Abstract
The aim of this study was to investigate the relationship between clinical findings and bacterial isolation in milk samples of meat-producing ewes. The study was conducted in 17 commercial flocks and 550 udder halves from suckling Santa Ines ewes. Initially, the clinical examination of the mammary glands and teats was performed by visual inspection and palpation of the teats and udder halves; then a scoring system was devised for all the findings. After that, the strip cup test and the California mastitis test (CMT) were performed. Then, milk samples for somatic cell counts (SCCs) and bacteriological analyses were collected. Staphylococci bacteria were the main etiological agent isolated in the present study. Upon investigation of the correlations between bacterial isolation and the clinical findings, only the presence of teat injury, pendulous udder, and alterations in the palpation of the teat were associated with bacterial isolation. A significant correlation between bacteriologically positive milk samples and CMT and SCC was also found. Thus, some clinical findings appeared as a risk factor for bacteriologically positive milk samples and can be used as a tool in mastitis control programs. However, a complete and extensive diagnosis, an appropriate therapy, and an efficient mastitis control program will require the combination of clinical examination, microbiological tests, and SCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis is an important disease in sheep, even in those used for meat production. The disease economically impacts the production system by increasing lamb mortality and decreasing lamb growth rates (Clements et al. 2003). Currently, the diagnosis of mastitis in small ruminants is mainly based on milk somatic cell count (MSCC) and on bacteriological assays (Bergonier et al. 2003; Clements et al. 2003; Souza et al. 2012). Very few reports have been presented in the literature concerning clinical examination of the udder as a diagnostic tool in small ruminants (Marogna et al. 2010, 2012), and most of the scientific conclusions drawn for cattle are often erroneously assumed to be the same for small ruminants (Marogna et al. 2012).
Systematic clinical examination of the udder and teat is relatively inexpensive and fast, and provides valuable information. Collection of such data is quite feasible, especially in flocks already applying a flock management system including other clinical records. Thus, both the ruminant practitioner and scientist conducting a field study can benefit from clinical udder examinations, especially when mastitis is expected (Klaas et al. 2004; Marogna et al. 2010).
In ewes that are not submitted to routine milking, physical inspection and palpation of the udders and teats may play an important role in the diagnosis of intramammary infections (IMI) or in the selection of animals to be submitted to MSCC and bacteriological examination. Furthermore, the majority of the information available on mammary gland health is concerned predominantly with dairy sheep from Mediterranean countries, and there are few data on nondairy breeds (Clements et al. 2003; Blagitz et al. 2012). Indeed, differences in climate, production forms, and management practices may give rise to differences in epidemiology, bacteriology, and manifestations of mastitis (Mork et al. 2007).
Here, we used Santa Ines ewes, a Brazilian breed mainly raised for meat production (Amarante et al. 2004; Ferreira et al. 2011), with a considerable milk production (Ferreira et al. 2011). In tropical conditions, these animals have a better ability to withstand environmental stressors than European breeds, which may be linked to greater resistance to some infections, as previously described (Amarante et al. 2004; Brincarello et al. 2005). As specialized dairy breeds are not well adapted to tropical climates and are susceptible to infections, crossbreeding with a resistant breed may enhance productivity. The Santa Ines ewe has, for instance, been widely crossbred with Ile de France (Amarante et al. 2004). This study aimed to identify alterations in the udders and teats associated with IMI in Santa Ines ewes.
Materials and methods
The study was conducted in 17 commercial flocks of Santa Ines ewes in São Paulo state, Brazil. The sample included 550 udder halves from 275 ewes at different lactation stages from extensive and semi-intensive management systems. We emphasized that the research complied with the Ethical Principles in Animal Research, approved by the Bioethics Commission.
Throughout the lactation period of the assessed ewes, the samples were grouped according to days after parturition as follows: stage 1, ewes from the 3rd to 5th day; stage 2, ewes from the 6th to the 15th day; stage 3, ewes from the 16th to the 30th day; stage 4, ewes from the 31st to the 45th day; stage 5, ewes from the 46th to the 60th day; stage 6, ewes from the 61st to the 75th day; stage 7, ewes from the 76th to the 90th day; and stage 8, ewes from the 91st to the 120th day after parturition.
Initially, a clinical examination of the mammary glands and teats was carried out based on the criteria described by Dirksen et al. (1993). Clinical examination of the udder and teats included a visual inspection for external appearance and absence of alterations in the teats and udder. The clinical examination included (1) palpation of the teats and the udder; (2) visual assessment of the volume and symmetry of each teat, presence of supernumerary teat(s), and presence of teat injury, such as pustules, scabs, ulcers, abscesses, rubor, vesicles, wounds, and warts; and (3) visual assessment of the volume and symmetry of the udder and presence of pendulous udder. Palpation of the teats was scored as follows: soft texture, thickened teat orifice, thelitis where all the layers were involved and the teat wall became very thickened and painful in acute stage, and cisternitis where the mucous membrane is involved and diagnosed by palpation of a dense, vertical cord in the center of the teat dip orifice obstruction. In an attempt to observe any alterations in udder palpability, the texture and presence of nodules in each udder half was recorded. Here, the presence of any signs of acute inflammation was also recorded by evaluating the presence of swelling, rubor, calor, pain, and reactivity of the supramammary lymph nodes.
A scoring system was devised, and numerical values were assigned for all findings as detailed in Tables 1 and 2. After that, the strip cup test was evaluated to identify the presence of clots, flakes, or other obviously abnormal secretion.
Milk samples for MSCC were collected in 40-mL vials containing microtablets of bronopol (2-bromo-2-nitropropane-1,3-diol). Then, MSCCs were performed in an automated fluorescent microscopic somatic cell counter (Somacount 300, Bentley Instruments®). For statistical analysis, the MSCCs were divided into milk samples with MSCC ≤ 200,000 cells/mL and those with MSCC ≥ 200,000 cells/mL (Paape et al. 2001; Souza et al. 2012).
The California mastitis test (CMT) was performed on foremilk samples, and the results were graded as follows: 0, no precipitate; 1, distinct precipitate/weak gel formation; 2, distinct gel formation; or 3, strong gel formation. Then, to analyze the data, the milk samples were divided into CMT negative (score 0) and positive (score ≤ 1) samples (Souza et al. 2012).
IMIs were identified based on bacteriological examination of the milk samples collected aseptically, which is considered to be the “gold standard” method for the diagnosis of IMI (Pyörälä 2003; Marogna et al. 2012). For bacteriological analysis, single milk samples from individual quarters were aseptically collected in sterile tubes after discarding the first three milk streams and scrubbing the teat ends with cotton with 70 % ethanol. Milk samples for bacteriological analysis were stored at −20 °C until the analysis. The bacteriological analysis was carried out by culturing 0.01 mL of each sample on 5 % ovine blood agar plates and incubating them aerobically for 24–72 h at 37 °C. The microbial strains were presumptively identified on the basis of morphology and Gram staining of the colonies. The methods used to identify the different organisms were those recommended by Oliver et al. (2004).
Initially, the association between the presence/absence of clinical signs and bacteriological examination results was evaluated using the chi-square test (GraphPad Software, Inc., San Diego, CA, USA). In this comparison, the significance level was set at P ≤ 0.05. Then, the statistical analysis of the data was performed with STATA statistical software version 12 (Stata Corp., College Station, Texas, USA) using a logistic regression model. The variables under examination were first analyzed individually to verify the significance and then in combination to assess the effect of the single variables on all others. In the first stage of the analysis, unconditional logistical model for each variable related to the clinical findings, with P values lower than 0.20, were considered as a select variable and passed to the next stage of the analysis. In the final model, variables selected in the first stage were used to develop a multivariate logistical model in which variables with P ≤ 0.05 were retained in the final model, except for MSCC and CMT that were only analyzed individually and did not enter in the final model.
Results
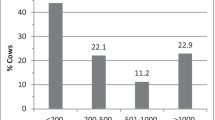
Most of the collected milk samples (76 %) were bacteriologically negative (n = 419 of 550 samples), and among the positive samples, most of the isolates (93 %) were Staphylococcus spp. (n = 122 of 131 bacteriologically positive samples), followed by Streptococcus spp. (n = 7 of 131 positive samples, 5 %) and Staphylococcus spp. and Streptococcus spp. (n = 2 of 131 positive samples, 1.5 %).
The association between the presence/absence of clinical signs in the udder and bacteriological examination results is reported in Table 3. Only the presence of teat injury (P = 0.003) and pendulous udder (P < 0.0001) were significantly associated with bacteriological results by chi-square test.
A logistic regression model was adjusted, taking the following as explanatory variables (P ≤ 0.20): lactation stage, strip cup test, udder palpation score, teat palpation score, udder depth, teat injury, and the score of physical examination, which were eligible for entry in the logistic regression analyses (Table 4). In this model, it was found that only three variables were statistically significant (P ≤ 0.05) for estimating the likelihood of being bacteriologically positive (Table 5), and these remained in the final model. Furthermore, the MSCC and CMT thresholds used here were significantly associated with bacterial isolation (Table 4).
During the diagnostic examination of the final model in the logistic regression, it was observed that if we categorized the palpation of the teat as having a normal consistency (score 0), thickened teat canal or thelitis (score 1), and cisternitis or obstructed teat canal (score 2), the model becomes better fitted. Therefore, this classification was used in the final model.
Discussion
Assessment of the udder and teat morphology can be performed by direct measurement of udder and teat traits or by subjective evaluation of the udder and teats traits using linear scales. Direct measurements provide objective information, but they consume time and are laborious for application on a large scale. On the other hand, linear traits are more useful for large-scale evaluations, although they rely on subjective information (Gelasakis et al. 2012). To the best of our knowledge, this is the first report that has investigated the relationship between subclinical IMI and several physical examination variables using a logistic analysis model in meat-producing ewes.
Staphylococci were the most commonly isolated bacteria in the present study, consistent with reports by several authors (Bergonier et al. 2003; Fragkou et al. 2007; Souza et al. 2012). It is well known that coagulase-negative staphylococci (CNS) are the principal organism present as bacterial flora in sheep teats, which may suggest that perhaps and under certain circumstances, this microorganism can cause IMI (Fragkou et al. 2007). This fact may explain why teat injury was significantly associated with IMI and the predominance of staphylococci infections found here.
The presence of teat injury as a risk factor demonstrated that the efficiency of the teat defense mechanisms largely depends on the integrity of the teat tissues. An association between teat injury and mastitis in goats was also observed by Ameh and Tari (2000). Indeed, an unhealthy teat skin condition could result in higher contamination of the teat surface with mastitis pathogens, resulting in an increased rate of IMI because the teat is the most common portal of entry of the various etiological agents of mastitis. Furthermore, it has been suggested that teat lesions appear to adversely affect mammary gland defense mechanisms (Mavrogianni et al. 2006, 2007; Fragkou et al. 2007; Mavrogianni and Fthenakis 2007). It is possible that under field conditions and on a longer-term basis, any factor affecting the immune status of the animals would affect the equilibrium of flora organisms within the teat, thus resulting in mastitis (Fragkou et al. 2007).
In Sarda ewes, Marogna et al. (2010) reported that other clinical findings, such as the presence of nodules in the udder by palpation, are associated with a higher risk for a positive bacterial culture. These authors described that the correlations between the clinical signs and bacterial isolation were limited to Streptococcus uberis, Staphylococcus aureus, and Staphylococcus epidermidis. S. epidermidis was only associated with the presence of pustules and ulcers, in contrast with S. aureus, which is associated with several signs of chronic mastitis: nodules, abscesses, sclerosis, and atrophy (Marogna et al. 2010). Conversely, our study indicated that alterations in teat palpations were associated with bacterial isolation. These discrepancies may be due to the bacterial species involved in IMI in these studies. In our study, staphylococci were the most frequent bacterial genera isolated. These alterations indicated that the target tissues of most of the pathogens isolated here are the teat cistern rather than the deep regions of the udder, as CNS are mostly associated with both the gland and teat cistern. Otherwise, S. aureus has a higher pathogenicity and is generally associated with the mammary parenchyma (Benites et al. 2003). It is possible that in herds and flocks not having a persistent udder health problem, the same etiology might not result in exactly the same outcome, so the hierarchy of risk factors differs depending on the particular circumstances (Marogna et al. 2012).
The inclusion of pendulous udder as a risk factor for IMI in the present study is in agreement with Sarker et al. (2013) in a study with cows. Similarly, Casu et al. (2006) described that the deep udders are more exposed to injury and are consequently more susceptible to IMI and may be liable to be culled.
In dairy ewes, the patterns of udder morphology should be specified in terms of machine milking efficiency (Gelasakis et al. 2012), although the same parameter cannot be applied to meat-producing ewes. Conversely, it should also be noted that selection based on the milk yield could lead to udders with excessive depth because udder depth was the only morphological mammary trait to have a high genetic correlation with milk yield (Serrano et al. 2002), and consequently, this feature may predispose ewes to IMI.
The stage of lactation is significantly associated with bacteriologically positive milk samples when analyzed individually Blagitz et al. (2008). On the other hand, when the effect of this variable against all other variables was investigated, no significant difference was detected, which suggested that this variable interacts with other variables. The non-significant difference in the lactation stage may be related to the high persistence of subclinical IMI during lactation due to the poor detection and elimination of infections during lactation and to their frequent staphylococcal origin (Bergonier et al. 2003; Blagitz et al. 2012).
Although some clinical findings appear to be risk factors for bacteriologically positive milk samples and can be used as a tool in a mastitis control program, many bacteriologically positive samples were not detected using only the clinical findings. Thus, a complete and extensive diagnosis and an appropriate therapy and mastitis control program require the combination of clinical examination, microbiological tests, and MSCCs.
Conclusion
The presence of teat injury and alterations in the palpation of the teat and pendulous udder were significantly associated with IMI. Therefore, the presence of one or more of these findings should indicate the need for submitting milk samples for microbiological evaluation and/or indirect tests, such as MSCC, to detect mastitis in meat-producing ewes, especially when underreporting of mastitis cases is expected.
References
Amarante, A.F.T., Brincarello, P.A., Rocha, A. and Gennari, S.M., 2004. Resistance of Santa Ines, Suffolk and Ile de France sheep to naturally acquired gastrointestinal nematode infections, Veterinary Parasitology, 120, 96–106.
Ameh, J.A. and Tari, I.S., 2000. Observations on the prevalence of caprine mastitis in relation to predisposing factors in Maiduguri, Small Ruminant Research, 35, 1–5.
Benites, N.R., Melville, P.A. and Costa E.O., 2003. Evaluation of the microbiological status of milk and various structures in mammary glands of from naturally infected dairy cows, Tropical Animal Health and Production, 35, 301–307.
Bergonier, D., De Crémoux, R., Rupp, R., Lagriffoul, G. and Berthelot, X., 2003. Mastitis in small ruminants, Veterinary Research, 34, 689–716.
Blagitz, M.G., Batista, C.F., Souza, F.N., Benites, N.R., Melville, P.A., Stricagnolo, C.R., Ricciardi, M., Gomes, V., Azedo, M.R., Sanches, B.G.S. and Della Libera, A.M.M.P., 2008. Cellular and microbiological profile of Santa Ines ewes in the lactation and the post-weaning period, Pesquisa Veterinária Brasileira, 28, 417–422.
Blagitz, M.G., Benites, N.R., Melville, P.A., Batista, C.F., Betiol, P.S., Azedo, M.R., Gomes, V., Souza, F.N. and Della Libera, A.M.M.P., 2012. Lactation stage and udder health status of Santa Ines ewes, Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 64, 495–498.
Brincarello, P.A., Amarante, A.F.T., Rocha, R.A., Cabral Filho, S.L., Huntley, J.F., Houdjik, J.G.M., Abdalla, A.L.A. and Gennari, S.M., 2005. Influence of dietary protein supply on resistance to experimental infections with Haemonchus contortus in Ile de France and Santa Ines lambs, Veterinary Parasitology, 134, 99–109.
Casu, S., Pernazza, I. and Carta, A., 2006. Feasibility of a linear scoring method of udder morphology for the selection scheme of the Sardinian sheep, Journal of Dairy Science, 89, 2200–2209.
Clements, A.C.A., Taylor, D.J. and Fitzpatrick, D.J., 2003. Evaluation of diagnostic procedures for subclinical mastitis in meat-producing sheep, Journal of Dairy Research, 70, 139–148.
Dirksen, G., Gründer, H-D. and Stöber, M., 1993. Rosenberg, Die Klinische Untersuchung des Rindes, (third ed., Verlagbuchhandlung Paul Parey, Berlin and Hamburg).
Ferreira, M.I.C., Borges, I., Macedo Júnior, G.L., Rodriguez, N.M., Penna, C.F.A.M., Souza, M.R., Gomes, M.G.T., Souza, F.A. and Cavalcanti, L.F., 2011. Milk production and composition of Santa Ines and Lacaune x Santa Ines crossbred ewes and performance of their lambs, Arquivo Brasileiro de Medicina Veterinária e Zootecnia, 63, 530–533.
Fragkou, I.A., Mavrogianni, V.S., Cripps, P.J., Gougoulis, D.A. and Fthenakis, G.C., 2007. The bacteria flora in the teat duct of ewes can protect against and can cause mastitis, Veterinary Research, 38, 525–545.
Gelasakis, A.I., Arsenos, G., Valergakis, G.E., Oikonomou, G., Kiossis, E., and Fthenakis, G.C., 2012. Study of factors affecting udder traits and assessment of their interrelationship with milking frequency in Chios breed ewes, Small Ruminant Research, 103, 232–239.
Klaas, I.C., Enevoldsen, C., Vaarst, M. and Houe, H., 2004. Systematic clinical examations for identification of latent udder health types in Danish dairy herds, Journal of Dairy Science, 87, 1217–1228.
Marogna, G., Rolesu, S., Lollai, S., Tola, S. and Leori, G., 2010. Clinical findings in sheep farms affected by recurrent bacterial mastitis, Small Ruminant Research, 88, 119–125.
Marogna, G., Pilo, C., Vidili, A., Tola, S., Schianchi, G. and Leori, S.G., 2012. Comparison of clinical findings, microbiological results, and farming parameters in goat herds affected by recurrent infectious mastitis, Small Ruminant Research, 102, 74–83.
Mavrogianni, V.S. and Fthenakis, G.C., 2007. Clinical, bacteriological, cytological and pathological features of teat disorders in ewes, Journal of Veterinary Medicine Series A, 54, 219–223.
Mavrogianni, V.S., Cripps, P.J. and Fthenakis, G.C., 2006. Description and validation of a novel technique to study bacterial flora of the teat duct of ewes, Small Ruminant Research, 66, 258–264.
Mavrogianni, M.V., Cripps, P.J. and Fthenakis, G.C., 2007. Bacterial flora and risk of infection of the ovine teat duct and mammary gland throughout lactation, Preventive Veterinary Medicine, 79, 163–173.
Mork, T., Waage, S., Tollersrud, T., Kvitle, B. and Sviland, S., 2007. Clinical mastitis in ewes; bacteriology, epidemiology and clinical features, Acta Veterinaria Scandinavia, 49:23.
Oliver, S.P., Lewis, M.J., Gillespie, B.E., Dowlen, H.H., Jaenicke, E.C. and Roberts, R.K., 2004. Microbiological procedures for the diagnosis of bovine udder infection and determination of milk quality, (fourth ed., National Mastitis Council, Verona).
Paape, M.J., Poutrel, B., Contreras, A., Marco, J.C, Capuco, A.V., 2001. Milk somatic cells and lactation in small ruminants, Journal of Dairy Science, 84, E237-E244.
Pyörälä, S., 2003. Indicators of inflammation in the diagnosis of mastitis, Veterinary Research, 34, 565–578.
Sarker, S.C., Parvin, M.S., Rahman, A.K.M.A. and Islam, M.T., 2013. Prevalence and risk factors of subclinical mastitis in lactating dairy cows in North and south regions of Bangladesh, Tropical Animal Health and Production, 45, 1171–1176.
Serrano, M., Pérez-Guzmán, M.D., Montoro, V. and Jurado, J.J., 2002. Genetic analysis of udder traits in Manchega ewes, Livestock Production Science, 77, 355–361.
Souza, F.N., Blagitz, M.G., Penna, C.F.A.M., Della Libera, A.M.M.P., Heinemann, M.B. and Cerqueira, M.M.O.P., 2012. Somatic cell count in small ruminants: friend or foe?, Small Ruminant Research, 107, 65–75.
Acknowledgments
The authors are grateful for the financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo.
Conflict of interest
All authors do not have financial, personal, or other relationships with other people or organizations that could inappropriately influence or bias the paper entitled “Clinical findings related to intramammary infections in meat-producing ewes”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Blagitz, M.G., Souza, F.N., Batista, C.F. et al. Clinical findings related to intramammary infections in meat-producing ewes. Trop Anim Health Prod 46, 127–132 (2014). https://doi.org/10.1007/s11250-013-0462-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-013-0462-8