Abstract
‘Wildlife-friendly’ gardening is a dominant theme in the media that readily engages public attention. However, there is little empirical evidence of the ecological benefits of increased habitat quality of individual domestic gardens. This study uses light-trapping to examine the response of moth assemblages to domestic gardens that are assessed in terms of their habitat complexity (simple and complex) both within the garden and extending out to a 30 m radius that includes surrounding habitats. The results clearly show that moth assemblages were influenced by complex habitats (particularly increasing levels of the variable shrubs and decreasing levels of artificial surfaces), but only at a scale that extended beyond the garden boundary to include the surrounding area. In other words, neither the complexity of the habitat within the garden or the size of the garden had any influence on the abundance or diversity of the moth assemblage. These results have implications for both garden management and landscape planning – if domestic gardens are to be a useful component of strategies to reduce biodiversity loss within the urban environment then they should provide good habitat quality and be managed as a network of interconnected patches rather than as individual units.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat plasticity in urban areas has been shown to provide a unique opportunity to develop urban green spaces as ecological biodiversity refuges that are under threat elsewhere (e.g. Pickett et al. 2001; Breuste 2004; Snep et al. 2005; Parsons et al. 2006). Smaller green spaces such as urban domestic gardens remain one of the least studied components of the urban green environment (Cameron et al. 2012) despite their potential to act as biodiversity refuges, increase matrix permeability as wildlife corridors and ‘stepping stones’ (Gaston et al. 2005; Goddard et al. 2010; Owen 2010), and to provide supplementary habitats for urban wildlife (Davies et al. 2009).
Domestic gardens can account for a substantial amount of the green spaces found in urban areas. In the UK, for example, 24% of London’s total land area is composed of domestic gardens (Smith 2010) and 87% of all UK households have access to a garden (Gibbons et al. 2011). Similarly, 25% of Dublin City and 50% of Dunedin, New Zealand, is composed of domestic gardens (Dublin City Council 2015; Mathieu et al. 2007). As such, the contribution of domestic gardens to the ecological value of urban environments should not be neglected. However, the on-going debate regarding the inherent biodiversity value of domestic gardens remains polarised into those that believe individual gardens are too small to be considered biologically significant, and those that consider their collective area is too large to be ignored (Goddard et al. 2010). Much of the debate is fuelled by the public perception of ‘wildlife-friendly’ gardening (e.g. Baines 2000; Packham 2001; Harris 2002; Gaston et al. 2007) that is actively encouraged by various non-governmental organizations.
The potential of domestic gardens to support a variety of different taxa has been demonstrated previously (e.g. Davies et al. 2009; Vergnes et al. 2012), with a focus on insect pollinators (Baldock et al. 2015; Levé et al. 2019), specifically diurnal Lepidoptera (butterflies) (Di Mauro et al. 2007; Fontaine et al. 2016; Toms et al. 2010), but the underlying mechanisms that influence garden biodiversity have not been clearly identified (Smith et al. 2006a and 2006b; Gaublomme et al. 2008; Prevedello and Vieira 2010; Lizée et al. 2011). Whilst the conservation potential of domestic gardens is hindered by a lack of ecological research (Gaston et al. 2005; Goddard et al. 2010), it is has been shown that habitat quality and urban greenspace interactions at differing scales are important factors in urban ecological systems. As examples, biodiversity in urban green spaces can be increased with the simple addition of more understorey vegetation (Threlfall et al. 2017); the presence of domestic gardens adjacent to urban parks has a positive influence on the species richness of birds (Chamberlain et al. 2004); and citizen science data suggests that pollinator richness in urban environments benefits from close proximity to domestic gardens (Levé et al. 2019). Clearly there are important implications for urban planning – maximising total patch area and minimising isolation of domestic gardens and other urban green spaces will result in benefits to urban biodiversity.
A major issue with domestic garden research is one of ‘scale mismatches’ (Borgstrom et al. 2006) where the scale of management practice within the garden does not match the scale of ecological patterns and processes. This is particularly relevant when studying taxa such as Lepidoptera that vary in their dispersal ability and habitat fidelity (Greenleaf et al. 2007; Hostetler 2001; Lizée et al. 2011). In addition, linkages between individual gardens within the urban greenspace remain unclear, and this can hinder their conservation potential. Indeed, the current emphasis on wildlife-friendly gardening often overlooks the potentially important collaborative aspect that might be required for gardens to provide the necessary habitat to support biodiversity.
Urban domestic gardens have been shown to have positive impacts on Lepidoptera assemblages (e.g. Di Mauro et al. 2007; Fontaine et al. 2016; Toms et al. 2010), although noctural moths are largely overlooked when assessing insect assemblages in urban areas, despite the potential for rigorous, standardised sampling protocols with light trapping (e.g. Bates et al. 2013; Merckx and Slade 2014). Moths are a key component of urban ecosystems, being important pollinators (Macgregor et al. 2019), herbivores and also as food for higher trophic levels. Their short generation time, their high habitat specificity and their mobility (Jones 2014) result in rapid responses to environmental changes (Groenendijk and Ellis 2011), and variation in the structure of species-rich moth assemblages can be easily linked to habitat and landscape changes in vegetation induced by human development (Buse et al. 1999; Ricketts et al. 2001; Visser et al. 2006). Consequently, their abundance and diversity can be used to assess the potential of different habitats to support biodiversity within domestic gardens.
Although domestic gardens differ considerably in terms of planting and floral diversity, the overall vegetation structure (or habitat complexity) within a garden has been shown to be a useful indicator of habitat quality. The diversity of various taxa responds positively to increasing habitat complexity (such as layered vegetation composed of trees and shrubs), whilst being negatively affected by simple structures (such as lawns or artificial surfaces; e.g., Beninde et al. 2015; Dylewski et al. 2019).
The aim of this study was to investigate the ecological benefit of domestic gardens through the response of nocturnal moth assemblages (Insecta: Lepidoptera) to habitat complexity both within and surrounding the garden, with the specific objective of providing empirical evidence that supports conservation initiatives that seek to harmonise the co-operative management actions of householders and communities. Moth assemblages in gardens with simple habitat (consisting mainly of lawns and artificial surfaces) and with complex habitat (containing a large proportion of shrubs, trees and layered vegetation) were assessed using light trapping to address the following linked hypotheses: (i) gardens with complex habitat support a more diverse moth assemblage than gardens with simple habitat; and (ii) the habitat surrounding a garden has a greater influence on the moth assemblage than the habitat within the garden.
Materials and methods
Study area description and site selection
Twelve domestic gardens were selected as study sites (using a convenience sampling method; Etikan et al. 2016) within the administrative region of Dún Laoghaire-Rathdown (53.3° N, 6.2° W; area 127.31km2, population approximately 218,018) in County Dublin, Ireland (Fig. 1(a) and (b)). The garden study sites had an average area of 364m2 (± 182m2 s.e.) and ranged from 15 to 2082 m2. The mean distance between gardens was 4.7 km (± 0.31 km s.e.). Although the dispersal abilities of some moth taxa (such as noctuids; Jones 2014) is greater than the distance between each site, previous studies have demonstrated that recaptures are extremely rare when moths are released 25 m from a light trap (Jones 2014; Truxa and Fiedler 2012) suggesting limited dispersal ability between the sampled habitats. In addition, habitat fragmentation and topographic barriers (e.g. buildings, unsuitable habitat, grey infrastructure and light pollution) between sites further strengthens their independence.
(a) Location of the Dún Laoghaire-Rathdown County Council area (DLRCC) within the Dublin Region on Ireland’s east coast. (b) Map of the DLRCC area showing the location of the twelve private gardens study sites (indicated by white dots). (c) Example of how spatial scale was defined at garden site 9; smaller scale ‘within garden’ denoted by garden boundaries (white line) and larger scale ‘outside garden’ defined by a circle of 30 m radius (red line) with light trap location as centre of the circle (red dot). (d) Example of a garden classified as ‘simple’ based on habitat variables at smaller spatial scale. (e) Example of a garden classified as ‘complex’ based on habitat variables at smaller spatial scale
Habitat and vegetation surveys
At each study site (i.e. an individual urban domestic garden), habitat complexity and vegetation composition were measured at two scales to understand the influence of surrounding habitat on the biodiversity of the domestic garden. The large-scale ‘outside garden’ survey was defined by a circular area of 30 m radius that included the garden site but extended beyond its borders (total area = 2827 m2, which exceeded the area of the largest garden site), with the position of a light trap within the garden as the centre of the circle. The small-scale ‘within garden’ habitat survey was defined by the garden boundaries (Fig. 1(c)). The percentage cover of five habitat variables (artificial surfaces, grass, shrub, tree and layered vegetation, the latter defined as multiple understorey plant species of >0.5 m height growing in the same area) was measured to assess habitat complexity. Within the habitat variables, seventeen vegetation types were identified using codes modified from Fossitt (2000) to assess vegetation composition (habitat variables and vegetation types are henceforth indicated by italics; see supplementary material S1 for full list).
Moth sampling protocol
Moth assemblages within each garden were sampled from dusk to dawn using a 12-V portable Heath Trap with a 15 W actinic bulb (Anglian Lepidopterist Supplies; www.angleps.com). This specific trap was chosen because of the low attraction radius that monitors moth assemblages within the selected habitat (Jones 2014; Merckx and Slade 2014). The sampling period was divided into two periods in the summer of 2017; June–July and September–October. Each sampling period consisted of five consecutive weeks with a gap of four weeks in between. Each garden site was sampled once a week (ten times in total) with at least four days between consecutive samples to avoid recaptures of the same individual, resulting in a total of 120 single light trapping events. The location of the light trap was fixed at a central point in each garden to standardise the area illuminated by the trap and to minimise the effects of shelter or windbreaks. The traps were placed before dusk and collected at dawn to avoid predation and escapees (Bates et al. 2013). The majority of moths within each trap were identified to species level (including the so-called ‘micro-moths’ since these species are less mobile and therefore appropriate for detecting treatment effects) apart from those species requiring genital dissection for accurate identification, which were aggregated following standard practice (see species list in supplementary material S3). The total number of each species/group was recorded.
Data analysis
Habitat data was inputted into Quantum GIS version 2.18.9 (QGIS Developmental Team 2017) to classify each garden as either simple or complex based on habitat complexity at the ‘within-’ and ‘outside garden’ scale, as determined by non-metric multidimensional scaling (NMDS) using Euclidian distance in CANOCO (version 5; ter Braak and Smilauer 2012). A hierarchical decision-making method was also employed to classify the sites as simple and complex based on their habitat complexity, with thresholds generated based on garden habitat complexity classifications previously described in Mathieu et al. (2007). The percentage of the surface area cover (m2) of each habitat variable at the two spatial scales (see supplementary material, S2) were used to classify sites. Habitat variables were grouped based on how they add structure to the vegetation, e.g. short cut lawns and artificial surfaces were grouped together since the contribution to habitat complexity or vegetation structure is the same, i.e. they have a poor structure (see Dylewski et al. 2019).
Mao Tau sample-based rarefaction curves were used to indicate whether sufficient sampling effort had been undertaken to accurately represent the assemblages of moth present at each site using PAST 3 software version 3.16 (Hammer et al. 2001). Diversity indices were used to describe the assemblage structure and highlight any differences between sites. The total number of moth species (S) and total abundance (a), Shannon-Wiener index (H′), Simpson’s index (λ) and Buzas and Gibson’s Evenness index (eH/S; Buzas and Gibson 1969) were calculated using PAST 3 and differences between simple and complex garden habitats were examined using t-tests at both small ‘within garden’ and large ‘outside garden’ spatial scales.
To visualise trends in moth diversity and abundance patterns across the twelve garden sites based on habitat classification, the diversity and abundance was plotted with ggplot2 package (Wickham 2009) in R version 3.5.1 (The R Core Team 2018). Generalised linear models (GLMs) were constructed (using negative binomial distribution assumptions) to demonstrate the effect of habitat complexity (and garden size as a covariate) at the two spatial scales.
The habitat variables and vegetation type were initially compared using Pearson’s correlation. Any two variables with a correlation coefficient > 0.7 were deemed highly correlated (Garden et al. 2007) and one of the variables was removed from the data set. The remaining habitat variables were used for statistical analysis. The assemblage response to both habitat variables and vegetation type was investigated using redundancy analysis (RDA) in CANOCO with forward stepwise variable selection (based on Akaike information criterion, AIC) using only variables that explained a significant (p < 0.05) proportion of the variation. Species abundance (aggregated by site, i.e. total moths sampled over ten weeks) was log (Y + 1) transformed to reduce the impact of dominant groups and to avoid log(0). Singleton species were excluded prior to analysis as RDA multivariate analysis is not well equipped to interpret a large number of single occurrences. Their addition can cause the model to be overfitted rendering the model unreliable (ter Braak and Smilauer 2012).
Results
Dataset description
A total of 1154 individuals belonging to 130 species of moth were caught and identified, with the majority belonging to the families Noctuidae (38 species; 29% of total number of individuals), Geometridae (37 species; 28% of total) and Tortricidae (20 species, 15% of total). Most species were rare with singletons accounting for 39% of species caught, and species occurring with an abundance of three or less individuals made up 56% of the total species caught (full species list in supplementary material S3).
Classifying sites
Initial habitat classifications of the study sites into simple and complex by NMDS (Fig. 2) were confirmed by hierarchical decision-making to generate five sites classified as simple and seven as complex at the ‘within garden’ scale, and six sites classified as simple and six as complex at the ‘outside garden’ scale. An examination of an outlier revealed a large area of complex habitat immediately outside the study area. This site was consequently removed from the analysis of diversity indices and models of the ‘outside garden’ dataset (see discussion for further exploration of this outlier).
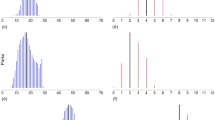
Categorisation of domestic garden sites in suburban Dublin using non-metric multidimensional biplots based on (a) ‘within garden’ habitat variables and (b) ‘outside garden’ habitat variables. Numbers refer to individual garden sites. Triangles, complex gardens; squares, simple gardens. Abbreviations: Art, artificial surfaces; Gra, grass; Shr, shrub; Tre, trees; Lay, layered vegetation
Sampling effort
Sample rarefaction curves revealed that in both five-week sampling periods (June–July and September–October), the sampling effort successfully captured the majority of the moths at each site (65–95%; see supplementary material, S4).
Moth assemblage structure and habitat complexity
The biodiversity metrics did not vary significantly between simple and complex garden sites when considered within the garden boundary (i.e. at the ‘within garden’ spatial scale; p > 0.05; Table 1). However, when garden sites were categorised as simple and complex based on an area that includes habitat complexity immediately surrounding the garden (the ‘outside garden’ scale), there was an increase in species richness (S), total abundance (a) and Shannon-Weiner index (H’) in complex gardens compared to simple gardens (Table 1).
The influence of habitat complexity and garden size on moth assemblages
Further support for a lack of response to habitat complexity at the ‘within garden’ spatial scale was provided by GLMs that indicated no difference between moth abundance or diversity (p = 0.63, p = 0.73 respectively) (Fig. 3(a) and (b); Table 2). However, when sites were classified based on the larger area that included surrounding habitats, complex sites had significantly higher moth abundance and diversity (both p-values <0.01) (Fig. 3(c) and (d); Table 2). There was no interactive effect of garden size on moth abundance or species richness at either spatial scale (p > 0.05; Table 2).
Moth abundance aggregated by garden site (grey dots) and grouped by habitat complexity based on habitat variables at (a) ‘within garden’ spatial scale and (b) ‘outside garden’ spatial scale. Moth species richness (number of species) aggregated by garden site (grey dots) and grouped by habitat complexity based on habitat variables at (c) within garden spatial scale and (d) outside garden spatial scale. Red dots denote mean values, error bars show standard error
Assemblage response to habitat and vegetation
When analysed at a scale within the garden boundary all habitat variables were correlated (with a Pearson’s correlation coefficient P of >0.7), and the single variable tree was selected as the explanatory variable since it accounted for the most variation in the moth assemblages by forward-stepwise RDA (see Fig. 4(a)). At the larger ‘outside garden’ scale that included habitat surrounding the garden, shrub was the only variable that was not highly correlated (P < 0.7). Similarly, when analysing the response of the moth assemblage to vegetation type, five vegetation categories were not highly correlated (P < 0.7) and therefore were adopted for use in the RDA (correlated variables for habitat and vegetation types are shown in supplementary material S5).
The response of moth assemblages in domestic gardens to habitat variables (panels a and b) and vegetation type (panels c and d), analysed using redundancy analysis (RDA) of log-transformed moth taxa (with singletons removed). (a) At the ‘within garden’ spatial scale, the habitat variable trees accounts for 15.8% of the total variance. (b) At the ‘outside garden’ spatial scale, the habitat variable shrub accounts for 18.3% of the total variance. (c) At the ‘within garden’ spatial scale, the vegetation types built land (BL1), fruit trees (WF1) and light-blocking structures (LB1) account for 43.7% of the total variance. (d) At the ‘outside garden’ spatial scale, the vegetation types unkempt shrubs (WS2) and built land (BL1) account for 30.2% of the variance (see text for detailed breakdown of variance). Numbers correspond to individual moth species as indicated in species lists in supplementary material, S3 and S4)
At the small spatial scale within the garden boundary, the percentage cover of the habitat classified as tree explained 15.8% of the variability in moth assemblage response (Fig. 4(a); pseudo F = 1.9, p < 0.05), although there was no clear direction to the assemblage response. When the gardens were classified based on the ‘outside garden’ scale (i.e. habitat complexity within and surrounding the garden), the percentage cover of the habitat classified as shrub accounted for 18.3% of the variation (Fig. 4(b); pseudo F = 2.2, p < 0.01) with all but one of the moth species responding positively to increasing area of shrub.
The assemblage response to vegetation type within the garden boundary (Fig. 4(c)) indicated that three explanatory variables accounted for 43.7% of the total variation: fruit trees (WF1: 16.2%; pseudo F = 1.9, p = 0.01), light blocking structures (LB1: 15.5%; pseudo F = 2.0, p = 0.01) and built land (BL1: 12%; pseudo F = 1.7, p = 0.03). However, there was no clear direction in the response to any one of the explanatory variables at this scale suggesting either a non-linear relationship or more likely that interaction effects between the explanatory variables could be shaping the moth assemblage. When gardens were classified based on the habitat complexity within and surrounding the garden the assemblage response to vegetation type was driven by two explanatory variables accounting for 30.2% of the observed variation (Fig. 4(d)); built land (BL1: 16.8%; pseudo F = 2.0, p = 0.01) and unkempt shrub (WS2: 13.4%; pseudo F = 1.7, p = 0.03). At this scale, the majority of the moth assemblage was positively associated with increasing percentage cover of unkempt shrub, and all but one of the remaining species responded to decreasing percentage cover of built land.
Discussion
The argument for ‘wildlife-friendly’ gardening is both persuasive and pervasive, yet empirical studies that directly address the ecological benefits of increased habitat structure within and around a domestic garden are scarce (Cabral et al. 2017; Gaston et al. 2005; Tresch et al. 2019) The data presented here suggest that moth assemblages are not influenced by either garden size or habitat quality within individual urban domestic gardens. The issue is one of scale, and the evidence is three-fold. First, habitat complexity (defined here as either simple or complex) only has an impact on the abundance and diversity of moth species when considered in an area that includes the garden and its immediate surrounding habitat (Table 1). Second, modelling approaches indicated that moth diversity and abundance were only impacted by habitat complexity at a scale that included the surrounding habitat (Fig. 3, Table 2), with no response within the garden. Finally, responses within gardens were difficult to interpret (such as the positive response of the moth assemblage to built structures in Fig. 4(c)), or spread across a variety of variables with no clear direction. Taken together, the moth assemblages in this study were influenced by habitat complexity at a scale that includes the garden habitat but crucially extends beyond its borders.
Moth assemblage structure and implications for garden management
The moths recorded at each garden site were dominated by a few species, irrespective of habitat complexity, reflecting the abundance of generalist species (accounting for approximately 84% of the species recorded based on larval food plant preferences; (Parson et al. 2012; Waring and Townsend 2017; supplementary material, S3) that are adapted to urban environments (McIntyre 2000; McIntyre et al. 2001). The relatively homogenous urban habitat does not support a diverse assemblage of specialist moth species because it cannot provide the variety of plant species found in natural habitats (Davey et al. 2012). Nevertheless, despite lower overall diversity compared to natural habitats, the moth assemblages in the present study responded positively to increasing habitat complexity, as has been observed previously in moths and other taxa (e.g. McIntyre 2000; McIntyre et al. 2001; White et al. 2005; Palomino and Carrascal 2006; Smith et al. 2006a, b; Kadlec et al. 2008).
The current trend for ‘wildlife-friendly’ gardening, as encouraged by organisations in the UK such as The Royal Society for the Protection of Birds and Woodland Trust (see ‘Giving Nature a Home in Your Garden’ https://www.rspb.org.uk, and ‘11 Essentials for the Perfect Wildlife Garden’, https://www.woodlandtrust.org.uk, respectively) is often criticised for a lack of empirical evidence (Gaston et al. 2005). In the current study, moth assemblages were positively associated with increasing areas of shrub (Fig. 4) and unkempt shrubs (Fig. 4(d)) and showed an overall negative association to increasing cover of built land and artificial surfaces (Fig. 4(d)), supporting the widely-documented observation that moth communities decline with increasing urbanisation (Bates et al. 2014; Rosch and McGeoch 2001). There is undoubtedly a role for wildlife-friendly gardening in promoting the urban biodiversity of moth and other invertebrate communities, but the importance of scale in garden management should not be overlooked – a domestic garden oasis in a desert of urban homogeneity will not benefit biodiversity, particularly for organisms with limited dispersal ability (Thomas et al. 2001; Vergnes et al. 2012). As a consequence, gardeners should be encouraged to collaborate with their neighbours to create a community of wildlife-friendly gardens that are managed together as an interconnected network of patches acting at multiple spatial scales across the urban landscape, with an ethos that favours shrubs and wild patches over pristine lawns and patios.
In order to truly inform management practices and optimise the urban garden’s ability to support rich moth assemblages future research needs to specifically address and quantify 1) the limitations of light trapping protocols in urban areas, 2) the exact scale at which gardens should be managed and 3) the effect garden size has on moth assemblages. These are addressed in turn below.
Using a light trapping protocol within highly urban areas can be affected by the light blocking structures surrounding it (and various other things such as artificial lighting (Macgregor et al. 2015)). Light blocking structure such as high walls and houses can interfere with the perception of the light emitted from the trap. Although this was not directly addressed in this study, it was found that one of the sites, which had relatively low species richness and abundance (25 and 39 respectively), was classified as complex. This garden in question was surrounded by high concrete walls and terrace houses, it is probable that the light trap under performed. There is clearly a need for research to explicitly examine the limitations of light trapping protocols in urban areas (see Conway et al. (2014) for a forest habitat example of this issue).
The approach used in this study was unique in the fact that it used a relatively small-scale buffer surrounding the garden (30 m) and the habitat classification protocol focused on fine-scale measurements. Previous research testing the influence of surrounding habitat on pollinator assemblages within domestic gardens/ allotment gardens, begin at a scale much larger than the ‘outside garden’ scale looked at here (e.g. 300 m–1000 m (Bennett and Lovell 2019); 2 km (Quistberg et al. 2016)), it is also common that only course habitat variables extracted from GIS landcover maps are used to find correlates (e.g. garden size, total area of green space; Smith et al. (2006a, b)) which cannot yield the same structural data that enabled the classification of habitats as simple or complex. Whilst the results above highlight the potential of using habitat complexity at a 30 m scale as an effective measurement of habitat quality for moth assemblages, one site remained an outlier following the classification protocol. This site had one of the highest number of species and abundance (46 and 155 respectively) and yet, was classified as simple. Closer examination of this outlier revealed that there was a large area of complex habitat 22 m beyond the 30 m radius, this site was subsequently removed from the dataset as its inclusion deemed all results inconclusive (data not shown). This shows that the methods for classifying habitat as simple and complex were effective but there is a need for further study to be undertaken to examine habitat complexity at multiple scales to pinpoint the best scale at which gardens should be managed. Although a 30 m radius captured much of the variation in moth assemblages, there is still room for improvement.
Garden size was found to have no interactive effect on the moth diversity or abundance at the ‘within garden’ scale or the ‘outside garden’ scale. This result contradicts previous observations, most notably Bates et al. (2014) which shows garden size being a significant variable to explain moth diversity within gardens. As the influence of garden size was not a primary aim of the research presented here, there was not enough variation in size across the sites to definitively state that garden size had no effect. However, the indication that garden size in not influencing moth communities, coupled with the lack of previous studies specifically examining the effects garden size has on moths shows a clear need for further research on this topic.
Implications for landscape and urban planning
Urban planning is probably not an effective tool for the management of individual urban domestic gardens, but it could play an important role at the landscape level to enhance their biodiversity value. Researchers and planners have begun using landscape ecology principles to develop green space networks and increase connectivity to preserve and restore biodiversity in urban green spaces, although domestic gardens are still largely overlooked (Scott et al. 2017). Indeed, current practice when constructing new housing developments is to incorporate communal green areas (i.e. mown lawns with minimal ecological contribution; Dylewski et al. 2019), and either omit or minimise areas for domestic gardens (Cameron et al. 2012).
The ecological land-use complementation model described by Colding (2007) outlines how urban habitats could interact synergistically to support biodiversity when clustered together. This model has been previously tested in an urban context using habitat patch size and fragmentation (e.g. urban parks, green corridors and domestic gardens in Vergnes et al. (2012). The results presented here suggest that managing domestic gardens and other green spaces to maximise habitat patch quality (through wildlife-friendly gardening) and optimising connectivity to minimise isolation (through effective urban planning) could result in tangible benefits to urban biodiversity.
Urban domestic gardens can be incorporated into landscape and urban planning initiatives both through a strategic planning approach and an opportunistic approach (Ahern 2007). Ideally a strategic planning approach would be proactive, with a prior vision of the ecological green space established before development occurs (such as the Green Heart of Holland; (Koomen et al. 2008). In the current context, planners should design garden configurations first and then subsequently incorporate residential areas. More realistically, an opportunistic approach may be necessary which identifies pre-existing configurations that represent special opportunities for sustainable landscape planning, such as the urban habitat ‘zonation’ conservation planning tool for protection of threatened species in Melbourne, Australia (Gordon et al. 2009). The individual components, in this case domestic gardens, may or may not be optimally located, but they represent the potential to provide a desired function such as promoting biodiversity. A fundamental challenge and impediment to applying landscape ecology-based principles is the common lack of empirical evidence of the effectiveness of a given intervention in a specific location. As a consequence, successful planning interventions require an adaptive approach with effective long-term monitoring (Ahern 2007).
Conclusion
The results presented here demonstrate the utility of moth assemblages for indicating the habitat quality of a specific patch of urban green space and their potential as a target group for monitoring biodiversity changes, and highlight the importance of placing the urban domestic garden within a landscape ecology framework. More broadly, realising the potential of domestic gardens for promoting biodiversity in an urban context requires a multidisciplinary effort involving ecologists, stakeholders, decision makers and planning and design professionals. Domestic gardens have largely been avoided in urban green infrastructure planning due to the perceived view that private ownership prevents intervention. Whilst this undoubtedly poses a logistic barrier, further work is required to clarify the ecological scale over which urban domestic gardens can make a difference, so that mitigating biodiversity loss in urban environments can move away from mere platitudes to a solid empirical evidence base. Urban domestic gardens are a vital component of the urban green environment and as such can no longer be ignored in future planning initiatives to promote urban biodiversity.
References
Ahern J (2007) Green infrastructure for cities: the spatial dimension. IWA, London
Baines, C. (2000). How to Make a Wildlife Garden. London
Baldock, K. C. R., Goddard, M. A., Hicks, D. M., Kunin, W. E., Mitschunas, N., Osgathorpe, L. M., … Memmott, J. (2015). Where is the UK’s pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. Biol. Sci., 282(1803), 20142849. https://doi.org/10.1098/rspb.2014.2849
Bates AJ, Sadler JP, Everett G, Grundy D, Lowe N, Davis G, Baker D, Bridge M, Clifton J, Freestone R, Gardner D, Gibson C, Hemming R, Howarth S, Orridge S, Shaw M, Tams T, Young H (2013) Assessing the value of the Garden moth scheme citizen science dataset: how does light trap type affect catch? Entomologia Experimentalis et Applicata 146(3):386–397. https://doi.org/10.1111/eea.12038
Bates AJ, Sadler JP, Grundy D, Lowe N, Davis G, Baker D, Bridge M, Freestone R, Gardner D, Gibson C, Hemming R, Howarth S, Orridge S, Shaw M, Tams T, Young H (2014) Garden and landscape-scale correlates of moths of differing conservation status: significant effects of urbanization and habitat diversity. PLoS One 9(1):e86925. https://doi.org/10.1371/journal.pone.0086925
Beninde J, Veith M, Hochkirch A (2015) Biodiversity in cities needs space: a meta-analysis of factors determining intra-urban biodiversity variation. Ecol Lett 18(6):581–592. https://doi.org/10.1111/ele.12427
Bennett AB, Lovell S (2019) Landscape and local site variables differentially influence pollinators and pollination services in urban agricultural sites. PLoS One 14(2):e0212034. https://doi.org/10.1371/journal.pone.0212034
Borgstrom ST, Elmqvist T, Angelstam P, Alfsen-Norodom C (2006) Scale mismatches in Management of Urban Landscapes. Ecol Soc 11(2)
Breuste JH (2004) Decision making, planning and design for the conservation of indigenous vegetation within urban development. Landsc Urban Plan 68:439–452. https://doi.org/10.1016/S0169-2046(03)00150-6
Buse A, Dury SJ, Woodburn RJW, Perrins CM, Good JEG (1999) Introduction effects of elevated temperature on multi-species interactions: the case of Pedunculate oak, winter moth and tits. Funct Ecol 13:74–82. https://doi.org/10.1046/j.1365-2435.1999.00010.x
Buzas MA, Gibson TG (1969) Species diversity: benthonic foraminifera in western North Atlantic. Science 163:72–75
Cabral I, Keim J, Engelmann R, Kraemer R, Siebert J, Bonn A (2017) Ecosystem services of allotment and community gardens: a Leipzig, Germany case study. Urban For Urban Green 23:44–53. https://doi.org/10.1016/J.UFUG.2017.02.008
Cameron RWF, Blanuša T, Taylor JE, Salisbury A, Halstead AJ, Henricot B, Thompson K (2012) The domestic garden – its contribution to urban green infrastructure. Urban For Urban Green 11:129–137. https://doi.org/10.1016/j.ufug.2012.01.002
Chamberlain DE, Cannon AR, Toms MP (2004) Associations of garden birds with gradients in garden habitat and local habitat. Ecography 27(5):589–600. https://doi.org/10.1111/j.0906-7590.2004.03984.x
Colding J (2007) ‘Ecological land-use complementation’ for building resilience in urban ecosystems. Landsc Urban Plan 81(1–2):46–55. https://doi.org/10.1016/J.LANDURBPLAN.2006.10.016
Conway, G. J., Plummer, K. E., Sadler, J. P., & Siriwardena, G. M. (2014). Appendix 2 the influence of light type and habitat barriers on moth flight-to-light responses
Davey CM, Chamberlain DE, Newson SE, Noble DG, Johnston A (2012) Rise of the generalists: evidence for climate driven homogenization in avian communities. Glob Ecol Biogeogr 21(5):568–578. https://doi.org/10.1111/j.1466-8238.2011.00693.x
Davies ZG, Fuller RA, Loram A, Irvine KN, Sims V, Gaston KJ (2009) A national scale inventory of resource provision for biodiversity within domestic gardens. Biol Conserv 142:761–771. https://doi.org/10.1016/j.biocon.2008.12.016
Di Mauro D, Dietz T, Rockwood L (2007) Determining the effect of urbanization on generalist butterfly species diversity in butterfly gardens. Urban Ecosyst 10(4):427–439. https://doi.org/10.1007/s11252-007-0039-2
Dublin City Council. (2015). Dublin City biodiversity action plan 2015–2020. Dublin
Dylewski Ł, Maćkowiak Ł, Banaszak-Cibicka W (2019) Are all urban green spaces a favourable habitat for pollinator communities? Bees, butterflies and hoverflies in different urban green areas. Ecological Entomology 44(5):678–689. https://doi.org/10.1111/een.12744
Etikan I, Musa SA, Alkassim RS (2016) Comparison of convenience sampling and purposive sampling. Am J Theor Appl Stat 5(1):1–4
Fontaine B, Bergerot B, Le Viol I, Julliard R (2016) Impact of urbanization and gardening practices on common butterfly communities in France. Ecol Evol 6(22):8174–8180. https://doi.org/10.1002/ece3.2526
Fossitt JA (2000) A guide to habitats in Ireland. Kilkenny Heritage Council, Kilkenny
Garden JG, McAlpine CA, Possingham HP, Jones DN (2007) Habitat structure is more important than vegetation composition for local-level management of native terrestrial reptile and small mammal species living in urban remnants: a case study from Brisbane, Australia. Austral Ecol 32(6):669–685. https://doi.org/10.1111/j.1442-9993.2007.01750.x
Gaston KJ, Ae RAF, Loram A, Charlotte AE, Ae M, Ae SP et al (2007) Urban domestic gardens (XI): variation in urban wildlife gardening in the United Kingdom. Biodivers Conserv 16:3227–3238. https://doi.org/10.1007/s10531-007-9174-6
Gaston KJ, Smith RM, Thompson K, Warren PH (2005) Urban domestic gardens (II): experimental tests of methods for increasing biodiversity. Biodivers Conserv 14(2):395–413
Gaublomme, E., Hendrickx, F., Dhuyvetter, H., & Desender, K. (2008). The effects of forest patch size and matrix type on changes in carabid beetle assemblages in an urbanized landscape. https://doi.org/10.1016/j.biocon.2008.07.022
Gibbons, S., Mourato, S., & Guilherme, R. (2011). The amenity value of English nature: a hedonic Price approach. Spatial Economics Research Centre (SERC), Discuss (74):
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25(2):90–98. https://doi.org/10.1016/J.TREE.2009.07.016
Gordon A, Simondson D, White M, Moilanen A, Bekessy SA (2009) Integrating conservation planning and landuse planning in urban landscapes. Landsc Urban Plan 91:183–194. https://doi.org/10.1016/j.landurbplan.2008.12.011
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationship to body size. Oecologia 153(3):589–596. https://doi.org/10.1007/s00442-007-0752-9
Groenendijk D, Ellis WN (2011) The state of the Dutch larger moth fauna. J Insect Conserv 15(1–2):95–101. https://doi.org/10.1007/s10841-010-9326-y
Hammer, O., Harper, D. A. T., & Ryan, P. D. (2001). PAST: paleontological statistics software package for education. University of Oslo: Natural History Museum
Harris, S. (2002). Mammals in your garden? London
Hostetler M (2001) The importance of multi-scale analyses in avian habitat selection studies in urban environments. In: Avian ecology and conservation in an urbanizing world. Springer US, Boston, MA, pp 139–154. https://doi.org/10.1007/978-1-4615-1531-9_7
Jones, H. B. C. (2014). Quantifying dispersal in British noctuid moths. University of York
Kadlec T, Benes J, Jarosik V, Konvicka M (2008) Revisiting urban refuges: changes of butterfly and burnet fauna in Prague reserves over three decades. Landsc Urban Plan 85(1):1–11. https://doi.org/10.1016/J.LANDURBPLAN.2007.07.007
Koomen E, Dekkers J, Van Dijk T (2008) Open-space preservation in the Netherlands: planning, practice and prospects. Land Use Policy 25(3):361–377
Levé M, Baudry E, Bessa-Gomes C (2019) Domestic gardens as favorable pollinator habitats in impervious landscapes. Sci Total Environ 647:420–430. https://doi.org/10.1016/j.scitotenv.2018.07.310
Lizée M-H, Manel S, Mauffrey J-F, Tatoni T, Deschamps-Cottin M (2011) Matrix configuration and patch isolation influences override the species-area relationship for urban butterfly communities. Lanscape Ecol 27(2):159–169. https://doi.org/10.1007/s10980-011-9651-x
Macgregor CJ, Kitson JJN, Fox R, Hahn C, Lunt DH, Pocock MJO, Evans DM (2019) Construction, validation, and application of nocturnal pollen transport networks in an agro-ecosystem: a comparison using light microscopy and DNA metabarcoding. Ecological Entomology 44(1):17–29. https://doi.org/10.1111/een.12674
Macgregor CJ, Pocock MJO, Fox R, Evans DM (2015) Pollination by nocturnal Lepidoptera, and the effects of light pollution: a review. Ecol Entomol Blackwell Publishing Ltd 40:187–198. https://doi.org/10.1111/een.12174
Mathieu R, Freeman C, Aryal J (2007) Mapping private gardens in urban areas using object-oriented techniques and very high-resolution satellite imagery. Landsc Urban Plan 81:179–192. https://doi.org/10.1016/j.landurbplan.2006.11.009
McIntyre, N E, Rango, J., Fagan, W. F., & Faeth, S. H. (2001). Ground arthropod community structure in a heterogeneous urban environment. Landsc Urban Plan, 52, 257–274
McIntyre NE (2000) Ecology of urban arthropods: a review and a call to action. Ann Entomol Soc Am 93(4):825–835. https://doi.org/10.1603/0013-8746(2000)093[0825:eouaar]2.0.co;2
Merckx T, Slade EM (2014) Macro-moth families differ in their attraction to light: implications for light-trap monitoring programmes. Insect Conserv Divers 7(5):453–461. https://doi.org/10.1111/icad.12068
Owen, J. (2010). Wildlife in a Garden: a thirty-year study. Royal Horticultural Society. London
Packham C (2001) Chris Packham’s Back Garden nature reserve, London
Palomino D, Carrascal LM (2006) Urban influence on birds at a regional scale: a case study with the avifauna of northern Madrid province. Landsc Urban Plan 77:276–290. https://doi.org/10.1016/j.landurbplan.2005.04.003
Parson, M., Sterlnig, P., & Lewington, R. (2012). Field Guide to the Micro- moths of Great Britain and Ireland. (P. Sterling, Ed.). London: Bloomsbury Natural History
Parsons H, Major RE, French K (2006) Species interactions and habitat associations of birds inhabiting urban areas of Sydney, Australia. Austral Ecology 31(2):217–227. https://doi.org/10.1111/j.1442-9993.2006.01584.x
Pickett STA, Cadenasso ML, Grove JM, Nilon CH, Pouyat RV, Zipperer WC, Costanza R (2001) Urban ecological systems: linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu Rev Ecol Evol Syst 32:127–157
Prevedello JA, Vieira MV (2010) Does the type of matrix matter? A quantitative review of the evidence. Biodivers Conserv 19:1205–1223. https://doi.org/10.1007/s10531-009-9750-z
QGIS Developmental Team. (2017). QGIS geographic information system. Geospatial Foundation Project
Quistberg RD, Bichier P, Philpott SM (2016) Community and ecosystem ecology landscape and local correlates of bee abundance and species richness in urban gardens. Community and Ecosystem Ecology, (45(3)), 592=601. https://doi.org/10.1093/ee/nvw025
Ricketts TH, Daily GC, Ehrlich PR, Fay JP (2001) Countryside biogeography of moths in a fragmented landscape: biodiversity in native and agricultural habitats. Conserv Biol 15(2):378–388
Rosch M, McGeoch MA (2001) African entomology - testing a bioindicator assemblage : gall-inhabiting moths and urbanization. African Entomol 9(1):85–94 Retrieved from https://journals.co.za/content/ento/9/1/EJC29844
Scott, A., Hölzinger, O., & Sadler, J. (2017). Making Plans for Green Infrastructure in England: Review of National Planning and Environmental Policies and Project Partners’ Plans. Retrieved from http://ncptool.com/Downloads/Making plans for green infrastructure in England 2017.Pdf
Smith, C. (2010). London: Garden City? Investigating the changing anatomy of London’s private gardens, and the scale of their loss. London
Smith RM, Gaston KJ, Warren PH, Thompson K (2006a) Urban domestic gardens (VIII) : environmental correlates of invertebrate abundance. Biodivers Conserv 15(8):2515–2545. https://doi.org/10.1007/s10531-005-2784-y
Smith RM, Warren PH, Thompson K, Gaston KJ (2006b) Urban domestic gardens (VI): environmental correlates of invertebrate species richness. Biodivers Conserv 15(8):2415–2438. https://doi.org/10.1007/s10531-004-5014-0
Snep, R. P. H., Opdam, P. F. M., Baveco, J. M., Wallisdevries, M. F., Timmermans, W., Kwak, R. G. M., & Kuypers, V. (2005). How peri-urban areas can strengthen animal populations within cities: a modeling approach. https://doi.org/10.1016/j.biocon.2005.06.034
ter Braak, C. J. ., & Smilauer, P. (2012). CANOCO reference manual and User’s guide to Canoco for windows: software for ordination. (version 5.0)
The R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS et al (2001) The quality and isolation of habitat patches both determine where butterlies persist in fragmented landscapes. https://doi.org/10.1098/rspb.2001.1693
Threlfall CG, Mata L, Mackie JA, Hahs AK, Stork NE, Williams NSG, Livesley SJ (2017) Increasing biodiversity in urban green spaces through simple vegetation interventions. J Appl Ecol 54(6):1874–1883. https://doi.org/10.1111/1365-2664.12876
Toms MP, Humphreys L, Kirkland P (2010) Monitoring butterflies within an urbanised landscape: the role of garden butterfly populations in a wider context. In: Warren M, Dover J (eds) The 2010 target and beyond for Lepidoptera. Butterfly Conservation Europe, Reading, pp 26–28
Tresch S, Frey D, Le Bayon RC, Mäder P, Stehle B, Fliessbach A, Moretti M (2019) Direct and indirect effects of urban gardening on aboveground and belowground diversity influencing soil multifunctionality. Sci Rep 9(1):1–13. https://doi.org/10.1038/s41598-019-46024-y
Truxa C, Fiedler K (2012) Attraction to light - from how far do moths (Lepidoptera) return to weak artificial sources of light? Eur J Entomol 109(1):77–84. https://doi.org/10.14411/eje.2012.010
Vergnes A, Le Viol I, Clergeau P (2012) Green corridors in urban landscapes affect the arthropod communities of domestic gardens. Biol Conserv 145:171–178. https://doi.org/10.1016/j.biocon.2011.11.002
Visser ME, Holleman LJM, Gienapp P (2006) Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147(1):164–172. https://doi.org/10.1007/s00442-005-0299-6
Waring, P., & Townsend, M. (2017). Field guide to the moths of Great Britain and Ireland (3rd ed.). London: Bloomsbury Natural History
White JG, Antos MJ, Fitzsimons JA, Palmer GC (2005) Non-uniform bird assemblages in urban environments: the influence of streetscape vegetation. Landsc Urban Plan 71(2–4):123–135. https://doi.org/10.1016/J.LANDURBPLAN.2004.02.006
Wickham, H. (2009). Ggplot2 : elegant graphics for data analysis. Springer
Acknowledgements
EEE would like to thank the numerous households who generously allowed access to their gardens, usually at unsocial hours, Moths Ireland (www.mothsireland.com) for assistance with species identification, and the UCD School of Biology and Environmental Science for the purchase of equipment. We would also like to thank all the reviewers for their constructive comments and feedback.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 2646 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ellis, E.E., Wilkinson, T.L. Moth assemblages within urban domestic gardens respond positively to habitat complexity, but only at a scale that extends beyond the garden boundary. Urban Ecosyst 24, 469–479 (2021). https://doi.org/10.1007/s11252-020-01050-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-01050-x