Abstract
Behavioural adaptations are recognized as crucial determinants for successful establishment and persistence of animal populations in a novel urban environment. Here, we examined mechanisms responsible for the development of urban behavioural type in a common waterbird, the Eurasian coot Fulica atra. We compared the behaviour of coots from a rural population and two urban populations that differed in the timing of colonization event (1960s vs. 2000s). We found that some behavioural characters associated with urban life (aggression during nest defence and boldness towards humans during foraging) were more strongly expressed in the older urban population when compared with the recently established urban population. By contrast, coots from the two urban populations showed a similar likelihood of exploiting human-derived food resources, as well as they showed similar levels of physiological stress. Urban coots were generally more aggressive, bolder, and less stressed than their rural conspecifics. Large behavioural and physiological divergence of coots from the recently established urban population and their rural conspecifics suggested that phenotypic plasticity and phenotype sorting may play a key role in the initial stages of urban colonization. On the other hand, increasing expression of boldness and aggression with the time since urbanization may suggest the role of microevolutionary adaptation in response to novel selective forces associated with the urban environment. Our results indicate that a combination of different processes (phenotypic plasticity, phenotype sorting, and microevolution) can determine successful colonization of urban areas by the Eurasian coots, and possibly other bird species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most natural environments are subject to sustained anthropogenic alterations. In some animal populations, the capacity of developmental, genetic, and demographic mechanisms can be insufficient to deal with this unusual rate of environment change (Chevin et al. 2010). Persistence of such populations might be threatened if the critical rate of environmental change is exceeded. On the other hand, some populations are able to effectively adapt to increasing anthropogenic pressure, but the mechanisms of these adaptations are far from being well understood (Lowry et al. 2013). First, a sorting process can be in operation, as only individuals with proper phenotypes might be able to successfully colonize novel environments (Sol et al. 2013). In this sense, urban invaders can be phenotypically or genetically pre-adapted for colonization of environments that remain under strong anthropogenic pressure (Carrete and Tella 2011), although it seems possible that some urban population can also be successfully established by a random set of individuals. Second, the environment can directly affect development of individual phenotypes via phenotypic plasticity. In fact, it is generally agreed that phenotypic plasticity plays a key role in the initial stages of urban colonization events, which require a rapid, but not-necessarily heritable adaptation (Sol et al. 2013; Tryjanowski et al. 2016). However, as an optimum phenotype changes with the environment, strong selection can result in a series of microevolutionary genetic adaptations (Miranda et al. 2013; Mueller et al. 2013). Heritable basis of adaptations to urban landscape would imply a gradual change in phenotype over longer period of time, likely resulting in a positive correlation between the expression of phenotypic trait and the time since urbanization (Møller and Ibáñez-Álamo 2012).
Behavioural adaptations are recognized as crucial determinants for successful establishment and persistence of populations in each novel environment, including an urbanized landscape (Yeh et al. 2007; Møller 2008; Newman et al. 2008; Atwell et al. 2012). Since different behavioural traits are likely to be inter-correlated and their expression is usually governed by similar physiological characters, an exposure to a novel environment may produce a novel suit of correlated behaviours, often referred to as a ‘behavioural syndrome’ (Sih et al. 2004). The most notable features of the ‘urban wildlife syndrome’ include elevated levels of boldness in risky situations, aggression towards humans and conspecifics, and more explorative behaviour (Evans et al. 2010). Consequently, urban-dwelling birds show reduced flight initiation distance (Carrete and Tella 2011; Møller et al. 2015) and different antipredator responses (Møller and Ibáñez-Álamo 2012), as well as they more eagerly exploit novel resources, such as human-derived food (Sol et al. 2013). Urban behavioural syndrome is also associated with attenuated stress response mediated by lower acute corticosterone levels, which allow urban birds to avoid detrimental consequences of chronic physiological stress (Partecke et al. 2006; Atwell et al. 2012).
The aim of this study was to investigate mechanisms responsible for the development of urban behavioural type in a common waterbird, the Eurasian coot Fulica atra. For this purpose we examined behaviour of coots from three populations in central Poland: an old urban population from Warszawa established probably in the 1960s or earlier (Luniak et al. 1964), a new urban population from Łódź established in the 2000s (Minias 2016), and a rural population. Expression of the following behavioural characters was assessed: aggression at the nest, exploitation of human-derived food resources, boldness towards humans during foraging, and stress responsiveness. In the previous work, we used population genetic methods to show that the new urban coot population from Łódź has been established via an influx of individuals from the nearby rural populations (Minias et al. 2017). Thus, here we hypothesized that any behavioural differences between coots from Łódź and adjacent wildland should be interpreted as an effect of phenotype sorting or phenotypic plasticity. On the other hand, any differences between coots from the old (Warszawa) and new (Łódź) urban population would indicate that the urban behavioural type develops with the time since urbanization, being consistent with the mechanism of microevolutionary adaptations.
Material and methods
Study area and populations
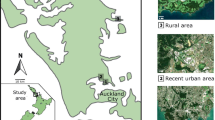
The study was conducted in 2016–2017 in three populations of the Eurasian coot from central Poland. The two urban populations markedly differed in the time since urbanization. The urban coot population from Warszawa (52° 16’ N, 21° 01′E) was probably among the first established in Poland. First observations of coots breeding the central parts of Warszawa date back to 1960s (Luniak et al. 1964) and by the end of 1980s the size of the urban population was estimated at 190–210 pairs (Luniak et al. 2001). Early colonization of this city could have been facilitated by the presence of a major river, Vistula, in the city centre. The second urban population from Łódź (51° 406’ N, 19° 28′E) was established several decades later. Here, the process of colonization probably started in the late 1990s or early 2000s. City-wide bird surveys in 1994–2002 revealed 19 breeding coot sites located in the outer zone of the city with low or moderate urbanization level (Janiszewski et al. 2009). The surveys were repeated in 2009–2014, providing evidence for nearly 15 new breeding sites of coots in the city centre (Minias 2016). The two cities, Warszawa and Łódź, are among the largest in Poland, both in terms of the area (517 and 293 km2, respectively) and the number of inhabitants (1,700,000 and 700,000, respectively). Data from urban populations were collected exclusively in the central parts of the cities characterized by compact settlement and strong anthropogenic pressure, mostly in urban parks. Peripheral breeding sites were not included in this study. Data were collected at 18 and 20 artificial water reservoirs in Warszawa and Łódź, respectively. Data from the rural population were collected at 11 water reservoirs from two nearby fishpond complexes in Sarnów (51° 51’ N, 19° 07′E) and Żeromin (51° 37’ N, 19° 37′E). Both rural sites were located on the private properties with restricted trespassing for unauthorized personnel, resulting in low anthropogenic pressure. Total surface area and the relative area of emergent vegetation (with respect to the total surface area) were measured for each urban and rural reservoir. We also collected data on the following nest-site characteristics: 1) distance from the nest to shore (± 0.5 m); 2) vegetation cover at the nest (distance from the nest to open water; ± 0.5 m); and 3) water depth at the nest (± 5 cm).
Behavioural observations
We performed two behavioural experiments on the Eurasian coots from all three populations. In the first experiment, we assessed components of aggressive nest defence behaviour by measuring the response of birds to the presence of a human intruder at the nest. During the incubation period, each nest was approached by a researcher with an upright posture and at a medium pace (following recommendations by Knight and Temple 1986). The researcher stayed for 5 min in a direct proximity of the nest (within 0.5 m) and observed birds with binoculars, if possible. Two components of behaviour were recorded for each pair: 1) occurrence of an active nest defence (threat posture, alarm vocalization, or splattering display) and 2) minimum approach distance (closest approach by any of mates to the human intruder). In territories with dense reed vegetation where no birds were observed at the nest-site during the experiment, the temperature of eggs was checked to assess whether the nest was left by a bird just prior to researcher approach. If eggs were warm, we assumed parental escape in response to the intruder and lack of active nest defence, otherwise, we excluded the nest from the experiment. The minimum approach distance for individuals that physically attacked the intruder with claws or bill was set to zero. Clutch size was recorded for each pair. Data on nest defence behaviour was collected for 42–49 pairs per population (139 pairs in total).
In the second experiment, we assessed the response of coot families to the intentional provisioning of food by humans. In general, waterfowl from our two study urban areas are mostly fed by people during winter, but anthropogenic feeding was also frequently recorded during the breeding season. No anthropogenic feeding was observed at the rural site. For the purpose of the experiment, we imitated the behaviour of people that fed waterbirds in urban parks. At the beginning of each trial, a researcher threw small pieces of bread into the water from the place at the shore that lacked emergent reed-like vegetation and was well visible for an experimental coot family. The initial distance from the researcher to birds ranged from 10 to 50 m. We first assessed whether birds responded to feeding with a directional approach towards the supplemented food. If no behavioural response was observed within 5 min, the trial was ended. If birds actively responded to feeding and approached, a researcher stopped throwing bread into the water and deployed small pieces of bread at the shore in a visible place within 0.5 m from the waterline. Behaviour of coots was observed at a distance of ca. 3 m from deployed food items. We assessed whether adults or chicks left the water and fed on the shore in the close presence of the researcher. We also assessed whether adults used alarm vocalization while watching their offspring feeding on bread. Age of chicks at the moment of experiment was assessed using a four-point scale based on the following criteria: 1) conspicuous downy red and orange feathers on head and breast, red bill; ca. 5–30% of adult size 2) entire body covered with greyish downy feathers, flight-feathers not yet growing, pinkish bill, ca. 30–60% of adult size; 3) entire body covered with grey structural fathers, flight-feathers growing, greyish bill, ca. 60–90% of adult size; 4) juvenile plumage fully developed, whitish bill, ca. 90–100% of adult size. Each of the first three stages lasts approximately 2–2.5 weeks and full juvenile plumage (stage 4) develops after ca. 1.5 months after hatching (PM, personal observ.). Fully developed juveniles usually remain under parental supervision for several weeks. Families with youngest chicks (stage 1) were excluded from the experiments, as small hatchlings are fed almost exclusively by parents, stay in the nest or its close vicinity, and usually avoid open water. Three-week chicks (stage 2) already feed on their own, although they can be still occasionally provisioned with food by parents (PM, personal observ.). All trials were conducted on week-days when public feeding of waterbirds was limited. Experiments were not conducted in rainy or foggy conditions, so that behavioural activity of birds was not restricted by inclement weather. In total, we performed 148 experiments (40–60 per population). Only one trial per breeding territory per year was conducted.
Stress responsiveness
The level of physiological stress was assessed with the heterophil/lymphocyte (H/L) ratio. In the environment that is perceived as stressful, the number of lymphocytes in peripheral circulating blood decreases, while the number of heterophils increases, resulting in an elevated H/L ratio (Davis et al. 2008). This, so called, white blood cell trafficking reflects physiological adaptation of an organism to an environment that has a higher risk of injury, for example via greater predator activity, and the magnitude of this response is mediated by glucocorticoids (Johnstone et al. 2012). Here, we assessed H/L ratios for 88 adult coots from the three studied populations (18–35 individuals per population). All coots were captured from late March to late December, although large majority of individuals (83%) were captured during the breeding season (from mid-April till the end of July). Birds were captured on nests or while feeding on the ground with noose traps made of monofilament nylon line. Upon capture, tarsus length was measured with callipers (± 0.1 mm) and used in the analyses as an indicator of the structural size of birds. The ulnar vein of each bird was punctured with a disposable needle. Ca. 50 μl of blood was preserved in 96% ethanol for molecular sexing and one drop of blood was transferred to a slide to prepare blood smears. Blood sampling was conducted within half an hour since capture to avoid any effect of acute handling stress on H/L ratio measurements (Davis 2005). DNA from samples stored in ethanol was extracted using GeneJet Genomic DNA Purification Kit (Thermo Fisher Scientific, Waltham, MA, USA) and the sex-specific chromohelicase-DNA-binding gene was amplified using a modified protocol by Griffiths et al. (1998), as described in Minias (2015). The PCR products were separated on 2% agarose gel and males were identified by one band only while females were identified by two bands. Blood smears were stained using the May-Grünewald-Giemsa method and scanned at 1000× magnification under a light microscope. A random sample of 100 white blood cells from each smear was counted and differentiated into five types: heterophils, lymphocytes, eosinophils, basophils, and monocytes. The H/L ratio was calculated by dividing the number of heterophils with the number of lymphocytes. To reduce variability, all blood smears were assessed by one of the authors (RW). Twenty two randomly chosen smears were assessed twice to estimate repeatability of H/L ratio measurements. As indicated by the intra-class correlation coefficient (ICC), measurement repeatability was high (ICC = 0.85, P < 0.001).
Statistical analyses
Between-population differences in the proportion of pairs that used active nest defence and responded to bread-feeding by humans were analysed with G tests. Differences in habitat characteristics (reservoir area and relative area of emergent vegetation) between populations were analysed with one-way ANOVA. Nest-site characteristics (vegetation cover at the nest, distance from the nest to shore, and water depth at the nest), minimum approach distance to a human intruder at the nest, and H/L ratios were analysed with the linear mixed-effects models. Since measurements of multiple nests or pairs from the same waterbodies (henceforth breeding sites) were non-independent, we entered breeding site as a random factor to avoid pseudoreplication in each model. We also included year as a second random factor and population as a fixed factor. Additionally, date and clutch size were entered as covariates in the analysis of minimum approach distance, while date of capture, tarsus size, and sex were entered as independent variables in the analysis of H/L ratios. The probability of bread-feeding on the shore in a close presence of humans and probability of parental alarms calls during bread-feeding were analysed with generalized linear mixed-effects models for binomial response variables. Breeding site and year were entered as random factors and population was entered as a fixed factor. Date, hour, and chick age were entered as covariates. All models were run with lmer and glmer functions in the lme4 package (Bates et al. 2015) developed for R statistical environment (R Development Core Team 2013). We used the car package (Fox and Weisberg 2011) to obtain Wald χ2 statistics (W) and P values for independent variables in all mixed models. Post-hoc comparisons were performed with the Tukey’s HSD test. All values are reported as means ± SE.
Results
Habitat and nest-site characteristics
Rural coots bred on significantly larger reservoirs (F2,46 = 23.2, P < 0.001) with larger relative area of emergent vegetation (F2,46 = 6.24, P = 0.004) than coots from urban populations (Tukey’s HSD: all P < 0.05; Table 1). No differences were found in the reservoir area and the relative area of emergent vegetation between the old and new urban populations (Tukey’s HSD: P = 0.76 and P = 0.92, respectively; Table 1). Larger availability of emergent reed-like vegetation in rural populations was associated with different nest-site characteristics of rural and urban coots. We found that rural coots nested further from shore (W = 17.21, P < 0.001) and had more vegetation cover at the nest (W = 9.10, P = 0.011) than coots from the two urban populations (Tukey’s HSD: all P < 0.05; Table 1). There were no significant differences in the distance from the nest to shore and vegetation cover at the nest between the old and new urban population (Tukey’s HSD: P = 0.17 and P = 0.50, respectively; Table 1). No between-population differences in water depth at the nest were found (W = 0.48, P = 0.79).
Nest defence behaviour
The proportion of pairs that showed active nest defence behaviour against a human intruder differed significantly between populations (G = 47.6, df = 2, P < 0.001; Fig. 1a). In the old urban population, all pairs showed active nest defence, while the proportion of coots actively defending their nests in the newly established urban population was significantly lower (85.7%; G = 4.82; df = 1; P = 0.028; Fig. 1a). Active nest defence was recorded in only 18.4% rural pairs. Similar differences were found for the minimum approach distance to a human intruder at the nest. The proportion of birds that approached a human intruder at a close distance (≤ 2 m) was nearly twice higher in old than new urban population (89.5% vs. 45.2%; G = 10.8, df = 1, P < 0.001; Fig. 1a). There were also significant between-population differences in the minimum approach distance (W = 22.70, P < 0.001; Fig. 1b). Coots from the old urban population had shorter approach distance than birds from the newly established urban population (1.22 ± 0.24 m vs. 3.07 ± 0.46 m; Tukey’s HSD: P = 0.036; Fig. 1b). Coots from the rural population had significantly longer approach distance (8.39 ± 1.77 m) than birds from the two urban populations (Tukey’s HSD: all P < 0.005; Fig. 1b). Minimum approach distance did not vary with date (W = 0.03, P = 0.86) or clutch size (W = 0.12, P = 0.73).
Proportion of pairs showing active nest defence (a) and the minimum approach distance (mean ± SE) to a human intruder at the nest (b) in urban (old and new) and rural populations of the Eurasian coot. Dark- and light-grey bars indicate proportion of coots actively defending their nests at a close (≤ 2 m) and distant (> 2 m) range from a human intruder, respectively
Exploitation of human-derived food
No behavioural reaction to bread-feeding by humans was observed in the rural population (N = 48 families). In contrast, most coot families from urban sites actively responded to an intentional bread-feeding by humans, and the proportion of such families was similar in the old and new urban population (90% vs. 85%; G = 0.28, P = 0.60). We found that adult coots from the old urban population were more likely to leave water and feed on the shore in the close presence of humans (0.74 ± 0.06 in Warsaw vs. 0.47 ± 0.09 in Łódź; W = 7.94, P = 0.005; Fig. 2). No such relationship was found for offspring, as they showed a similar probability of feeding on shore in both urban populations (0.58 ± 0.07 in Warsaw vs. 0.62 ± 0.08 in Łódź; W = 3.27, P = 0.071; Fig. 2). Probability of feeding on the shore increased with chick age (W = 6.61, P = 0.010; β = 1.07 ± 0.42). Also, both adults and chicks were more likely to feed on the shore in the morning hours (adults: W = 8.11, P = 0.004, β = −0.68 ± 0.24; chicks: W = 7.48, P = 0.006, β = −0.32 ± 0.12). Adult coots from the recently established urban population more frequently used alarm calls while watching their offspring feeding on bread in the close presence of humans (0.09 ± 0.04 in Warsaw vs. 0.44 ± 0.09 in Łódź; W = 7.23, P = 0.007).
Stress responsiveness
There were significant differences in H/L ratios of coots from urban and rural populations (W = 15.45, P < 0.001). Specifically, coots from urban populations had significantly or nearly significantly lower H/L ratios than coots from the rural population (Tukey’s HSD: P < 0.001 for Łódź, P = 0.072 for Warszawa; Fig. 3), indicating their higher resistance to stress. In contrast, there were no significant differences in the stress response of coots from the old and new urban population (Tukey’s HSD: P = 0.78). Coot H/L ratios were not affected by date (W = 2.49, P = 0.11), sex (W = 0.18, P = 0.67), or tarsus length (W = 0.01, P = 0.94).
Discussion
The results of this study provided strong support for the hypothesis that the urban behavioural type develops with time since urbanization in the Eurasian coot. We found that the expression of some behavioural characters associated with urban life was significantly stronger in the old urban population of coots when compared with the recently established urban population. Most importantly, adult coots from the old urban population were more aggressive during nest defence and were bolder during foraging. Adult coots from the recently established population were also more likely to give alarm calls in a risky situation. This behavioural divergence was apparent despite the lack of habitat differences between the two urban sites. By contrast, coots from the two urban populations showed a similar likelihood of exploiting human-derived food resources, as well as they showed similar levels of physiological stress. Urban coots were generally more aggressive, bolder, and less stressed than their rural conspecifics.
The fact that the expression of key behavioural characters of urban-dwelling birds, boldness and aggression, increased with the time since urbanization may suggest that these adaptations were due to microevolutionary changes. Similar pattern has been recently reported for different components of escape behaviour in 15 European bird species, in which differences in behaviour between rural and urban habitats increased as more time elapsed since urbanization (Møller and Ibáñez-Álamo 2012). Another inter-specific study on birds showed that the levels of antioxidants increased with the number of generations since urbanization, as an adaptation for elevated oxidative stress resulting from higher temperatures and pollution in urbanized landscape (Møller et al. 2010). Finally, an analysis of historical urban colonization by the European blackbird Turdus merula provided evidence for a negative correlation between the time since urbanization and migratoriness, where recent urban populations were more migratory than the old ones (Møller et al. 2014). All these gradual changes in behaviour and physiology are consistent with a scenario of microevolution rather than phenotypic plasticity or phenotype sorting. Our hypothesis that elevated boldness and aggression of urban coots might have a genetic basis is consistent with previous studies on the blackbird, showing that divergence at SERT gene responsible for aggressive behaviours and anxiety exhibited strong association with habitat (urban vs. rural) type resulting from selection pressures during urbanization events (Mueller et al. 2013). Also, elevated neophobia and reduced neophilia of urban blackbirds were identified as intrinsic personality traits likely resulting from microevolutionary changes (Miranda et al. 2013).
The hypothesis that some behavioural characters become genetically fixed in urban populations does not preclude the mechanisms of phenotype sorting or phenotypic plasticity taking place at the initial stages of urban colonization events. In fact, a large behavioural divergence between the recently established urban population in Łódź and an adjacent rural population indicates that these processes are very likely to be in operation. Our recent study of microsatellite divergence between several urban and rural coot populations from Poland suggested that Łódź was colonized by rural individuals rather than by birds originating from other urban populations, providing support for an independent model of colonization (Minias et al. 2017). This means that coots which colonized the city of Łódź within the last 15 years were either behaviourally and physiologically pre-adapted to urban life (phenotype sorting) or were extremely plastic in their behaviour. Actually, we cannot exclude that the co-occurrence of these two mechanisms determined the successful colonization of urban environment by the Eurasian coots. Some behavioural traits associated with urban life, such as an exploitation of human-derived food resources, almost certainly constituted a plastic response to a novel environment. We found that ca. 90% of birds from the new and old urban population readily responded to bread-feeding, while no such behaviours were observed in the rural population. Thus, it seems likely that coots can quickly learn to exploit this new source of food from conspecifics or from other urban waterfowl, e.g. mallards Anas platyrhynchos. On the other hand, urban environments are likely to be colonized by rural individuals that are inherently bold, aggressive, and resistant to stress. In fact, we observed a huge variance in boldness and aggression of rural coots, as measured during nest defence experiments. While most rural birds secretively left the nest at the human approach, ca. 20% of breeding pairs engaged in active nest defence and only one pair defended their nest at a close range (< 2 m) from a human intruder. In accordance with the mechanism of phenotype sorting, rural birds with the latter personality traits (bold and aggressive) might have been more likely to act as urban invaders. This is consistent with the previous study by Carrete and Tella (2011), who found that large inter-individual variability in fear of humans within rural populations was a key determinant of urban invasiveness.
Our experiments indicate that urban behaviour in the Eurasian coots could not be a mere product of habituation, defined as the gradual decrease in response to repeated stimuli (Evans et al. 2010). Behavioural habituation is often invoked to explain the loss of fearfulness towards human disturbance in urban animal populations (Evans et al. 2010). For instance, birds can reduce their flight initiation distances (FID) as a response to frequent exposure to humans (Fernández-Juricic 2004, Bjørvik et al. 2015; but see Carrete and Tella 2011, Møller et al. 2013) and there is experimental evidence that habituation may complement the mechanisms of risk allocation in the responses of urban birds towards humans (Rodriguez-Prieto et al. 2009). During the process of habituation urban animals can learn that humans are unlikely to harm them and they eventually come to ignore humans approaching at relatively short distances (Myers and Hyman 2016). In contrast, rural individuals that are not accustomed to human disturbance should perceive humans as potential predators. Consistently with the latter assumption, coots from our rural population seemed to view humans only as a threat, while urban birds were flexible enough to view humans as both threat (at the nest) and potential food source (via bread-feeding). However, it seems that behavioural responses of urban coots to humans were a result of plastic adaptation rather than habituation, as coots were actively approaching a human stimulus, rather than ignoring it.
Finally, we also found that urban coots had lower average stress responsiveness than rural conspecifics, which could likely have arisen via phenotype sorting. Specifically, we showed that coots from the recently established urban population had significantly lower H/L ratios when compared with rural conspecifics, while no differences in H/L ratios were found between the two (new and old) urban populations. This means that high resistance to stress might be a crucial prerequisite for a successful urban colonization, which is consistent with the recent findings on the urban colonization patterns by the dark-eyed juncos Juno hyemalis in California, USA. It has been found that juncos from the recently established (1980s) urban population in San Diego had reduced acute corticosterone response to handling when compared with a nearby wildland population and these differences had a genetic basis (Atwell et al. 2012). Physiological adaptations in urban juncos have been explained by non-random founder phenotypes (phenotype sorting) in combination with rapid microevolution resulting from novel selective forces associated with the urban environment (Atwell et al. 2012).
In conclusion, our study of urban colonization by the Eurasian coots provided rare evidence for the development of urban behaviour with the time since urbanization (see also Møller et al. 2012). Some behavioural components (boldness and aggressiveness) were more strongly expressed in the older urban population, suggesting that they have become genetically fixed via novel selective pressures associated with urban life. On the other hand, large behavioural and physiological divergence of coots from the recently established urban population and an adjacent rural population suggested that phenotypic plasticity and phenotype sorting may play a key role in the initial stages of urban colonization. Our results suggest that adaptation to urban life may require a highly context-dependent response to humans and emphasize high complexity of processes involved in the avian invasions into urban areas.
References
Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED (2012) Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol 23:960–969
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bjørvik LM, Dale S, Hermansen GH, Munishi PK, Moe SR (2015) Bird flight initiation distances in relation to distance from human settlements in a Tanzanian floodplain habitat. J Ornithol 156:239–246
Carrete M, Tella JL (2011) Inter-individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS One 6:e18859
Chevin L-M, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8:e100357
Davis AK (2005) Effects of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76:334–338
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772
Evans J, Boudreau K, Hyman J (2010) Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116:588–595
Fernández-Juricic E (2004) Spatial and temporal responses of forest birds to human approaches in a protected area and implications for two management strategies. Biol Conserv 117:407–416
Fox J, Weisberg S (2011) An R companion to applied regression. Second Edition, Sadge, Thousand Oaks, CA
Griffiths R, Double MC, Orr k DRJ (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Janiszewski T, Wojciechowski Z, Markowski J (2009) The atlas of breeding birds in Łódź. Łódź Univ. Press, Poland, in Polish with English summary
Johnstone CP, Reina RD, Lill AJ (2012) Interpreting indices of physiological stress in free-living vertebrates. J Comp Physiol B 182:861–879
Knight RL, Temple SA (1986) Methodological problems in studies of avian nest defence. Anim Behav 34:561–566
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environemnts. Biol Rev 88:537–549
Luniak M, Kalbarczyk W, Pawłowski W (1964) Birds of Warsaw. Acta Ornithol 8:175–285
Luniak M, Kozłowski P, Nowicki W, Plit J (2001) Birds of Warsaw 1962–2000. Polish Academy of Sciences, Warszawa, Poland, in Polish with English summary
Minias P (2015) Sex determination of adult Eurasian coot (Fulica atra) by morphometric measurements. Waterbirds 38:191–194
Minias P (2016) Reproduction and survival in the city: which fitness components drive urban colonization in a reed-nesting waterbird? Curr Zool 62:79–87
Minias P, Włodarczyk R, Minias A, Dziadek J (2017) How birds colonize cities: genetic evidence from a common waterbird, the Eurasian coot. J Avian Biol 48:1095–1103
Miranda AC, Schielzeth H, Sonntag T, Partecke J (2013) Urbanization and its effects on personality traits: a result of microevolution or phenotypic plasticity? Glob Chang Biol 19:2634–2644
Møller AP (2008) Flight distance of urban birds, predation, and selection for urban life. Behav Ecol Sociobiol 63:63–75
Møller AP, Ibáñez-Álamo JD (2012) Escape behaviour of birds provides evidence of predation being involved in urbanization. Anim Behav 84:341–348
Møller AP, Erritzøe J, Karadas F (2010) Levels of antioxidants in rural and urban birds and their consequences. Oecologia 163:35–45
Møller AP, Diaz M, Flensted-Jensen E, Grim T, Ibáñez-Álamo JD, Jokimäki J, Mänd R, Markó G, Tryjanowski P (2012) High urban population density of birds reflects their timing of urbanization. Oecologia 170:867–875
Møller AP, Grim T, Ibáñez-Álamo JD, Markó G, Tryjanowski P (2013) Change in flight initiation distance between urban and rural habitats following a cold winter. Behav Ecol 24:1211–1217
Møller AP, Jokimäki J, Skorka P, Tryjanowski P (2014) Loss of migration and urbanization in birds: a case study of the blackbird (Turdus merula). Oecologia 175:1019–1027
Møller AP, Tryjanowski P, Díaz M, Kwieciński Z, Indykiewicz P, Mitrus C, Goławski A, Polakowski M (2015) Urban habitats and feeders both contribute to flight initiation distance reduction in birds. Behav Ecol 26:861–865
Mueller JC, Partecke J, Hatchwell BJ, Gaston KJ, Evans KL (2013) Candidate gene polymorphisms for behavioural adaptations during urbanization in blackbirds. Mol Ecol 22:3629–3637
Myers RE, Hyman J (2016) Differences in measures of boldness even when underlying behavioral syndromes are present in two populations of the song sparrow (Melospiza melodia). J Ethol 34:197–206
Newman MM, Yeh PJ, Price TD (2008) Song variation in a recently founded population of the dark-eyed junco (Junco hyemalis). Ethology 114:164–173
Partecke J, Schwabl I, Gwinner E (2006) Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87:1945–1952
R Development Core Team (2013) R: A language and environment for statistical Computing R Foundation for Statistical Computing, Vienna, Austria
Rodriguez-Prieto I, Fernández-Juricic E, Martín J, Regis Y (2009) Antipredator behavior in blackbirds: habituation complements risk allocation. Behav Ecol 20:371–377
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112
Tryjanowski P, Møller AP, Morelli F, Biaduń W, Brauze T, Ciach M, Czechowski P, Czyż S, Dulisz B, Goławski A, Hetmański T, Indykiewicz P, Mitrus C, Myczko Ł, Nowakowski JJ, Polakowski M, Takacs V, Wysocki D, Zduniak P (2016) Urbanization affects neophilia and risk-taking at bird-feeders. Sci Rep 6:28575
Yeh PJ, Hauber ME, Price TD (2007) Alternative nesting behaviours following colonisation of a novel environment by a passerine bird. Oikos 116:1473–1480
Acknowledgments
We are grateful to all field volunteers who participated in data collection. We thank two anonymous reviewers for their constructive comments on the earlier draft of the manuscript. The study was performed by the permissions of the Local Bioethical Commission in Łódź and the General Environmental Protection Directorate in Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Minias, P., Jedlikowski, J. & Włodarczyk, R. Development of urban behaviour is associated with time since urbanization in a reed-nesting waterbird. Urban Ecosyst 21, 1021–1028 (2018). https://doi.org/10.1007/s11252-018-0781-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-018-0781-7