Abstract
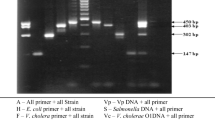
Group A rotaviruses play an important role in causing gastroenteritis and mortality in buffalo (Bubalus bubalis) calves. A number of assays like RNA-polyacrylamide gel electrophoresis (RNA-PAGE), enzyme-linked immunosorbent assay (ELISA), reverse transcription polymerase chain reaction (RT-PCR) and virus isolation have been employed for rotavirus diagnosis. We evaluated the comparative efficacy of different assays for detection of group A rotavirus in buffalo calves. A total of 455 faecal samples collected from five organized farms in northern India were screened by monoclonal antibody based ELISA, 33 (7.25%) samples were positive for group A rotavirus. The percent positivity ranged from 3.22% to 28% in different organized farms. The same samples were also tested by RNA-PAGE, which revealed classical 11 segments with 4:2:3:2 migration patterns in 14 faecal samples showing 3.08% positivity. Virus isolation was successfully done from 21 (4.61%) samples. However, only 15 (3.3%) samples yielded a specific product of 864 and 1,011 bp for VP4 and VP7 genes, respectively, by RT-PCR. The sensitivity and specificity of ELISA, RNA-PAGE and RT-PCR was 100%, 66.67% and 71.43% and 97%, 100% and 100%, respectively, considering virus isolation as standard test. ELISA being simple, fast and sensitive assay can be used as routine laboratory test for the diagnosis of group A rotavirus and field epidemiological studies.
Similar content being viewed by others
References
Beards, G.M. and Desselberger, U., 1989. Determination of rotavirus serotype-specific antibodies in sera by competitive enhanced enzyme immunoassay. Journal of Virological Methods, 24, 103–110.
Buesa, J., Colomina, J., Raga, J., Villanueva, A. and Prat, J., 1996. Evaluation of reverse transcription and polymerase cahain reaction (RT/PCR) for the detection of rotaviruses: applications of the assay, Research in Virology, 147(6): 353–61
Chandra, N.P.S., and Mahalingam, S. 1995. Study of natural rotavirus infection in buffalo calves in Sri Lanka,Tropical Animal Health and Production, 27, 221–224.
Chomoczynski, P. and Sacchi, N., 1987. Single step method of RNA isolation by acid Guanidinium thiocyanate-phenol-chloroform extraction, Analytical Biochemistry, 162, 156–159.
Desselberger, U., Iturriza-Gomara, M. and Gray, J.J., 2001. Rotavirus epidemiology and surveillance. Novartis Foundation Symposium, 238, 125–147.
Espejo, R.T., Puerto, F., Soler, C. and Gonzalez, N., 1984. Characterization of a human pararotavirus, Infection and Immunity, 44, 112–116.
Estes, M.K., Graham, D.Y. and Dimitrov, D.H., 1984. The molecular epidemiology of rotavirus gastroenteritis. Progress in Medical Virology, 29, 1–22.
Fagiolo A., Cristina R., Olga L. and Antonio B., 2005. Buffalo Pathologies. In Borghese Antonio ed, Buffalo Production and Research. FAO, Rome, 249–296.
Gouvea, V., Glass, R.I. Woods, P., Taniguchi, K., Clark, H.F., Forrester, B. and Fang, Z.Y. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool samples. Journal of Clinical Microbiology, 28, 276–282.
Grover, Y.P., Minakshi, Patnayak, D.P. and Pandey, R. 1998. Epidemiological studies on bovine rotavirus in organised dairy farms in Haryana and adjoining areas during 1996–97, Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, 19, 121–123.
Gulati, B.R, Meherchandani, S. and Pandey, R., 1995. Electrophoretic typing of the genomic RNA of rotaviruses from diarrhoeic calves. Indian Journal of Virology, 11, 7–12.
Gulati, B. R., Singh, B. K. and Kumar, D., 2006. Development of a monoclonal antibody-based sandwich ELISA for detection of equine rotavirus from diarrhoeic foals Indian Journal of Microbiology, 46, 349–354
Herring, A.J., Inglis, N.F., Ojeh, C.K., Snodgrass, D.R. and Menzies, J.D., 1982. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver stained polyacrylamide gels. Journal of Clinical Microbiology, 16, 473–477.
Isegawa, Y., Nakagomi, O., Nakagomi, T., Ishida, S., Uesugi, S., and Ueda, S. 1993. Determination of bovine rotavirus G and P serotype by polymerase chain reaction, Molecular and Cellular Probes, 7, 277–284
Jindal, S.R, Maiti, N.K and Oberoi, M.S., 2000. Genomic diversity and prevalence of rotavirus in cow and buffalo calves in northern India, Revue scientifique et technique (International Office of Epizootics), 193, 871–876
Laemmli, U.K., 1970. Cleavage of structural proteins during the assembly of head of bacteriophage T4, Nature, 227, 680–685.
Manuja, Balvinder K., Prasad, M, Manuja, A, Gulati, B. R., and Prasad, G., 2008. A novel genomic constellation (G10P[3]) of Group A rotavirus detected from buffalo calves in northern India, Virus Research, 138, 36–42
Martella, V., Pratelli, A., Pinto, O., Ferrara, G., Tempesta, M., Buonavoglia, D. 1999. Typing by polymerase chain reaction of buffalo rotaviruses isolated in Italy. Zentralbl Veterinarmed B., 46, 499–502.
Martella, V., Ciarlet, M., Baselga R, Arista S, Elia G, Lorusso E, Banyai K, TerioV, Madio A, Ruggeri FM, Falcone E, Camero M, Decaro N, Buonavoglia C., 2005. Sequence analysis of the VP7 and VP4 genes identifies a novel VP7 gene allele of porcine rotaviruses, sharing a common evolutionary origin with human G2 rotaviruses, Virology, 337, 111–23
Minakshi, 1999. Studies on relative frequencies of G (VP7) and P (VP4) genotypes of group ‘A’ rotavirus from diarrhoeic bovine calves by RT-PCR and their restriction endonuclease profile. (Unpublished Ph.D. thesis, CCS HAU, Hisar).
Mittal, S.K., Srivastava, R.N. and Prasad, S., 1986. Rotavirus infection in buffalo calves: Detection by agar-gel precipitation test and enzyme-linked immunosorbent assay, Indian Journal of Animal Sciences, 56, 1127–1131
Nataraju, S.M., Chattopadhyay, U.K. and Krishnan T., 2009, A study on the possibility of zoonotic infection in rotaviral diarrhoea among calves and buffalo calves in and around Kolkata, India, European Review for Medical and Pharmacological Sciences, 13 (1), 7–11
Pereira, H.G., Linhares, A.C., Candeias, J.A. and Glass, R.I., 1983. National laboratory surveillance of viral agents of gastroenteritis in Brazil, Bulletin of the Pan American Health Organization, 27, 224–233.
Pisanelli, G., Martella, V., Pagnini, U., De Martino, L., Lorusso, E., Iovane, G., Buonavoglia, C., 2005. Distribution of G (VP7) and P (VP4) genotypes in buffalo group A rotaviruses, Veterinary Microbiology, 110, 1–6.
Rathore, B.S., 1998. An epidemiological study on buffalo morbidity and mortality based on four year observations on 18,630 buffaloes maintained at 28 livestock farms in India, Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, 191, 43–49.
Saif, L.J. and Jiang, B. 1994. Nongroup A rotaviruses of humans and animals, Current Topics in Microbiology and Immunology, 185, 339–71.
Singh, A., and Pandey, R., 1988. Analysis of electrophoretypes of rotavirus from diarrhoeic faeces of neonatal buffalo calves in India. Acta Virologica, 322,156–159.
Svensson, L., Uhnoo, I., Grandien, M. and Wadell, G., 1986. Molecular epidemiology of rotavirus infections in Upsala, Sweden in 1981: disappearance of a predominant electropherotype, Journal of Medical Virology, 18, 101–111.
Winiarczyk, S. and Gradzki, Z. 1999, Comparison of polymerase chain reaction and dot hybridization with enzyme-linked immunoassay, virological examination and polyacrylamide gel electrophoresis for the detection of porcine rotavirus in faecal specimen, Zentralbl Veterinarmed B., 46(9), 623–634.
Acknowledgments
The authors are thankful to ICAR, New Delhi for providing financial support in the form of research project on “Molecular studies on rotaviruses of avian, bovine and human origins and development of multiplex RT-PCR for diagnosis” and CCSHAU, Hisar for providing infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manuja, B.K., Prasad, M., Gulati, B.R. et al. Comparative efficacy of immunological, molecular and culture assays for detection of group A rotavirus from faecal samples of buffalo (Bubalus bubalis) calves. Trop Anim Health Prod 42, 1817–1820 (2010). https://doi.org/10.1007/s11250-010-9642-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-010-9642-y