Abstract
While ZDDP tribofilm formation has been widely studied, the mechanism of ZDDP tribofilm removal during rubbing is still unclear. The study employs a ball on disc tribometer to monitor ZDDP tribofilm development in rolling-sliding, mixed lubrication conditions. It is found that when ZDDP tribofilms are formed very rapidly, as is the case with short alkyl chain, secondary ZDDPs, a large proportion of the initially-formed tribofilm is suddenly lost during rubbing. By contrast, the tribofilms that form more slowly from primary ZDDPs and longer chain secondaries are not partially lost during rubbing. XPS analysis showed that a rapidly-formed tribofilm before its partial removal has a very small Zn/O ratio, and a high BO/NBO. This suggests that such tribofilm contains a significant proportion of ultraphosphate, which is likely to have a relatively weak structure due to lack of stabilising cations. This may result in the tribofilm being partially removed when it reaches a certain thickness. By comparison, the remaining tribofilm, and also tribofilms that form slowly, have high Zn/O and low BO/NBO. This suggests that they consist of short chain polyphosphates and are thus stronger and more durable.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years the role of lubricant antiwear additives such as ZDDPs and ashless phosphorus additives has become increasingly important because of the growing use of low viscosity oils. These are being introduced with the aim of reducing power losses from hydrodynamic friction, churning and pumping to increase machine efficiency, but can result in machine components operating for long periods in thin film, mixed and boundary lubrication conditions. Under these conditions, surface failure including wear and scuffing is more likely to occur, but can be avoided by using additives that form antiwear tribofilms. Thus, it has become important to understand the ability of antiwear additives, including ZDDPs, to form tribofilms to protect rubbing surfaces.
Generally, ZDDPs with lower thermal stability form tribofilms more rapidly [1] and two molecular characteristics of ZDDPs have been suggested to influence these properties. One is the structure of the alkyl group, so that ZDDPs with secondary alkyl groups are less thermally stable but form tribofilms faster than primary ones, while aryl ZDDPs are very thermally stable but form tribofilms only very slowly [2, 3]. The second is alkyl chain length, so that ZDDPs with very short chains form tribofilms faster than those with higher molecular weight alkyl groups [4]. In consequence, the rate of tribofilm formation is;
secondary alkyl > primary alkyl > aryl. &
short chains > long chains.
ZDDP tribofilms increase friction coefficient in mixed and boundary lubrication conditions compared to the base oil alone [4, 5]. This friction increase has been correlated with the thickness of the ZDDP tribofilms and it has been proposed to originate from the roughness of tribofilms [6, 7]. Thus, ZDDPs that form tribofilms very rapidly also quickly increase boundary friction [4].
The reaction sequence to form ZDDP tribofilms has been well studied. The first stage of this must be diffusion to and adsorption of ZDDP molecules onto the rubbing surface. It has been shown that ZDDP molecules adsorb on Fe via the sulphur atoms of the P = S bonds [8], suggesting Fe can play an important role in promoting tribofilm formation on steel by acting as an adsorption site for ZDDP molecules. After adsorption onto the surface, an intermolecular reaction between dialkyl dithiophosphate (or dithioalkyl phosphate) species is believed to lead to rapid polymerization to form long chain polyphosphates or metaphosphates. This is then followed by slower depolymerization of the long phosphate chains to form short chain phosphates such as pyro- and orthophosphate. This is achieved by loss of thioalkyl or alkoxy groups [9, 10] and/or ingress of Zn or Fe cations to balance the phosphate ion charge as P-O-P bonds break [11,12,13]. It should be noted that the chain length of phosphates in ZDDP tribofilms can be estimated from the ratio of bridging oxygen, BO (P–O–P), to non-bridging oxygen, NBO (P = O and P–O–M), intensity and this BO/NBO ratio can be measured by XPS [11, 14].
As a ZDDP tribofilm develops, its physical properties change. Parsaeian et al. [14] have suggested that the strength or durability of a ZDDP tribofilm may depend on its phosphate chain length and how this changes with rubbing time. While a durable tribofilm was composed of shorter polyphosphates, vulnerable tribofilm was composed of longer polyphosphates. Ueda et al. [15] showed that ZDDP tribofilms undergo a structural transformation during rubbing from a predominantly amorphous structure to one that is nanocrystalline. This occurs due to the depolymerization of initially-formed polyphosphate chains to much shorter phosphates. Importantly, this amorphous to nanocrystalline structural transformation results in the tribofilm becoming more durable after extended rubbing. This structural transformation may also affect the hardness of tribofilm as Kalin et al. [16] found that tribofilms became harder with rubbing time.
Together with tribofilm formation, its removal can also occur during ZDDP tribofilm growth. In some conditions tribofilm removal rate may exceed tribofilm formation rate so that the total tribofilm thickness decreases. For example, it appears that a sudden increase in contact pressure increases both tribofilm formation and removal rate [17]. Dorgham et al. [18] recently showed that ZDDP tribofilm tends to be removed when it forms rapidly. It was suggested that fast formation of ZDDP tribofilm results in thicker but less compact tribofilm layers that can be removed easily under shear. With the role of tribofilm becoming more important due to the introduction of low viscosity, tribofilm removal, especially drastic removal, must be understood and avoided. However, the mechanism of tribofilm removal is not well investigated.
Therefore, the aim of this study is to understand what factors influence tribofilm removal by using a series of ZDDPs with varied alkyl structures and concentrations. Possible mechanisms of ZDDP tribofilm formation and removal are then suggested.
2 Test Lubricants
Solutions of ZDDPs in a gas to liquid (GTL) base oil were studied. The base oil (GTL 3) had kinematic viscosity 9.9 mm2/s at 40 °C and 2.7 mm2/s at 100 °C. Four ZDDPs with different alkyl groups were investigated, as listed in Table 1. ZDDP2-C3/C6 is a mixture of C3 secondary ZDDP and C6 secondary ZDDP at the ratio of 3:2. The other three ZDDPs were synthesised from single alcohols and should thus contain just one type of alkyl group. Selected tests were performed at ZDDP concentrations of 50, 100, 300 and 800 ppm phosphorus. This is indicated in the relevant figures as necessary. The amounts of ZDDP in GTL are not high enough to significantly impact the blend viscosity so that all blends can be considered to have the same EHD film-forming capability.
3 Test Methods
3.1 MTM Test Rig
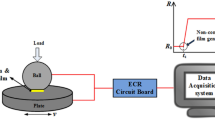
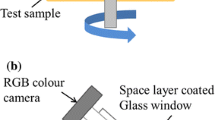
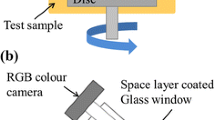
A mini-traction machine (MTM) ball on disc tribometer with a spacer layer imaging attachment (SLIM) was employed to observe ZDDP tribofilm growth. A schematic of the rig is shown in Fig. 1. A 19.05-mm-diameter ball is loaded against the flat surface of a 46-mm-diameter-disc which is immersed in the test lubricant. The ball and the disc are driven by separate electric motors at specified rolling/sliding conditions.
To capture SLIM images, the rubbing test is paused and the ball is raised and loaded against a glass flat. The glass flat surface is coated with a semi-reflective chromium layer on top of which is a transparent silica spacer layer. Light waves reflected from the chromium layer and the ball surface undergo optical interference upon recombination, generating an interference image of the contact between the ball and the glass surface which is then captured by an RGB camera. Tribofilm thickness is determined from this interferometry image using calibrated RGB values.
3.2 Test Conditions and Procedures
The contact conditions used in the MTM-SLIM tests are listed in Table 2. Under these conditions, the initial lambda ratio (ratio of EHD film thickness to composite surface roughness) was calculated to be 0.1; thus these rubbing contacts were in the boundary and mixed lubrication regime. The development of ZDDP tribofilm on the ball was monitored by SLIM measurements performed at set intervals throughout each 4 h test. All tests shown in this paper were repeated twice and the differences of tribofilm thickness and friction coefficient between repeat tests were always less than 10%. The values plotted on the relevant graphs are representative values obtained from one result.
Some tests consisted of two stages, one in which the ball and disc were rubbed in ZDDP solutions and the second in which the ball and disc, having pre-formed ZDDP tribofilms, were rubbed in additive-free base oil (GTL 3). Before this second stage the ball and disc were removed from the test rig, rinsed in hexane to remove all ZDDP solution and then three times in GTL 3. They were then returned to the rig to be rubbed in GTL 3.
3.3 Tribofilm Analysis
At the end of the 4-h rubbing tests, tribofilms on the balls were analysed using XPS and nanoindentation. Before analysis, discs were lightly rinsed in toluene without any wiping, to remove supernatant liquid but avoid removing tribofilm from the surfaces.
3.3.1 Tribofilm Chemical Characterization (XPS)
A Thermo Fisher K-Alpha spectrophotometer was used for X-ray photoelectron spectroscopy (XPS) to analyse the chemical structure of ZDDP tribofilms. A monochromatic Al-Kα X-ray source (energy = 1486.6 eV) was employed. The X-ray source was operated at 6 mA emission current and 12 kV anode bias. The calibration of the spectrometer was performed using Au (4f7/2), Cu (2p3/2) and Ag (3d5/2) signals. The spot size was an elliptical shape with a long axis of 200 µm to make sure that the analysis area was within the wear track. From this method, the majority of photoemission comes from tribofilm, but some photoemission from outside the wear track might not be completely ruled out. Samples were positioned at 0º emission angle. Base pressure in the main chamber of the spectrometer was 2 × 10−9 mbar. A survey spectrum was acquired in a high-pass energy (200 eV) scanned mode. A narrow scan, to determine the relative atomic composition as well as for determination of chemical information, was then acquired in a low-pass energy (20 eV) mode. The charging of the specimen was corrected by referring aliphatic carbon binding energy to 284.8 eV (C 1 s signal) [19]. This applied a small correction of the measured C 1 s signals from 285.2 ± 0.2 to 284.8 eV.
3.3.2 Hardness Measurement (Nano Indentation)
The hardness depth profiles of MTM balls with tribofilms were measured using a nanoindentation method. A Nano Indenter G 200 with a Berkovich tip manufactured by Keysight Technologies was employed. Tests were carried out in the Continuous Stiffness Measurement (CSM) mode, which allows measurement of the contact stiffness at any point along the loading curve [20]. The hardness at each indentation depth was determined by analysing the amplitude and phase response. The frequency and displacement amplitude values were 45 Hz and 2 nm, respectively. Surface approach velocity was 10 nm/s. With a load range of 50 mN, a target depth of 2 µm and a strain rate of 0.01 s−1, 25 points at 25 µm intervals were obtained. All measurements were carried out at room temperature. Generally, the true mechanical properties of surface-treated materials can only be determined from indentation data originating from the outermost 10% of the surface treatment because of the well-known substrate effect [21], so the hardness of tribofilms were quantified at such an outermost region.
4 Results
4.1 Effect of ZDDP Structure on Tribofilm Formation
Figure 2 shows the evolution of ZDDP tribofilm thickness over 4 h rubbing tests, determined using SLIM. Four ZDDPs are compared, all at a concentration of 300 ppm P. ZDDP2-C6(300) and ZDDP1-C8(300) gradually form tribofilm to reach ca. 140 nm after 2 h rubbing and ca. 80 nm after 2 h rubbing, respectively. By comparison, ZDDP2-C3/C6(300) and ZDDP2-C4(300) very quickly form ca. 120 nm tribofilm after 0.5 h rubbing, and then, interestingly, most of this tribofilm is removed before 1 h rubbing, to leave only ca. 25 nm. After this, no more tribofilm is developed until the end of the test so the tribofilm thickness stays at 25 nm. This result suggests that the chemical structure of ZDDP significantly influences ZDDP tribofilm formation and removal. The observed impact of ZDDP structure on ZDDP tribofilm growth and loss broadly agrees with previous studies. For example, Fujita et al. [22] found that while ZDDP2-C3/C6 gave some tribofilm removal after rubbing, no loss of tribofilm with ZDDP1-C8 was seen. Dawczyk et al. [2] also showed that a ZDDP composed mainly from secondary alkyl structure, believed to be a ZDDP2-C3/C6, formed a tribofilm very rapidly, but this tribofilm became unstable after about 1 h of rubbing to be partially removed in the later stages of a test. It should also be noted that commercial mixed ZDDP2-C3/C6s are available having various ratios of the two alkyl groups and the proportions are very rarely stated in publications, so direct comparison is difficult. It should also be noted that, based on the measured surface profiles of test specimens after rubbing and tribofilm removal, no wear was observed on test specimens after rubbing in any of the tests.
Figure 3 shows optical micrographs of tribofilms on the MTM discs after 4 h rubbing. While ZDDP2-C6(300) and ZDDP1-C8(300) formed rough but evenly distributed tribofilms in the wear track, ZDDP2-C3/C6(300) and ZDDP2-C4(300) formed very patchy tribofilms, with areas where almost the complete tribofilm were lost.
Figure 4 shows how MTM friction coefficient versus entrainment speed evolves with rubbing time. For ZDDP2-C6(300) and ZDDP1-C8(300), the two characteristic features of friction behaviour with ZDDP tribofilm growth can be seen; (i) a shift of the mixed lubrication region of the friction curves to higher entrainment speed, and (ii) an increase in slow speed boundary friction. Recently, Dawczyk et al. [2] showed that the increase in MTM friction in mixed lubrication conditions originates from the increase in effective roughness of the rubbing surfaces due to the formation of ZDDP tribofilms. By comparison, ZDDP2-C3/C6(300) and ZDDP2-C4(300) shows a similar trend as above until 0.5 h rubbing, then a different trend is observed; (i) a shift of the mixed lubrication regime of the friction curve to lower entrainment speed and (ii) a very high boundary friction at slow speed. This transition is seen only after partial tribofilm removal after 1 h rubbing. Generally, the trends in increasing friction coefficient follows the tribofilm growth shown in Fig. 2 with high friction in mixed lubrication only when a thick tribofilm is present.
4.2 Effect of ZDDP Concentration on Tribofilm Formation
The effect of varying ZDDP concentration on tribofilm formation was studied for ZDDP2-C6 and ZDDP2-C3/C6. Results are shown in Fig. 5. For ZDDP2-C6 no tribofilm removal is seen at any P concentration. Instead, tribofilm formation rate increases steadily with rubbing time, with rate of formation increasing from 50 to 300 ppm P concentration. Above 300 ppm the system appears to saturate since both rate of formation and eventual thickness of tribofilm are same at 300 and 800 ppm P. This saturation of tribofilm growth with increased ZDDP concentration was previously observed by Fujita et al. [22]. By contrast, for ZDDP2-C3/C6, at low concentrations the rate of tribofilm formation increases with P concentration to reach 45 nm and 161 nm maximum tribofilm thickness at 50 ppm and 100 ppm P, respectively. No sudden loss of tribofilm is seen. However, at 300 ppm and 800 ppm P a tribofilm initially forms much more rapidly, and then tribofilm is partially removed after 30 min rubbing. Figure 6 shows how MTM friction coefficient versus entrainment speed evolves before and after rubbing at different P concentrations for the same two ZDDPs. Initial friction coefficient was equivalent in all cases. ZDDP2-C6 all showed similar friction curves which matched those of ZDDP2-C3/C6 with 100 ppm of P. By comparison, for ZDDP2-C3/C6, 300 and 800 ppm P gave a higher boundary friction and gave a transition to mixed lubrication regime at slower speed than those with 50 ppm and 100 ppm P.
4.3 Effect of Load and Temperature on Tribofilm Development
Figure 7 shows the influence of load and temperature on the formation and removal of ZDDP2-C3/C6 tribofilm. When ZDDP2-C3/C6(100) is rubbed at higher load (75N) and higher temperature (130 °C) than shown previously, the behaviour of a 100 ppm P solution becomes similar to that seen for 300 and 800 ppm P, i.e. rapid initial tribofilm growth followed by removal to ca. 30 nm with no further change thereafter.
4.4 Hardness of ZDDP Tribofilms
The harnesses of some of the ZDDP tribofilms on the balls were measured using nano-indentation. Figure 8 shows the depth profile of tribofilm hardness from ZDDP2-C3/C6, while Fig. 9 shows the measured hardness calculated from the depth profile. Tribofilms of ZDDP2-C3/C6(100) after 240 min rubbing and ZDDP2-C3/C6(300) after 30 min rubbing have ca. 150 nm and 120 nm thickness, respectively. The tribofilm of ZDDP2-C3/C6(100) after 240 min has higher hardness (4 GPa) than the one formed from ZDDP2-C3/C6(300) after 30 min, 3 GPa. These results agree with a previous study as Kalin et al. [16] who showed that ZDDP tribofilms became harder with extended rubbing. It has been shown previously that ZDDP tribofilms become more durable after extended rubbing [14] due to a change in structure [15] and this would be expected to be accompanied by an increase in hardness.
4.5 Effect of Replacing ZDDP Solution by Base Oil and by Fresh ZDDP Solution
To further compare the properties of the tribofilms formed under different conditions, tests were carried out in which the test was halted, and the ZDDP solution drained and replaced by GTL 3 base oil, as described above in Sect. 3.2. Figure 10 shows the effect of this on tribofilm development. Two curves show tests on ZDDP-C3/C6(100) and ZDDP-C3/C6(300) without replacement of the ZDDP solution and emphasise the large difference in behaviour at the two concentrations. The other two curves show the effect of replacing ZDDP solution by GTL 3 base oil and then resuming rubbing for a further 30 min. For the 100 ppm solution this is done after 3.5 h rubbing and it is evident that further rubbing in base oil has no effect on the tribofilm–the tribofilm is strong enough to withstand rubbing. For the 300 ppm solution, replacement is made after 30 min rubbing in ZDDP solution and it can be seen that the tribofilm is now partially lost during rubbing in base oil–indicating that it is relatively weak. This behaviour is similar to that observed previously by Parsaeian et al. [14] and Ueda et al. [15].
Figure 11 shows a test with ZDD2-C3/C6(300) in which the ZDDP solution was drained after 1 h rubbing (at which stage most of the tribofilm had been lost) and was replaced by a fresh solution of the same ZDDP. No further film growth occurred. This eliminates the possibility that a tribofilm does not reform after being lost because the original ZDDP had been consumed.
5 Discussion
This study has provided clear evidence that ZDDPs having primary alkyl groups and long chain secondary alkyl groups form tribofilms that grow steadily and monotonically until they reach thick and stable thicknesses. By contrast, in the conditions studied, very reactive ZDDPs having short chain secondary alkyl groups such as C3 and C4 form tribofilms very rapidly but these tribofilms are then suddenly partially removed after approximately 30 min rubbing. After this partial removal no further tribofilm grows. These two responses produce very different steady-state tribofilm thicknesses and friction behaviour. It is therefore of interest to understand the origin of these differences and (i) why such tribofilms that form rapidly are partially removed and (ii) why tribofilm does not grow again after this sudden removal.
To identify any differences in chemical structure of the ZDDP tribofilms, the outermost few nanometers of some of the tribofilms on the balls were analysed using XPS. Figure 12 shows the Zn/O and BO/NBO ratios of the tribofilms (i) after 30 min rubbing of ZDDP2-C3/C6(300) before any tribofilm removal (ii) after 50 min rubbing of ZDDP2-C3/C6(300) which is after tribofilm removal, and (iii) after 240 min rubbing of ZDDP2-C3/C6(100) without any tribofilm removal. The tribofilm formation of these three blends was shown in Fig. 5. The ratios of bridging oxygen (BO) to non-bridging oxygen (NBO) were calculated from the O 1 s BO bond peak area (533.4 ± 0.1 eV, red line) and NBO peak area (531.8 ± 0.1 eV, blue line).
XPS analysis shows significant differences in both Zn/O and BO/NBO and in particular it can be seen that prior to tribofilm removal in the test using ZDDP2-C3/C6(300), the Zn/O ratio is very low and the BO/NBO ratio is unusually high.
To help interpret these results, Fig. 13, adapted from Appendix A in [9], shows the main forms of phosphate that might form from ZDDP.
As outlined in the Introduction, ZDDP is generally believed to form initially a polyphosphate tribofilm, but this then converts to a short chain ortho- or pyrophosphate after prolonged rubbing [11, 14, 15, 23]. The theoretical Zn/O and BO/NBO ratios of the various phosphate structures in Fig, 13 can be calculated as listed in Table 3. Based on this, the Zn/O ratios measured for the partial ZDDP2-C3/C6(300) after removal and for the ZDDP2-C3/C6(100) tribofilm that is not removed at all are both indicative of polyphosphate, as suggested in much previous work [11, 14, 15, 23]. However, the Zn/O for the ZDDP2-C3/C6(300) prior to removal is extremely low. This is strongly suggestive of a high proportion of ultraphosphate. Deductions from the BO/NBO are more problematic since this does not take account of any possible bridging or non-bridging S, but it is still higher than expected for a metaphosphate.
It thus appears that the tribofilms that are formed very rapidly by the C3/C6 and C4 ZDDPs contain a significant proportion of ultraphosphate. The formation of this phosphate structure has been suggested previously by Heuberger et al. [11] in thermal films (who termed it “cross-linked polyphosphate”) and by Zhou et al. [24]. Ultraphosphate may be produced when very reactive ZDDP molecules connect together extremely rapidly to saturate all the potentially available linkage sites on each P atom. Structurally and compositionally, in the absence of any Zn it is identical to the polymeric, tetragonal allotrope of P2O5 [25]. Because the tribofilm has very few, if any, stabilizing Zn cations it is likely to be relatively weak, which explains its sudden loss once it reaches a critical thickness. This was previously suggested by Dorgham et al. [18]. Its removal then exposes a more conventional, mature subsurface ZDDP tribofilm composition with BO/NBO typical of a short to medium chain polyphosphate. The quite low Zn/O probably reflects some replacement of Zn ions by Fe ions in this lower region of the tribofilm, as noted in previous studies [26].
By comparison, when tribofilm develops more slowly, a conventional tribofilm mainly consisting of short chain polyphosphates based on Zn and/or Fe cations is formed. Such a phosphate is stronger, with higher hardness than that formed very rapidly, as shown in Fig. 9, and does not experience a sudden partial removal; instead it grows steadily to form a very thick tribofilm. This slower tribofilm formation occurs with long chain secondary and with primary ZDDPs but also with short chain secondary ZDDPs at low concentrations and low load/temperature combinations, as shown in Fig. 5 and Fig. 7.
An important question that arises is why ZDDP tribofilm growth does not resume after its sudden and partial removal. This is clearly not due to all the ZDDP in solution being consumed since, as shown in Fig. 11, tribofilm growth does not resume even when fresh ZDDP is added. One possibility is that ZDDP molecules do not adsorb on the exposed phosphate sub-surface. Alternatively, this surface, unlike steel, is unable to provide the required chemical environment to initiate the ZDDP reaction sequence. A third possibility is that tribofilm does form, but that this is only weakly bonded to the substrate and is thus removed as rapidly as it forms. Previous work has shown that ZDDPs can form tribofilms on some ceramic surfaces but not on others such as SiC and a-C:H DLC, and that when they do form on such surfaces they are often quite weak [27].
It is not clear whether this rapid formation and partial loss of ZDDP tribofilm is a desirable or undesirable feature in practical terms. Clearly the final tribofilm formed by short chain secondary ZDDPs after partial loss is much thinner than that formed more slowly from primary and long chain secondary ZDDPs, but we do not yet know how thick a ZDDP tribofilm needs to be to provide effective protection against wear. Also, the partial loss of the tribofilm appears to produce a smoother tribofilm that gives lower friction in mixed lubrication conditions, although higher boundary friction.
6 Conclusions
In order to further understand what factors influence ZDDP tribofilm removal, a series of ZDDP with varied alkyl structure and concentrations was studied using MTM-SLIM. Key conclusions are as follows;
-
While long chain secondary ZDDP2-C6 and primary ZDDP1-C8 form tribofilms whose thicknesses increases monotonically during extended rubbing, short chain secondary ZDDP2-C3/C6 and ZDDP2-C4 form very thick tribofilms extremely rapidly and then lose most of these tribofilms quite suddenly after about one hour of rubbing. The residual tribofilms are typically only ca. 25 nm thick and remain at this thickness until the end of test.
-
For ZDDP2-C3/C6 this sudden, partial loss of tribofilm thickness is concentration-dependent and only occurs at relatively high ZDDP concentrations. It also occurs more readily at high loads and temperatures and is thus most likely to occur when the tribofilm is formed very rapidly.
-
XPS analysis showed that rapidly-formed tribofilm of ZDDP2-C3/C6(300) present after 30 min has a very little Zn/O and high BO/NBO ratio. This suggests that such tribofilm contains some ultraphosphate, which is likely to have a relatively weak structure due to lack of stabilising cations. This results in the tribofilm being partially removed when it reaches a certain thickness. By comparison, the remaining tribofilm, and also tribofilms that form slowly, have high Zn/O and low BO/NBO. This suggests that they consist of relatively short chain phosphates and are thus stronger and more durable.
-
The finding that the speed of tribofilm growth has an influence on tribofilm removal has practical implications for optimizing the lubricant additive system to protect components from surface failure such as wear.
Data Availability
The research data will be made available upon request.
References
Born, M., Hipeaux, J.C., Marchand, P., Pbtrole, F.: The Relationship between chemical structure and effectiveness of some metallic dialkyl- and diaryl-dithiophosphates in different lubricated mechanisms. Lubr. Sci. 4–2, 93–116 (1992)
Dawczyk, J., Morgan, N., Russo, J., Spikes, H.: Film thickness and friction of ZDDP tribofilms. Tribol. Lett. 67, 34 (2019)
Bennett, P.A.: A surface effect associated with the use of oils containing zinc dialkyl dithiophosphate. ASLE Trans. 2, 78–90 (1959)
Zhang, J., Ueda, M., Campen, S., Spikes, H.: Boundary friction of ZDDP tribofilms. Tribol. Lett. 69, 1–17 (2021)
Tripaldi, G., Vettor, A., Spikes, H.: Friction behaviour of ZDDP films in the mixed, boundary/EHD regime. SAE Trans. 1819–1830 (1996)
Taylor, L.J., Spikes, H.A.: Friction-enhancing properties of ZDDP antiwear additive: part I—friction and morphology of ZDDP reaction films. Tribol. Trans. 46, 303–309 (2003)
Taylor, L., Dratva, A., Spikes, H.A.: Friction and wear behavior of zinc dialkyldithiophosphate additive. Tribol. Trans. 43, 469–479 (2000)
Yamaguchi, E.S., Ryason, P.R.: Inelastic electron tunneling spectra of lubricant oil additives on native aluminum oxide surfaces. Tribol. Trans. 36, 367–374 (1993)
Spikes, H.: The history and mechanisms of ZDDP. Tribol. Lett. 17, 469–489 (2004)
Jones, R.B., Coy, R.C.: The chemistry of the thermal degradation of zinc dialkyldithiophosphate additives. ASLE Trans. 24, 91–97 (1981)
Heuberger, R., Rossi, A., Spencer, N.D.: XPS study of the influence of temperature on ZnDTP tribofilm composition. Tribol. Lett. 25, 185–196 (2007)
Willermet, P.A., Dailey, D.P., Carter, R.O., Schmitz, P.J., Zhu, W., Bell, J.C., Park, D.: The composition of lubricant-derived surface layers formed in a lubricated cam/tappet contact II. Effects of adding overbased detergent and dispersant to a simple ZDTP solution. Tribol. Int. 28, 163–175 (1995)
Philippon, D., De Barros-Bouchet, M.I., Le Mogne, T., Vacher, B., Lerasle, O., Martin, J.M.: A multi-technique approach to the characterization of iron phosphide tribofilm. Thin Solid Films 524, 191–196 (2012)
Parsaeian, P., Ghanbarzadeh, A., Van Eijk, M.C.P., Nedelcu, I., Neville, A., Morina, A.: A new insight into the interfacial mechanisms of the tribofilm formed by zinc dialkyl dithiophosphate. Appl. Surf. Sci. 403, 472–486 (2017)
Ueda, M., Kadiric, A., Spikes, H.A.: On the crystallinity and durability of ZDDP tribofilm. Tribol. Lett. 67, 1–13 (2019)
Kalin, M., Oblak, E., Akbari, S.: Evolution of the nano-scale mechanical properties of tribofilms formed from low- and high-SAPS oils and ZDDP on DLC coatings and steel. Tribol. Int. 96, 43–56 (2016)
Zhang, J., Spikes, H.: On the mechanism of ZDDP antiwear film formation. Tribol. Lett. 63, 1–15 (2016)
Dorgham, A., Parsaeian, P., Azam, A., Wang, C., Morina, A., Neville, A.: Single-asperity study of the reaction kinetics of P-based triboreactive films. Tribol. Int. 133, 288–296 (2019)
Chastain, J., Jr, R.C.K., Handbook of X-ray photoelectron spectroscopy, 1992.
Li, X., Bhushan, B.: A review of nanoindentation continuous stiffness measurement technique and its applications. Mater Charact. 48.1, 11–36 (2002)
Saha, R., Nix, W.D.: Effects of the substrate on the determination of thin film mechanical properties by nanoindentation. Acta Mater. 50, 23–38 (2002)
Fujita, H., Glovnea, R.P., Spikes, H.A.: Study of zinc dialkydithiophosphate antiwear film formation and removal processes, part I: experimental. Tribol. Trans. 48, 558–566 (2005)
Ueda, M., Kadiric, A., Spikes, H.: Influence of steel surface composition on ZDDP tribofilm growth using ion implantation. Tribol. Lett. 1–14 (2021)
Zhou, J.G., Thompson, J., Cutler, J., Blyth, R., Kasrai, M., Bancroft, G.M., Yamaguchi, E.: Resolving the chemical variation of phosphates in thin ZDDP tribofilms by X-ray photoelectron spectroscopy using synchrotron radiation: evidence for ultraphosphates and organic phosphates. Tribol. Lett. 39, 101–107 (2010)
Cormia, R.L., MacKenzie, J.D., Turnbull, D.: Viscous flow and melt allotropy of phosphorus pentoxide. J. Appl. Phys. 34, 2245–2248 (1963)
Ito, K., Martin, J.M., Minfray, C., Kato, K.: Formation mechanism of a low friction ZDDP tribofilm on iron oxide. Tribol. Trans. 50, 211–216 (2007)
Ueda, M., Kadiric, A., Spikes, H.: “ZDDP Tribofilm Formation on Non-Ferrous Surfaces”, Tribol. Online 15, 318–331 (2020)
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mao Ueda. The first draft of the manuscript was written by Mao Ueda and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ueda, M., Spikes, H. ZDDP Tribofilm Formation and Removal. Tribol Lett 72, 109 (2024). https://doi.org/10.1007/s11249-024-01915-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-024-01915-8