958
Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Sci (80-) 358:eaam8327. https://doi.org/10.1126/science.aam8327
Jose VS, Nameer PO (2020) The expanding distribution of the Indian Peafowl (Pavo cristatus) as an indicator of changing climate in Kerala, southern India: A modelling study using MaxEnt. Ecol Indic 110:105930. https://doi.org/10.1016/j.ecolind.2019.105930
Kawabata M, Shen Q (2006) Job Accessibility as an Indicator of Auto-Oriented Urban Structure: A Comparison of Boston and Los Angeles with Tokyo. Environ Plan B Plan Des 33:115–130. https://doi.org/10.1068/b31144
Keller CME, Scallan JT (1999) Potential roadside biases due to habitat changes along breeding bird survey routes. Condor 101:50–57
Kendal D, Dobbs C, Gallagher RV et al (2018) A global comparison of the climatic niches of urban and native tree populations. Glob Ecol Biogeogr 27:629–637. https://doi.org/10.1111/geb.12728
Kennedy CM, Oakleaf JR, Theobald DM et al (2019) Managing the middle: A shift in conservation priorities based on the global human modification gradient. Glob Chang Biol 25:811–826. https://doi.org/10.1111/gcb.14549
Kimm H, Guan K, Jiang C et al (2020) Deriving high-spatiotemporal-resolution leaf area index for agroecosystems in the US Corn Belt using Planet Labs CubeSat and STAIR fusion data. Remote Sens Environ 239:111615
Kobori H, Dickinson JL, Washitani I et al (2016) Citizen science: a new approach to advance ecology, education, and conservation. Ecol Res 31:1–19. https://doi.org/10.1007/s11284-015-1314-y
Kolbe SE, Miller AI, Cameron GN, Culley TM (2016) Effects of natural and anthropogenic environmental influences on tree community composition and structure in forests along an urban-wildland gradient in southwestern Ohio. Urban Ecosyst 19:915–938
Kühn I, Brandl R, Klotz S (2004) The flora of German cities is naturally species rich. Evol Ecol Res 6:749–764
Landis JR, Koch GG(1977) The measurement of observer agreement for categorical data.Biometrics159–174
LASAN (2021) Los Angeles Native Fauna. Los Angeles
Lathrop EW, Thorne RF (1978) A flora of the Santa Ana Mountains, California. Aliso A J Syst Florist Bot 9:197–278
Le Louarn M, Clergeau P, Strubbe D, Deschamps-Cottin M (2018) Dynamic species distribution models reveal spatiotemporal habitat shifts in native range‐expanding versus non‐native invasive birds in an urban area. J Avian Biol 49:jav–01527
Le Viol I, Jiguet F, Brotons L et al (2012) More and more generalists: two decades of changes in the European avifauna. Biol Lett 8:780–782. https://doi.org/10.1098/rsbl.2012.0496
Leong M, Trautwein M (2019) A citizen science approach to evaluating US cities for biotic homogenization. PeerJ 7:e6879. https://doi.org/10.7717/peerj.6879
Leroy B, Meynard CN, Bellard C, Courchamp F (2016) virtualspecies, an R package to generate virtual species distributions. Ecography (Cop) 39:599–607. https://doi.org/10.1111/ecog.01388
Li E, Parker SS, Pauly GB et al (2019) An Urban Biodiversity Assessment Framework That Combines an Urban Habitat Classification Scheme and Citizen Science Data. https://doi.org/10.3389/fevo.2019.00277. Front Ecol Evol 7:
Lian X, Jiao L, Zhong J et al (2021) Artificial light pollution inhibits plant phenology advance induced by climate warming. Environ Pollut 291:118110
Liaw A, Wiener M (2002) Classification and regression by randomForest. R news 2:18–22
Liddicoat C, Krauss SL, Bissett A et al (2022) Next generation restoration metrics: Using soil eDNA bacterial community data to measure trajectories towards rehabilitation targets. J Environ Manage 310:114748
Lin M, Simons AL, Harrigan RJ et al (2021) Landscape analyses using eDNA metabarcoding and Earth observation predict community biodiversity in California. Ecol Appl 31. https://doi.org/10.1002/eap.2379
Liu C, White M, Newell G(2009) Measuring the accuracy of species distribution models: a review. In: Proceedings 18th World IMACs/MODSIM Congress. Cairns, Australia. p 4247
Liu G, Rowley JJL, Kingsford RT, Callaghan CT (2021) Species’ traits drive amphibian tolerance to anthropogenic habitat modification. Glob Chang Biol 27:3120–3132
Liu J, Yu M, Tomlinson K, Slik JWF (2017) Patterns and drivers of plant biodiversity in Chinese university campuses. Landsc Urban Plan 164:64–70. https://doi.org/10.1016/j.landurbplan.2017.04.008
Liu J, Bai H, Ma H, Feng G (2019) Bird diversity in Chinese urban parks was more associated with natural factors than anthropogenic factors. Urban For Urban Green 43:126358. https://doi.org/10.1016/j.ufug.2019.06.001
Lizée M-H, Mauffrey J-F, Tatoni T, Deschamps-Cottin M (2011) Monitoring urban environments on the basis of biological traits. Ecol Indic 11:353–361. https://doi.org/10.1016/j.ecolind.2010.06.003
Llop E, Pinho P, Matos P et al (2012) The use of lichen functional groups as indicators of air quality in a Mediterranean urban environment. Ecol Indic 13:215–221. https://doi.org/10.1016/j.ecolind.2011.06.005
Lopez B, Minor E, Crooks A (2020) Insights into human-wildlife interactions in cities from bird sightings recorded online. Landsc Urban Plan 196:103742. https://doi.org/10.1016/j.landurbplan.2019.103742
Luck GW (2007) A review of the relationships between human population density and biodiversity. Biol Rev 82:607–645. https://doi.org/10.1111/j.1469-185X.2007.00028.x
Mair L, Ruete A (2016) Explaining Spatial Variation in the Recording Effort of Citizen Science Data across Multiple Taxa. PLoS ONE 11:e0147796. https://doi.org/10.1371/journal.pone.0147796
Mas-Carrió E, Schneider J, Nasanbat B et al(2021) Assessing environmental DNA metabarcoding and camera trap surveys as complementary tools for biomonitoring of remote desert water bodies. Environ DNA
Matthies S, Kopel D, Rüter S et al (2013) Vascular Plant Species Richness Patterns in Urban Environments: Case Studies from Hannover, Germany and Haifa, Israel. Modeling of Land-Use and Ecological Dynamics. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 107–118
Maune DF (2006) Vertical accuracy assessment report, 2006 LiDAR Bare-Earth Dataset for Los Angeles Region Imagery Acquisition Consortium (LAR-IAC). Dewberry, Fairfax, VA
McCaffrey RE (2005) Using citizen science in urban bird studies. Urban habitats 3:70–86
McCrimmon DA, Ogden JC, Bancroft GT et al(2020) Great Egret (Ardea alba), version 1.0. Birds World (SM Billerman, Ed Cornell Lab Ornithol Ithaca, NY, USA https//doi.org/102173/bow greegr 1:
McGlynn TP, Meineke EK, Bahlai CA et al (2019) Temperature accounts for the biodiversity of a hyperdiverse group of insects in urban Los Angeles. Proc R Soc B Biol Sci 286:20191818. https://doi.org/10.1098/rspb.2019.1818
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453. https://doi.org/10.1016/S0169-5347(99)01679-1
Meehan August L, Faust JB, Cushing L et al (2012) Methodological considerations in screening for cumulative environmental health impacts: Lessons learned from a pilot study in California. Int J Environ Res Public Health 9:3069–3084
Mekonen S (2017) Birds as biodiversity and environmental indicator. J Nat Sci Res 7:28–34
Melles S, Glenn S, Martin K(2003) Urban bird diversity and landscape complexity: species–environment associations along a multiscale habitat gradient.Conserv Ecol7
Merow C, Smith MJ, Silander JA (2013) A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography (Cop) 36:1058–1069. https://doi.org/10.1111/j.1600-0587.2013.07872.x
Milanovich JR, Peterman WE, Barrett K, Hopton ME (2012) Do species distribution models predict species richness in urban and natural green spaces? A case study using amphibians. Landsc Urban Plan 107:409–418. https://doi.org/10.1016/j.landurbplan.2012.07.010
Molloy SW, Davis RA, Dunlop JA, van Etten EJB (2017) Applying surrogate species presences to correct sample bias in species distribution models: a case study using the Pilbara population of the Northern Quoll. Nat Conserv 18:25–46. https://doi.org/10.3897/natureconservation.18.12235
Montero S (2020) Leveraging Bogotá: Sustainable development, global philanthropy and the rise of urban solutionism. Urban Stud 57:2263–2281. https://doi.org/10.1177/0042098018798555
Mowbray TB, Sedinger JS, Trost RE (2002) Canada goose (Branta canadensis). Birds North Am
Mozgeris G, Juodkienė V, Jonikavičius D et al (2018) Ultra-Light Aircraft-Based Hyperspectral and Colour-Infrared Imaging to Identify Deciduous Tree Species in an Urban Environment. Remote Sens 10:1668. https://doi.org/10.3390/rs10101668
Muscarella R, Galante PJ, Soley-Guardia M et al (2014) ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol Evol 5:1198–1205. https://doi.org/10.1111/2041-210X.12261
Myers N, Mittermeier RA, Mittermeier CG et al (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Nell CS, Meza-Lopez MM, Croy JR et al (2018) Relative effects of genetic variation sensu lato and sexual dimorphism on plant traits and associated arthropod communities. Oecologia 187:389–400. https://doi.org/10.1007/s00442-018-4065-y
Nesom GL (2013) Taxonomic notes on Diplacus (Phrymaceae). Phytoneuron 66:1–8
Nielsen AB, Van Den Bosch M, Maruthaveeran S, van den Bosch CK (2014) Species richness in urban parks and its drivers: a review of empirical evidence. Urban Ecosyst 17:305–327
Nørgaard L, Olesen CR, Trøjelsgaard K et al (2021) eDNA metabarcoding for biodiversity assessment, generalist predators as sampling assistants. Sci Rep 11:1–12
Norton BA, Evans KL, Warren PH (2016) Urban Biodiversity and Landscape Ecology: Patterns, Processes and Planning. Curr Landsc Ecol Reports 1:178–192. https://doi.org/10.1007/s40823-016-0018-5
Orlando L, Ortega L, Defeo O (2020) Urbanization effects on sandy beach macrofauna along an estuarine gradient. Ecol Indic 111:106036
Panetsos CA, Baker HG (1967) The origin of variation in “wild” Raphanus sativus (Cruciferae) in California. Genetica 38:243–274
Parsons KC, Master TL (2020) Snowy Egret (Egretta thula). In: Poole AF, Gill FB (eds) Birds of the World. Cornell Lab of Ornithology
Pataki DE, McCarthy HR, Gillespie T et al (2013) A trait-based ecology of the Los Angeles urban forest. Ecosphere 4:1–20. https://doi.org/10.1890/ES13-00017.1
Pauwels J, Le Viol I, Azam C et al (2019) Accounting for artificial light impact on bat activity for a biodiversity-friendly urban planning. Landsc Urban Plan 183:12–25. https://doi.org/10.1016/j.landurbplan.2018.08.030
Pebesma E (2018) Simple features for R: standardized support for spatial vector data. R J 10:439–446 doi: 10.32614. RJ-2018-009
Perillo A, Mazzoni LG, Passos LF et al (2017) Anthropogenic noise reduces bird species richness and diversity in urban parks. Ibis (Lond 1859) 159:638–646. https://doi.org/10.1111/ibi.12481
Petersen TK, Speed JDM, Grøtan V, Austrheim G (2021) Species data for understanding biodiversity dynamics: The what, where and when of species occurrence data collection. Ecol Solut Evid. https://doi.org/10.1002/2688-8319.12048. 2:
Phillips BB, Bullock JM, Gaston KJ et al (2021) Impacts of multiple pollutants on pollinator activity in road verges. J Appl Ecol 58:1017–1029. https://doi.org/10.1111/1365-2664.13844
Phillips SJ, Dudík M, Elith J et al (2009) Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl 19:181–197. https://doi.org/10.1890/07-2153.1
Pincetl S (2015) Cities as Novel Biomes: Recognizing Urban Ecosystem Services as Anthropogenic. Front Ecol Evol 3. https://doi.org/10.3389/fevo.2015.00140
Pinho P, Correia O, Lecoq M et al (2016) Evaluating green infrastructure in urban environments using a multi-taxa and functional diversity approach. Environ Res 147:601–610. https://doi.org/10.1016/j.envres.2015.12.025
Planillo A, Kramer-Schadt S, Buchholz S et al (2021) Arthropod abundance modulates bird community responses to urbanization. Divers Distrib 27:34–49. https://doi.org/10.1111/ddi.13169
Polidori C, García-Gila J, Blasco-Aróstegui J, Gil-Tapetado D (2021) Urban areas are favouring the spread of an alien mud-dauber wasp into climatically non-optimal latitudes. Acta Oecol 110:103678
Pollock LJ, Tingley R, Morris WK et al (2014) Understanding co-occurrence by modelling species simultaneously with a Joint Species Distribution Model (JSDM). Methods Ecol Evol 5:397–406. https://doi.org/10.1111/2041-210X.12180
Proosdij ASJ, Sosef MSM, Wieringa JJ, Raes N (2016) Minimum required number of specimen records to develop accurate species distribution models. Ecography (Cop) 39:542–552. https://doi.org/10.1111/ecog.01509
Proppe DS, Sturdy CB, St. Clair CC (2013) Anthropogenic noise decreases urban songbird diversity and may contribute to homogenization. Glob Chang Biol 19:1075–1084. https://doi.org/10.1111/gcb.12098
Qian S, Qin D, Wu X et al (2020) Urban growth and topographical factors shape patterns of spontaneous plant community diversity in a mountainous city in southwest China. Urban For Urban Green 55:126814. https://doi.org/10.1016/j.ufug.2020.126814
Radeloff VC, Helmers DP, Kramer HA et al (2018) Rapid growth of the US wildland-urban interface raises wildfire risk. Proc Natl Acad Sci 115:3314–3319. https://doi.org/10.1073/pnas.1718850115
Rail S(1993) Black-Crowned Night Heron—Nycticorax nycticorax
Räsänen A, Juutinen S, Kalacska M et al (2020) Peatland leaf-area index and biomass estimation with ultra-high resolution remote sensing. GIScience Remote Sens 57:943–964
Rastandeh A, Pedersen Zari M, Brown D, Vale R (2019) Analysis of landform and land cover: Potentials for urban biodiversity conservation against rising temperatures. Urban Policy Res 37:338–349
Rauser C(2021) UCLA Sustainable LA Grand Challenge: Innovatively Applying Research to Create the First Sustainable Megacity. In: World Scientific Encyclopedia of Climate Change: Case Studies of Climate Risk, Action, and Opportunity Volume 1. World Scientific, pp 105–114
Rebman JP, Gibson J, Rich K (2016) Annotated checklist of the vascular plants of Baja California, Mexico. San Diego Society of Natural History
Rhodes JR, Wiegand T, McAlpine CA et al (2006) Modeling species’ distributions to improve conservation in semiurban landscapes: koala case study. Conserv Biol 20:449–459
Rhodes MW, Bennie JJ, Spalding A et al (2022) Recent advances in the remote sensing of insects. Biol Rev 97:343–360
Ripley B, Venables B, Bates DM et al (2013) Package ‘mass’. Cran r 538:113–120
Rodriguez-Galiano VF, Ghimire B, Rogan J et al (2012) An assessment of the effectiveness of a random forest classifier for land-cover classification. ISPRS J Photogramm Remote Sens 67:93–104. https://doi.org/10.1016/j.isprsjprs.2011.11.002
Roeland S, Moretti M, Amorim JH et al (2019) Towards an integrative approach to evaluate the environmental ecosystem services provided by urban forest. J For Res 30:1981–1996
Rogers JB, Stein ED, Beck MW, Ambrose RF (2020) The impact of climate change induced alterations of streamflow and stream temperature on the distribution of riparian species. PLoS ONE 15:e0242682. https://doi.org/10.1371/journal.pone.0242682
RStudio Team (2021) RStudio:Integrated Development Environment for R
Ruppert KM, Kline RJ, Rahman MS (2019) Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob Ecol Conserv 17:e00547
Salgado-Hernanz PM, Bauzà J, Alomar C et al (2021) Assessment of marine litter through remote sensing: recent approaches and future goals. Mar Pollut Bull 168:112347
Santorufo L, Van Gestel CAM, Rocco A, Maisto G (2012) Soil invertebrates as bioindicators of urban soil quality. Environ Pollut 161:57–63. https://doi.org/10.1016/j.envpol.2011.09.042
Schell CJ, Dyson K, Fuentes TL et al(2020) The ecological and evolutionary consequences of systemic racism in urban environments. Science (80-) 369:. https://doi.org/10.1126/science.aay4497
Sergio F, Newton IAN (2003) Occupancy as a measure of territory quality. J Anim Ecol 72:857–865
Seymour M, Edwards FK, Cosby BJ et al (2021) Environmental DNA provides higher resolution assessment of riverine biodiversity and ecosystem function via spatio-temporal nestedness and turnover partitioning. Commun Biol 4:1–12
Shaffer HB (2018) Urban biodiversity arks. Nat Sustain 1:725–727. https://doi.org/10.1038/s41893-018-0193-y
Siddig AAH, Ellison AM, Ochs A et al (2016) How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol Indic 60:223–230. https://doi.org/10.1016/j.ecolind.2015.06.036
Signorell A, Aho K, Alfons A et al (2019) DescTools: Tools for descriptive statistics. R Packag version 099 28:17
Sigsgaard EE, Olsen K, Hansen MDD et al (2021) Environmental DNA metabarcoding of cow dung reveals taxonomic and functional diversity of invertebrate assemblages. Mol Ecol 30:3374–3389
Silvertown J (2009) A new dawn for citizen science. Trends Ecol Evol 24:467–471. https://doi.org/10.1016/j.tree.2009.03.017
Simley JD, Carswell WJ Jr (2009) The National Map—Hydrography: US Geological Survey Fact Sheet 2009–3054. US Geol Surv Natl Center, Reston, VA
Simons AL, Martin KLM, Longcore T (2021) Determining the Effects of Artificial Light at Night on the Distributions of Western Snowy Plovers (Charadrius nivosus nivosus) and California Grunion (Leuresthes tenuis) in Southern California. J Coast Res
Simons AL, Yin X, Longcore T (2020) High correlation but high scale-dependent variance between satellite measured night lights and terrestrial exposure. Environ Res Commun 2:021006
Slatyer RA, Hirst M, Sexton JP (2013) Niche breadth predicts geographical range size: a general ecological pattern. Ecol Lett 16:1104–1114
Soberón J (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett 10:1115–1123. https://doi.org/10.1111/j.1461-0248.2007.01107.x
Sol D, González-Lagos C, Moreira D et al (2014) Urbanisation tolerance and the loss of avian diversity. Ecol Lett 17:942–950. https://doi.org/10.1111/ele.12297
Spear DM, Pauly GB, Kaiser K (2017) Citizen Science as a Tool for Augmenting Museum Collection Data from Urban Areas. Front Ecol Evol 5. https://doi.org/10.3389/fevo.2017.00086
Sperandii MG, Bazzichetto M, Acosta ATR et al (2019) Multiple drivers of plant diversity in coastal dunes: A Mediterranean experience. Sci Total Environ 652:1435–1444
Stas M, Aerts R, Hendrickx M et al (2020) An evaluation of species distribution models to estimate tree diversity at genus level in a heterogeneous urban-rural landscape. Landsc Urban Plan 198:103770. https://doi.org/10.1016/j.landurbplan.2020.103770
Stat M, John J, DiBattista JD et al (2019) Combined use of eDNA metabarcoding and video surveillance for the assessment of fish biodiversity. Conserv Biol 33:196–205
Stocking KM (1955) Some taxonomic and ecological considerations of the genus Marah (Cucurbitaceae). Madroño 13:113–137
Syfert MM, Smith MJ, Coomes DA (2013) The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 8:e55158. https://doi.org/10.1371/journal.pone.0055158
Tayyebi A, Darrel Jenerette G (2016) Increases in the climate change adaption effectiveness and availability of vegetation across a coastal to desert climate gradient in metropolitan Los Angeles, CA, USA. Sci Total Environ 548–549:60–71. https://doi.org/10.1016/j.scitotenv.2016.01.049
Teixeira CP, Fernandes CO (2020) Novel ecosystems: a review of the concept in non-urban and urban contexts. Landsc Ecol 35:23–39. https://doi.org/10.1007/s10980-019-00934-4
Terres JK(1980) Audubon Society Encyclopedia of North American Birds. Distributed by Random House
Thuiller W, Guéguen M, Renaud J et al (2019) Uncertainty in ensembles of global biodiversity scenarios. Nat Commun 10:1–9
Troudet J, Grandcolas P, Blin A et al (2017) Taxonomic bias in biodiversity data and societal preferences. Sci Rep 7:9132. https://doi.org/10.1038/s41598-017-09084-6
Uchida K, Blakey RV, Burger JR et al (2021) Urban Biodiversity and the Importance of Scale. Trends Ecol Evol 36:123–131. https://doi.org/10.1016/j.tree.2020.10.011
Vallecillo S, Maes J, Polce C, Lavalle C (2016) A habitat quality indicator for common birds in Europe based on species distribution models. Ecol Indic 69:488–499. https://doi.org/10.1016/j.ecolind.2016.05.008
Vendetti JE, Lee C, LaFollette P (2018) Five new records of introduced terrestrial gastropods in southern California discovered by citizen science. Am Malacol Bull 36:232–247
von Takach B, Scheele BC, Moore H et al (2020) Patterns of niche contraction identify vital refuge areas for declining mammals. Divers Distrib 26:1467–1482. https://doi.org/10.1111/ddi.13145
Warmerdam F (2008) The geospatial data abstraction library. Open source approaches in spatial data handling. Springer, pp 87–104
Wellmann T, Lausch A, Scheuer S, Haase D (2020) Earth observation based indication for avian species distribution models using the spectral trait concept and machine learning in an urban setting. Ecol Indic 111:106029. https://doi.org/10.1016/j.ecolind.2019.106029
Wenzel A, Grass I, Belavadi VV, Tscharntke T (2020) How urbanization is driving pollinator diversity and pollination – A systematic review. Biol Conserv 241:108321. https://doi.org/10.1016/j.biocon.2019.108321
Wickham H, Chang W, Wickham MH(2016) Package ‘ggplot2’Create Elegant Data Visualisations Using the Grammar of Graphics; Version 2. 2016
Williams K, Hobbs RJ, Hamburg SP (1987) Invasion of an annual grassland in Northern California by Baccharis pilularis ssp. consanguinea. Oecologia 72:461–465
Yan D, Mills JG, Gellie NJC et al (2018) High-throughput eDNA monitoring of fungi to track functional recovery in ecological restoration. Biol Conserv 217:113–120
Yonezawa S, Ushio M, Yamanaka H et al (2020) Environmental DNA metabarcoding reveals the presence of a small, quick-moving, nocturnal water shrew in a forest stream. Conserv Genet 21:1079–1084
Young DA(1972) The reproductive biology of Rhus integrifolia and Rhus ovata (Anacardiaceae). Evolution (N Y) 406–414
Zhang Y, Wu L, Ren H et al (2020) Mapping Water Quality Parameters in Urban Rivers from Hyperspectral Images Using a New Self-Adapting Selection of Multiple Artificial Neural Networks. Remote Sens 12:336. https://doi.org/10.3390/rs12020336
Zurell D, Pollock LJ, Thuiller W (2018) Do joint species distribution models reliably detect interspecific interactions from co-occurrence data in homogenous environments? Ecography (Cop) 41:1812–1819
Acknowledgements
We would like to acknowledge Michelle Barton, Dr. Travis Longcore, Dr. Rachel Meyer, and Dr. Sabrina Drill for their insights in landscape ecology used in this project.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contribution to the original draft, as well as subsequent edits. Conceptualization of this project was performed by Ariel Levi Simons. Methodology was performed by Ariel Levi Simons, Jose Gallegos, and Michael Gatheru. Software development was performed by Ariel Levi Simons, Laura Riccardelli, and Jose Gallegos. Validation was performed by Valeria Viera. Data curation was performed by Michelle Fu. Supervision was performed by Stevie Caldwell. Visualization was performed by Nhi Truong.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This orginal online version was revised:
The author requested to update figure as figure 1 published was out of date
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
SIMONS, A.L., CALDWELL, S., FU, M. et al. Constructing ecological indices for urban environments using species distribution models. Urban Ecosyst 25, 1745–1764 (2022). https://doi.org/10.1007/s11252-022-01265-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-022-01265-0