Abstract
The oil-separation properties of lubricating greases are responsible for transporting base oil to the bearing contacts. Therefore, a good understanding of these properties is necessary to predict grease life based on physical grease properties. Currently, oil separation for small, aged grease samples collected from bearings, is studied using so-called maintenance tools. These tools give qualitative insight into the grease properties, e.g., increases or decreases in oil separation after ageing of the grease. In this work, a quantitative, lab-scale method to study oil separation is presented. Using this method, the amount of base oil transferred from a grease sample to a piece of blotting paper is measured based on the difference in light transmission through the oil stain and the dry paper. Translation of transmitted light intensity to oil concentration is accomplished using a modified Lambert-Beer’s law. This enables the determination of the instantaneous bleed rate and oil content. In combination with a physical model, this method can help to improve the understanding of the driving forces behind oil separation, e.g., the affinity pressure and permeability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Of the extensive amount of bearings used worldwide, the majority are grease-lubricated [1]. However, rolling bearing life is generally much longer than grease life. Therefore, grease life predictions are of great interest to bearing manufacturers as well as end-users. So far, these predictions are predominantly (semi-)empirical [1,2,3,4,5,6,7]. However, during the last decades, much research has been done to develop more physics-based grease life models. This has led to models for the changes in grease properties, such as the flow properties (starting with the work of Spiegel [8]) or oxidation resistance [9]. The present paper contributes to this further by describing a method that can be used to study the evolution of one of the most important properties of grease in rolling bearings: “oil separation”.
A lubricating grease consists of a thickener that forms a reservoir for the lubricating oil. Depending on the conditions, oil is released into the running track of a rolling bearing, supplying the roller/ball-ring contacts with lubricant. The importance of this mechanism, also called “bleed”, has been recognized already in 1949 by Wilcock and Anderson [10] in their paper with the title “Grease-an oil storehouse for bearings”. This paper showed that the end of grease life is not so much determined by the bleed rate of fresh grease but by the bleed of aged grease in the bearing. However, this bleed depends on several parameters such as the temperature, shear and oil loss in the running track. These depend strongly on how the bearing is used and are thus difficult to specify in laboratory tests. Therefore, grease specifications currently only include the bleed properties of fresh grease.
It is only recently that more work has been published on the evolution/durability of grease bleed in rolling bearings [11,12,13] or in the laboratory [14].
The standardized methods to measure bleed [15, 16] cannot be used to measure the evolution of the bleed properties of small grease samples taken from bearings because these methods require large sample volumes. An often used method to monitor the grease condition is the SKF TKGT 1 test kit [17, 18]. With this test kit, the grease condition is monitored by taking a small grease sample (\(\sim 100\,mg\)) and placing it on a piece of blotting paper. The paper is then left on a heating plate, set to 55 °C, for 120 min. After this time, the diameter of the oil stain that is formed on the paper is measured using a ruler. The stain diameter gives an indication of the (average) bleed rate of the grease on blotting paper. Comparing grease samples of different ages, information is obtained about changes in bleed rate with sample age.
This work presents a more advanced version of the small-scale bleed test, which is able to capture the dynamics of the bleed process, showing the evolution of bleed properties over time. With this, it is possible to determine the bleed rate as a function of the remaining oil content in the grease. The method is based on the use of light absorption to quantify the amount of oil inside blotting paper, as previously used in the work of Zhang et al. [11, 12]. The theoretical background of the method as well as a step-by-step approach will be discussed, showing the experimental set up and result analysis. Compared to the work of Zhang et al. [11, 12] the calibration procedure has been improved, reducing the effects of stray light. Also, the analysis of results is adjusted to focus towards a more detailed analysis of the bleeding process.
2 Experimental Setup
The experimental setup used for this type of experiment, consists of 4 main parts; a light source, a diffuser to homogenize the illumination of the blotting paper, a sample holder and a CCD camera. A schematic representation of the setup is shown in Fig. 1. The light source and diffuser are combined in a Metaphase MB-CBL4X4-W-24CV backlight. The light is connected to a power supply, by which the light intensity can be adjusted by varying its power output. The sample holder is a set of 3D-printed, ABS rings with an inner diameter of 40 mm, outer diameter of 60 mm and a thickness of 3.5 mm. Both rings have four holes, evenly distributed over their perimeter to allow attachment using screws. The CCD camera is an IDS UI-3860LE-M-GL monochrome camera with a Ricoh FL-CC2514-2 M lens. The camera has been mounted above the sample holder and light source using a general laboratory stand. This allows for a consistent distance to the blotting paper, minimizing the need for adjustments to camera settings. The camera is connected to a PC using a standard micro-USB/USB-A type cable. To reduce the amount of stray light, the setup should be placed in a dark room or in a dark enclosure. To further reduce the stray light, non-translucent tape can be used to cover the light source outside the sample holder.
3 Theoretical Background
The translation from light intensity to concentration is based on the same theory as was used and described in the work of Zhang et al. [11, 12]. This same theoretical background is repeated in this work to allow for a complete overview of the presented method and its background.
To relate the transmitted light intensity with the local oil concentration, we use Lambert-Beer’s law:
where q is the extinction coefficient. The blotting paper through which the light will be transmitted will consist of dry paper regions as well as wet paper regions that contain absorbed base oil. Based on this, the extinction coefficient q can be described using three terms, for the paper pores filled with base oil, the empty pores and the paper fibres:
where \(\alpha (s)\) is the fraction of filled pores with radius s, n(s)ds is the number density of pores where \(s \le\) radius \(\le s + {\text {d}}s\), f(s) is the pore size dependence of attenuation and \(\lambda _1, \lambda _2\) and \(q_0\) are positive constants where \(\lambda _2 > \lambda _1\) due to larger attenuation of empty pores compared to filled pores. Substituting Eq. 2 in Eq. 1, integrating over the paper thickness and normalizing the intensity to the background gives:
where r is the distance from the centre of the stain, b the paper thickness and \(I_{\text{bg}}\) the light intensity transmitted through dry paper. The local volume fraction of oil in the paper is equal to:
Due to capillary effects the smaller paper pores will be filled with oil before the larger pores, therefore \(\alpha (s) = 1\) for \(s < s_{\phi }\), for all other values of s, \(\alpha (s) = 0\). The value of \(s_{\phi }\) depends on the local volume fraction of oil:
where \(n_0\) is the average number density of pores where \(s \le\) pore size \(\le s + {\text {d}}s\).
For the range of interest, i.e. \(0< s < S_{\infty }\), it is assumed that the function f(s) can be approximated by \(f(s) = f_0 + f_1 s^{\beta }\) with \(f_0\) and \(f_1\) being constants. Since a pore size of zero does not contribute \(f_0 = 0\) and \(\beta > 0\). Assuming a flat pore distribution, i.e. \(n(s){\text{d}s} = n_0\), Eq. 3 gives:
while \(s_{\phi } \propto \phi ^{1/4}\). Substituting this into Eq. 3 gives:
where \(\phi _*\) is a positive dimensionless constant, scaling with \([b(\lambda _2 - \lambda _1)]^{-w}\) where:
To calculate the total mass of oil in the paper, \(\rho \phi (r)\) is integrated over the volume of the paper:
with S, the integrated logarithmic intensity contrast:
where \(A_{\text{px}}\) is the pixel area and I(i, j) is the locally transmitted light intensity in pixel (i, j).
4 Validation and Calibration
To validate the method described above, the experiment is performed with a drop of oil instead of a grease sample, similar to experiments done to determine the viscosity of base oils [19]. When the oil drop is fully absorbed by the paper, the oil will keep spreading over the pores due to capillary effects which favour the wetting of smaller pores. However, the total amount of oil in the paper, and therefore the integrated logarithmic intensity contrast S, remains constant. This regime in which S remains constant, while the stain still grows, is called the redistribution regime.
Based on this, the assumption that \(f(s) = f_1 s^{\beta }\) from Sect. 3, can be validated and the value of \(w = \frac{4}{\beta + 1}\) (Eq. 8) can be determined. To achieve this, the S(t) curves of the oil drops are constructed for different values of w, to finally find the value where the slope of the curve in the redistribution regime is equal to zero. For the oil drops used in the example (Fig. 2) this resulted in a value of \(w = 1.69 \pm 0.06\).
In Fig. 2b, a calibration curve is constructed by plotting \(S_{\infty }\), which is the value of S in the redistribution regime, versus the mass \(m_{drop}\) of the oil drop. The linear relationship found is in agreement with Eq. 9 and further validates the power-law assumption.
Additionally, the modification of the Lambert-Beer’s law can be validated based on the porosity of the blotting paper. Defining
and
results in
Therefore,
This relation has been plotted (as the blue line) in Fig. 2b.
Using the paper porosity as measured by Zhang et al. [11] using mercury porosimetry, the slope of the \(S_{\infty }\) vs mass curve can be calculated. This is a prediction of the curve obtained from the data and should therefore be close to the best fit curve. Both curves are given in Fig. 2b and are within the expected error. This further validates the use of the modified Lambert-Beer’s law. Additionally, to show the reproducibility of the results, two series of experimental data as well as the datapoints from [11] are plotted in Fig. 2b. For the first series of experimental data \(R^2 = 0.99\) compared to the best fit line. For the second experimental data series and the data reproduced from Zhang et al. [11] \(R^2\) is equal to 0.94 and 0.90 respectively.
Besides the validation of the method, the calibration curve in Fig. 2b provides the conversion factor \(m_* = \rho b \phi _*\) from the slope of the graph \(m_*^{-1} = (\rho b \phi _*)^{-1}\). The conversion factor \(m_*\) is necessary to convert the measured transmitted light intensity to the mass of oil. The conversion factor is obtained by determining the slope of the best fitting linear curve through these data points. The slope (\(dS_{\infty }/dm = L_{\text{sat}}/(\rho b \phi _p)\)) of both curves in Fig. 2b can be given: 53.12 and \(49.91~mm^2/mg\).
5 Experimental Method
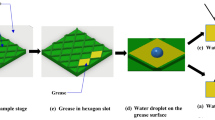
The minimal amount of grease sample required for this experiment is \(\sim 100~mg\). The program responsible for the capture of the images should be set up to be ready for capture. The camera as well as the light source should be turned on. A circular piece of blotting paper with a diameter equal to the outer diameter of the support ring and four holes along its perimeter will be used for the experiment. The support rings are left to the side for now. A plexiglass mask with a sample sized hole (\(\varnothing = 10~mm, h=1.1~mm\)) is used to apply the sample to the blotting paper. When the sample is applied to the paper, the capture program should be started immediately to define the starting time of the experiment properly. After starting the capture program, the support rings are placed around the blotting paper and attached using four screws. Subsequently, the sample holder is placed on top of the light source in a marked location. The setup enclosure is closed to prevent influx of external light and the sample is left for six hours, images of the sample are captured every 20 s. Examples of these images and their corresponding cross-sectional intensity profiles are shown in Fig. 3.
6 Data Analysis
To analyse the captured images and translate them into concentration profiles, several steps have to be taken:
-
1.
Calibrate the pixel size from the inner diameter of the support ring.
-
2.
Subtract the not transmitted background light.
-
3.
Determine the transmitted light intensity outside the stain in the dry paper. This intensity we call \(I_{\text{bg}}\).
-
4.
Measure the local transmitted light intensity in the stain. This intensity we call I(x, y).
-
5.
Convert the intensity profile to a concentration profile according to the relation
$$\begin{aligned} c(x,y)=\rho b \phi _* \left( \ln \left\{ \frac{I(x,y)}{I_{\text{bg}}}\right\} \right) ^w \end{aligned}$$(16) -
6.
Estimate the concentration under the grease patch, which can not be measured.
-
7.
Integrate the obtained concentration profile to get the absorbed mass of oil and the effective radius.
In the following sections, these steps will be discussed in detail.
6.1 Pixel Size
In case of square pixels, the pixel size \(L_{\text{px}} = \sqrt{A_{\text{px}}}\) can be determined from the inner diameter \(D_{\text{ring}}\) of the support ring, by counting the number of pixels \(N_{\text{px}}\) inside the image of the support ring:
So:
In this approach the pixels at the rim are not correctly taken into account. However, since the typical count of pixels inside the ring is \(N=7*10^5\) and the rim contains roughly \(2(\pi N)^{1/2}\) pixels, the relative error in pixel size is \(0.2\%\).
6.2 Non-transmitted Background
In the current setup, the emitting area of the light source is a lot larger than the piece of blotting paper that is used. This results in an unwanted contribution of stray light that was not transmitted through the paper. To minimize this, the parts of the light source outside the support ring, as well as the bottom of the support ring, are covered with non-translucent tape. Although this minimizes the amount of stray light, there might still be small amounts of this light in the images. This contribution has to be removed during the analysis. To do so, the inner contour of the support ring is located in the image using edge detection. The average intensity measured outside this contour \(\Delta I_{\text{nt}}\) is identified as stray light and subtracted from the image inside the contour. The image outside this contour is disregarded in the further analysis. \(\Delta I_{\text{nt}}\) is a measure of the accuracy of the measured transmitted intensities.
6.3 Background Intensity
The background intensity (\(I_{\text{bg}}\)) consists of the light that is transmitted through the dry sections of the blotting paper. To determine the background intensity, the area on and outside the support ring is excluded (which was detected in the previous step). Subsequently, the oil stain and grease patch are identified using edge detection and excluded as well. Afterwards, only the dry paper will remain in the image and the average background intensity can be calculated by taking the sum of all intensities divided by the number of pixels in this area. \(I_{\text{bg}}\) is determined for each image separately as the intensity depends slightly on the distance from the support ring which affects the average intensity as the area of dry paper decreases over time.
6.4 Local Intensity
To determine the local intensity, the irrelevant parts of the image are removed. This can be done based on the earlier detection of the grease patch, the support ring and the oil stain. After removal, an image containing the oil stain, i.e. the wetted part of the blotting paper outside the grease patch, is left. This image, is essentially an array containing the local transmitted light intensity for each pixel in the stain (\(I_{i,j}\)), which can be used for the calculations in the following steps.
6.5 From Intensity to Concentration
The intensity profile obtained in previous steps, can be translated to a concentration profile assuming a unique relation (see Eq. 7) between concentration and intensity reduction due to scattering and absorption. For an example of intensity profiles, see Fig. 4. The grey bar in Fig. 4a indicates the average intensity of a dry piece of blotting paper which matches with the background intensity as measured in all frames.
6.6 Under the Grease Patch
Once the concentration profile (c(r)) is known, the total mass of oil in the stain, outside the grease patch (i.e. for \(r > a\)) can be determined. However, the blotting paper that is covered by the grease patch will also contain a certain amount of oil. As it is not possible to determine the concentration of oil in this section of the paper based on the transmitted light intensity, an estimation has to be made. Since this section of the paper is in direct contact with the grease patch and the oil front only has to move a distance \(b = 186~ \mu m\) to saturate this part of the paper, it is assumed that this region is fully saturated. As a result of this, the volume of oil under the grease patch can be calculated according to:
As discussed in the work of Zhang et al. [11] this leads to a slight overestimation, especially in the initial stages of the experiment, as the paper under the grease patch is not yet fully saturated in this stage.
6.7 Total Mass and Effective Radius
As described in Sect. 3, the total mass of oil absorbed by the paper can be calculated by integrating the concentration over the volume of paper (Eq. 9), which can be written as:
with \(\rho = 0.91\) for the samples shown in Figs. 5 and 6.
As obtained from the value of \(m_*\) from the calibration curve in Fig. 2b.
Alternatively, instead of translating the intensity profile to a concentration profile and integrating it over the paper volume, the integrated logarithmic intensity contrast, S, can be calculated from the intensity profile according to Eq. 10, which can be translated to the total mass of absorbed oil according to Eq. 9. Subsequently, the effective radius, \(R_{\text{eff}}\), can be calculated based on the total mass of absorbed oil (Eq. 21). An example of the total mass as well as the effective radius versus time is given in Fig. 5. Figure 5b not only shows the effective radius as calculated from the total oil mass, it also shows the corresponding front radius which is the radius of the stain as detected in the images. The difference between these radii, for example in the blue curve is approximately \(16.2/12.8 = 1.26\) leading to \(m_1/m_2 = 1.26^2 \approx 1.60\). Which results in an error in oil mass of \(\sim 30\%\). This shows the importance of taking the oil distribution throughout the stain into account. As described in Sect. 1, improvements were made to reduce the effects of stray light. The effect of this correction on the effective radius is shown in Fig. 5c. Especially compared to the difference in effective radii for the different samples, as shown in Fig. 5b, the effect of the stray light correction is significant.
6.8 Bleed Rate and Oil Content
Knowing the total mass m of oil transferred from the grease to the paper over time it is possible to determine the instantaneous bleed rate of the grease:
Plotting the calculated bleed rate as a function of the remaining oil mass \(m_0 - m\), with \(m_0\) the initial oil mass in the sample, gives insight into how the flow of oil is impacted by the availability of oil in the sample. Figure 6 shows the relation between bleed rate and the remaining oil mass for seven grease samples with a different initial oil mass \(m_0\). The initial oil mass of the samples is determined experimentally, by taking a small sample of grease and separating the thickener and base oil. This is done by dissolving the grease in petroleum ether, filtering out the thickener, and evaporating the solvent from the oil-solvent mixture. By weighing the grease and the separate components before and after separation the initial amount of oil in the sample can be determined.
The initial bleed rate is determined based on extrapolation. This can be done by fitting a power law (\(R^2 > 0.99\)) or a second-order polynomial (\(R^2 > 0.99\)) to the final region (\(>45~mg\) remaining oil mass) of the curves. The average value of both fits is indicated in Fig. 6 using the star symbols, the error bars indicate the difference between the extrapolated values of both fits.
As can be observed from the curves in Fig. 6, the bleed rate becomes almost zero for a remaining oil content of about 35 mg, indicating that it becomes very hard to extract all oil from a grease sample. This is the result of changes in the forces that drive the flow of oil, e.g. the difference in oil-thickener affinity, oil-paper affinity and the permeability of the grease thickener matrix.
Example of a number of bleed rate vs. remaining oil content curves as obtained from the method described in this work. Smoothing has been applied to the data to improve readability. Star symbols indicate the initial mass of oil in the sample and the estimated initial bleed rate based on the average of a power law and a second-order polynomial extrapolation
7 Conclusion
Using an optical method, it is possible to determine the local oil concentration in an oil stain on blotting paper. This leads to a more accurate estimate of the total oil content in the paper compared to measuring the stain diameter. After validation of the method, the instantaneous bleed rate of lubricating greases on blotting paper could be measured. Compared to current standardized methods [15, 16] and maintenance tools, the present method is able to:
-
probe the dynamics of the bleed process
-
measure the bleed rate as a function of the remaining mass of oil \(m_0-m\), after determining the initial oil mass \(m_0\) in a separate experiment.
This leads to a better insight in the relation between the release of oil from a grease and the availability of oil in that grease. The obtained results will be useful as experimental input for our current modelling of the bleed rate as a function of the capillary pressure in the grease sample, due to the affinity of the oil for the thickener matrix.
Data Availability
Data supporting the findings in this work is provided within the manuscript, further data is available from the authors upon reasonable request.
Abbreviations
- \(\alpha (s){\text {d}}s\) :
-
Fraction of filled pores with radius between s and \(s + {\text {d}}s\) (-)
- \(\beta\) :
-
Constant (-)
- \(\dot{m}\) :
-
Bleed rate (\(\mu\) g s\(^{-1}\))
- \(\lambda _1, \lambda _2\) :
-
Positive constant (-)
- \(\phi\) :
-
Volume fraction of oil (-)
- \(\phi _*\) :
-
Positive constant (-)
- \(\phi _p\) :
-
Porosity of the paper (-)
- \(\rho\) :
-
Density of the base oil (mg mm\(^{-3}\))
- a :
-
Initial radius of the grease patch (mm)
- \(A_{\text{paper}}\) :
-
Paper area (mm\(^2\))
- \(A_{\text{px}}\) :
-
Pixel area (mm\(^2\))
- \(A_{\text{ring}}\) :
-
Area inside sample holder ring (mm\(^2\))
- b :
-
Paper thickness (mm)
- c :
-
Concentration (mg mm\(^{-3}\))
- \(D_{\text{ring}}\) :
-
Diameter of sample holder ring (mm)
- f(s)ds :
-
Pore size dependence of attenuation (-)
- I :
-
Intensity (-)
- i, j :
-
Pixel coordinates (-)
- \(I_{\text{bg}}\) :
-
Background intensity (-)
- \(I_{\text{nt}}\) :
-
Non-transmitted (stray) light intensity (-)
- \(I_{\text{sat}}\) :
-
Light intensity at full saturation (-)
- \(L_{\text{px}}\) :
-
Length of a pixel (mm)
- m :
-
Mass of oil (mg)
- \(m_*\) :
-
Mass conversion factor (mg mm\(^{-2}\))
- \(m_0\) :
-
Initial oil mass in the grease sample (mg)
- \(m_{drop}\) :
-
Mass of the oil drop (mg)
- \(m_{\text{patch}}\) :
-
Mass of the grease patch (mg)
- n(s)ds :
-
Number density of pores with radius between s and \(s + {\text {d}}s\) (-)
- \(n_0\) :
-
Average number density of pores (-)
- \(N_{\text{px}}\) :
-
Number of pixels (-)
- q :
-
Extinction coefficient (-)
- r, z :
-
Coordinates (-)
- \(R_{\text{eff}}\) :
-
Effective radius (mm)
- S :
-
Integrated logarithmic intensity contrast (mm\(^2\))
- s :
-
Pore radius (mm)
- \(S_{\infty }\) :
-
Integrated logarithmic intensity contrast in the redistribution regime (mm\(^2\))
- \(s_{\phi }\) :
-
Critical pore size (-)
- \(V_{\text{oil}}\) :
-
Volume of oil (mm\(^3\))
- w :
-
Constant (-)
References
Grease Life in Rolling Bearings, Chap. 4, pp. 71–98. Wiley, New York (2012). https://doi.org/10.1002/9781118483961.ch4
Naka, M., Yamazaki, M., Yokouchi, A., Yamamoto, Y.: Anti-seizure performance of lubricating greases in various types of rolling bearings. In: Proceedings of the International Tribology Conference, Nagasaki, pp. 1407–1412 (2000)
Booser, E.R., Khonsari, M.M.: Grease life in ball bearings: the effect of temperatures. Tribol. Lubr. Technol. 66, 36–44 (2010)
Kawamura, T., Minami, M., Hirata, M.: Grease life prediction for sealed ball bearings. Tribol. Trans. 44(2), 256–262 (2001). https://doi.org/10.1080/10402000108982456
Lugt, P.M., Holgerson, M., Reinholdsson, F.: Impact of oxidation on grease life in rolling bearings. Tribol. Int. 188, 108785 (2023). https://doi.org/10.1016/j.triboint.2023.108785
Huiskamp, B.: Grease life in lubricated-for-life deep groove ball bearings. Evolution 2, 26–28 (2004)
GfT, Gesellschaft für Tribologie e.V.: Arbeitsblatt 3, Wälzlagerschmierung, neue überarbeitete Auflage (2006)
Spiegel, K., Fricke, J., Meis, K.-R., Sonntag, F.: Die Fließeigenschaften von Schmierfetten in Abhängigkeit von Beanspruchungsdauer und Temperatur. TA Essl. 3, 21–4121411 (1992)
Rhee, I.S.: Prediction of high temperature grease life using a decomposition kinetic model. NLGI Spokesm. 74(2), 28–35 (2010)
Wilcock, D.F., Anderson, M.: Grease-an oil storehouse for bearings. In: Symposium on Functional Tests for Ball Bearing Greases, ASTM No 84 (1949)
Zhang, Q., Mugele, F., Lugt, P.M., Ende, D.: Characterizing the fluid-matrix affinity in an organogel from the growth dynamics of oil stains on blotting paper. Soft Matter 16(17), 4200–4209 (2020). https://doi.org/10.1039/C9SM01965K
Zhang, Q., Mugele, F., Ende, D., Lugt, P.M.: A model configuration for studying stationary grease bleed in rolling bearings. Tribol. Trans. 64(6), 1127–1137 (2021). https://doi.org/10.1080/10402004.2021.1904071
Akchurin, A., Ende, D., Lugt, P.M.: Modeling impact of grease mechanical ageing on bleed and permeability in rolling bearings. Tribol. Int. 170, 107507 (2022). https://doi.org/10.1016/j.triboint.2022.107507
Hogenberk, F., Osara, J.A., Ende, D., Lugt, P.M.: On the evolution of oil-separation properties of lubricating greases under shear degradation. Tribol. Int. 179, 108154 (2023). https://doi.org/10.1016/j.triboint.2022.108154
Deutsches Institut für Normung: Bestimmung der Ölabscheidung aus Schmierfetten unter statischen Bedingungen. DIN 51817 (1998)
Testing, A.S., Materials: ASTM D1742-88, Standard Test Method for Oil Separation from Lubricating Grease During Storage (1988)
Noordover, A., David, S., Fiddelaers, F., Van Den Kommer, A.: Grease test kit and methods of testing grease. US Patent 9,341,611 (2016)
SKF: SKF Grease Test Kit TKGT 1. Technical report (2022). https://www.skf.com/ph/products/lubrication-management/manual-lubrication-tools/lubricant-analysis-tools/grease-test-kit
De Laurentis, N., Yoe, L., Lugt, P.M.: Methods to measure viscosity of tiny volumes of oil with blotter paper. Tribol. Trans. (2023). https://doi.org/10.1080/10402004.2023.2291112
Acknowledgements
This research was carried out under project number T19021 in the framework of the Research Program of the Materials innovation institute (M2i) (www.m2i.nl) supported by the Dutch government. We would like to thank Robert Jan Meijer for valuable discussions regarding this work and SKF Research & Technology Development for the permission to publish this paper.
Funding
This research was performed at the SKF University Technology Centre (UTC) for Grease Lubrication at the University of Twente and was funded by SKF and M2i.
Author information
Authors and Affiliations
Contributions
F.H. wrote the main manuscript text, D.E. prepared figure 1a and F.H. prepared figures 1b-6. All authors edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hogenberk, F., van den Ende, D., de Rooij, M.B. et al. A Quantitative Method to Measure Oil-Separation Properties of Lubricating Greases. Tribol Lett 72, 102 (2024). https://doi.org/10.1007/s11249-024-01900-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-024-01900-1