Abstract
Many previous researchers have reported the formation of carbonaceous tribofilms from organic lubricants on rubbing metallic surfaces. This paper shows that a very important factor in the formation of such tribofilms is the presence or absence of molecular oxygen. When steel surfaces are rubbed in saturated hydrocarbon lubricants in the absence of oxygen, for example in nitrogen or hydrogen gas, carbonaceous films form very readily, resulting in low friction and wear. However, when a significant amount of oxygen is present, as is the case in air, carbonaceous tribofilms are not generally formed, so friction and wear are very high, with values comparable to those seen when no lubricant is present. In situ Raman analysis combined with gas-switching experiments show that the carbonaceous films formed during rubbing when no oxygen is present are rapidly removed during rubbing in air, while tests in which lubricant is removed during a test in N2 indicate that the films are quite weak. This suggests that these carbonaceous films are being continually removed and replenished during rubbing in oxygen-free conditions. It is proposed that these carbonaceous films are formed from hydrocarbyl free radicals that are generated mechanochemically from hydrocarbon molecules during rubbing. In the absence of oxygen, these free radicals then react together to form a carbonaceous film. However, when oxygen is present, the hydrocarbyl free radicals react extremely rapidly with oxygen molecules to produce hydroperoxyl free radicals and so are no longer available to generate a carbonaceous tribofilm.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It has long been known that when metal surfaces are rubbed together in a liquid or vapourised organic lubricant, carbonaceous tribofilms can form on the rubbing surfaces. This was first recognised in the 1950s in the context of the contamination of electric switch contacts by adventitious, airborne organics and in recent years has become a field of considerable research interest, driven in part by the possibility that such tribofilms might be beneficial in terms of reducing friction and wear.
The phenomenon is, however, relatively sporadic, with carbonaceous films forming easily on some rubbing surfaces and in some conditions but only with difficulty or not at all in others. The research described in this paper will suggest that some of this variation is because the extent to which a carbonaceous tribofilm can form is strongly dependent on the presence or absence of atmospheric oxygen. When lubricated steel surfaces are rubbed together in the boundary and mixed regime in an environment that contains little or no O2, a carbonaceous tribofilm generally forms rapidly. However, when surfaces are rubbed together in air, which contains 21% O2, carbon films form reliably only when catalytic metals such as Mo, Cu, Pt and Pd are present; they can form in the presence of engineering steels but only slowly and under a limited range of conditions.
The current research compares carbonaceous film formation from a variety of hydrocarbon lubricants in a nitrogen and dry air environment. It uses a new, sealed tribometer to extend the range of environments studied to illustrate the kinetics of carbon film formation and removal. It then employs an in situ Raman tribometer to monitor carbonaceous tribofilm formation and removal within a controlled atmospheric environment. Note that in this work, “carbonaceous tribofilm” and “carbon film” refer to tribofilms of organic nature, whose Raman spectra contains the characteristic D- and G-bands. The two terms are used interchangeably.
2 Background
The ability of relatively unreactive organic molecules such as hydrocarbons to form tribofilms on rubbing metal surfaces has been noted and explored several times over the last 70 years in the context of various research fields. It was first reported in electrical switchgear when, in 1958, Hermance and Egan noted the formation of insulating organic deposits on non-arcing palladium and other platinum-group electrical switches [1]. They ascribed these to the reaction of adventitious organic vapour contaminants on rubbing surfaces and termed the product “frictional polymer” [1]. Considerable further research followed in this area [2,3,4,5] and although ability to analyse the product was limited it was determined that the mechanism of formation was most probably a free radical process promoted by surface catalysis and rubbing [4]. The phenomenon is still being researched to the present day [6, 7].
In the 1960s, there was considerable interest in the boundary lubricating ability of hydrocarbon base oils and it was found that these formed carbon-containing films on rubbing steel ball surfaces in 4-ball testers in some conditions [8, 9]. The role of oxygen was explored and it was noted that carbon-based films were formed during rubbing in argon, resulting in superior tribological performance at low loads. However, an oxygen atmosphere gave better performance at very high loads due to oil oxidation products [8].
From ca 2000, there was concern that the release of active hydrogen from organic lubricants during rubbing might embrittle steel to promote rolling contact fatigue and, especially in recent years, might help generate white etching cracks. There was also interest in lubricants for use in space. Both these areas of interest led to research on the behaviour of lubricants in vacuum, where hydrogen and other molecular fragments generated during rubbing of steel surfaces in liquid lubricants and greases could be monitored using a mass spectrometer [10,11,12,13,14]. John et al. detected small hydrocarbon fragments formed from a high MWt hydrocarbon base oil and found that more and smaller fragments were detected when rubbing took place than without rubbing [10]. Tests were also carried out in nitrogen and produced similar hydrocarbon fragments and a black surface tribofilm, which Raman analysis showed to contain graphitic carbon. Kohara et al. tested a range of base fluid types and found that hydrogen was evolved during high friction asperity contact events. By comparing the behaviour of hydrocarbon and fluorocarbon base oils, they demonstrated that the hydrogen originated from the lubricant [11]. Lu et al. found that hydrogen evolution only occurred above a critical applied load and then was both load and sliding speed dependent. They proposed a mechanism based on hydrocarbon adsorption on nascent metal followed by surface-catalysed unzipping of the molecular chain [13].
Over the last decade, research on the formation of carbonaceous films has accelerated considerably. Now a key focus appears to be whether tribofilms formed in this way can be of practical value in terms of reducing friction and wear, although other work has simply reported the formation of such films.
Erdemir and co-workers have explored the possibility of using catalytically active, metal-containing coatings to promote the formation of carbonaceous tribofilms [15]. They found that steel surfaces coated with a composite of copper with molybdenum or vanadium nitride gave lower friction and surface damage than uncoated steel when lubricated with both PAO base oil and a formulated engine oil. A carbonaceous tribofilm was detected on the coated surfaces but not the uncoated ones. The work has subsequently been extended to other types of organic lubricant including methane and ethanol [16,17,18], and to other catalytic metals [19]. Molecular modelling was carried out to help elucidate the mechanism of film formation. This was performed at 1000K to mimic asperity contact temperatures and from it the authors suggested a combination of C–C bond and C–H bond scission leading to dehydrogenation followed by aromatisation. Normally C–H bonds are stronger than C–C ones, but it was suggested that catalysis served to promote a classical dehydrogenation reaction [20].
Wang and co-workers have studied strained ring molecules based on cyclopropane that might undergo C–C scission more easily than other hydrocarbons [21,22,23,24]. They found that the addition of 2.5% wt. of cyclopropyl-carboxylic acid to PAO produced a very large reduction in wear and a more modest reduction in friction. Raman surface analysis indicated the formation of a carbonaceous film on the rubbed surfaces. The researchers proposed that the acid group promotes adsorption on the metal surfaces, where pressure and flash temperature transform the additive to a carbon-based solid film.
Carbonaceous tribofilms have also been detected and credited with reducing wear in tests with an ester biofuel [25], and synthetic [26] and natural [27] ester lubricants, as well as on retrieved metal–metal hip joints, where they were presumed to be formed from denatured proteins [28].
Based on the above, it is evident that the formation of carbonaceous tribofilms can be strongly promoted by surface catalysis. This need not be from deliberately modified surface coatings but may also result from the presence of catalytic additives. Two studies have used Raman analysis to study tribofilm formation by the friction modifier additive molybdenum dialkydithiocarbamate (MoDTC) and noted that a carbon-based film was formed quite readily alongside MoS2 when MoDTC was present, but was not formed from MoDTC-free base oil [29, 30]. The propensity of carbonaceous tribofilm formation on Cu and Pt-group metals was noted above and it is also possible that the carbonaceous film observed on retrieved metal hip implants [29], and in wear tests on such implants [31], is promoted by the presence of catalytic metals in the CoCrMo alloy employed. Recently Khan et al. showed that the extent of carbonaceous film formation and also the level of wear in a reciprocating ball on a flat contact lubricated by PAO varied between steels and suggested that this variation originates from the presence of catalytically active metals and oxides in some alloys [32].
A key aspect of research on carbonaceous tribofilms since the 1990s [33] has been the use of surface Raman spectroscopy to detect a pair of adsorption bands at wavenumbers ca 1350 and 1570 cm−1. These bands, known, respectively, as the D- and G-bands, are indicative of the presence of amorphous or graphitic carbon [34] and their existence on cleaned, rubbed surfaces serves as a relatively straightforward way to establish the presence of a carbonaceous tribofilm. Indeed, detection of these bands was used in all the post-2000 references cited above except for refs 11 to 13. Recently, it has been suggested that these bands should not be taken as proving unequivocally that amorphous or graphitic carbon is produced during rubbing, since it is possible that other types of carbon-rich material may be converted into a graphitic structure during exposure to the intense laser beam used in Raman analysis [35]. This does not, of course, nullify the value of the method for demonstrating the presence of a carbonaceous tribofilm, simply the interpretation of this film as having a graphitic content.
Most of the experimental research outlined above, except for the work in vacuum, was carried out in laboratory air. In early work Vinogradov et al. compared wear and seizure of hydrocarbons in argon and oxygen in four ball tests and found that a hard coating which they ascribed to carbide was formed on the ball surface in argon [8]. Argibay and co-workers carried out controlled atmosphere tests in which a sapphire ball was rubbed against a flat coated in a thin film of Pt/Au alloy [6, 7, 36]. Organic lubricants were either adventitious trace hydrocarbons or, in some tests, a stream of isopropanol/water delivered via the vapour phase. Tests in air gave high friction, but in nitrogen friction dropped to a very low value during rubbing and a carbonaceous film that the authors suggested to be amorphous carbon DLC was identified using Raman. FIB/TEM showed this to be 50 to 100 nm thick.
Recently, the authors of the current paper have carried out a systematic study of the influence of oxygen level on the friction and wear of a reciprocating steel ball on steel disc contact lubricated by isooctane and hexadecane [37]. Typical results are shown in Fig. 1. They indicate that both friction and wear are much lower in nitrogen (and argon) than in dry air.
Raman surface analysis showed that at zero and very low oxygen levels, a carbonaceous film was always formed rapidly on the rubbing surfaces and was present inside and around the rubbed track. In tests in air and above about 5% O2, only iron oxides were detected on the rubbed surfaces.
The drop in friction as oxygen level is reduced shown in Fig. 1 appears at first sight to be progressive but, as can be seen in Fig. 2, when friction is monitored during a test at intermediate oxygen levels, it is evident that friction switches intermittently between a high and low value to give the average value seen in Fig. 1.
Very recently, Li et al. have studied the influence of carrier gas on the lubrication of stainless steel ball on flat contacts by hydrocarbons delivered using vapour phase lubrication [38]. They found similar behaviour to the above, i.e. saturated hydrocarbons produced carbonaceous tribofilms and gave low friction and wear in both 100% N2 and a blend of 10% H2 in N2 carrier gas, but not in O2. When an O2 stream was employed, only an unsaturated hydrocarbon was able to form such a tribofilm.
These studies, together with the work of Argibay [6] and also observations of tests in a vacuum chamber in air and N2 [10], suggest that carbon tribofilm formation can be greatly facilitated, even in the absence of overtly catalytic surfaces, by replacing the oxygen in the atmospheric environment by nitrogen. The current paper explores this further to consider the kinetics of carbonaceous film formation, the composition of the tribofilm, its mechanism of formation, and its potential utility.
3 Experimental Details
3.1 Tribometers
Three tribometer systems were employed in this study.
3.1.1 HFR in a Box
A conventional high-frequency reciprocating rig (HFR) from PCS Instruments was used for some tests. This loads and reciprocates a 6 mm diameter ball against the flat surface of a stationary disc immersed in a lubricant at a controlled temperature. Friction is monitored throughout the test and wear scar on the ball measured at the end of test using a microscope. For this study, the rig was enclosed in a sealed plexiglass box fitted with gas inlet and outlet ports. A zirconia ceramic oxygen sensor was positioned inside the box to monitor oxygen concentration and different levels of oxygen were obtained by combining dry air and nitrogen streams via a mixing valve. The setup is the same as that used in previous work where more details can be found [37]. For the current work, the plexiglass box was fitted with a pair of home-made glove ports to enable rubbed disc samples to be placed directly in a portable nitrogen-filled holder with a transparent window within the box so that they could then be transported and Raman-analysed without exposure to air.
3.1.2 Sealed HFR
To extend the comparison between nitrogen and air to less benign oxygen-free gases, a sealed HFR (HPR) from PCS instruments was employed. This has the same contact configuration as the conventional HFR and uses the same ball and disc specimens, but the lubricant bath and ball and disc holders are entirely enclosed in a stainless steel casing with a small internal volume of ca 60 ml. The test rig is shown in Fig. 3. Sealing of the reciprocating shaft is via two flexible bellows, while an external transducer monitors friction by displacement of the disc holder. The latter differs only from the conventional HFR in that the shaft to the transducer is attached to and passes through a flexible, metal membrane to enable complete sealing. The test rig can operate up to 10 bar pressure and 150 °C.
The small internal volume of this rig facilitates the safe use of gases such as hydrogen and ammonia but also makes it possible to switch gases between alternate flows to completely change the composition of the gas in the test chamber within less than one second, enabling some kinetics of film formation and removal to be explored, as described later in this paper.
3.1.3 MTM in a Box with In Situ Raman
The minitraction machine (MTM) is based on a 19 mm-diameter ball loaded and rotating against the flat surface of a rotating disc. The disc is fully immersed in a temperature-controlled test lubricant and friction is monitored via the lateral displacement of the ball shaft bearing housing. Unlike the HFR, this instrument provides rolling-sliding contact conditions, which are arguably more realistic of the bearing and gear contacts present in most lubricated machines. Because of the way that friction is measured it was not practicable to seal the MTM test chamber, so instead, to enable controlled atmospheric conditions the whole test rig was enclosed in a sealed plexiglass box. Air–nitrogen mixture was premixed to a desired O2 level before entering the MTM test chamber. An oxygen sensor was connected to the gas outlet of the test chamber to monitor the O2 level in the chamber.
Raman spectra of wear tracks on rubbed balls were obtained using in situ Raman spectroscopy (see Fig. 4). This employed a home-built setup described previously [30]. A 400 mW 532 nm continuous wave laser excitation was used. The beam was attenuated by an ND filter, reflected by several moveable mirrors and then expanded before it travelled through a collimator and dichroic. During tests, ball and disc motion were halted intermittently and the ball was raised and pressed upward against a CaF2 window. The laser beam was then focused onto the stationary surface of the ball via this window using a 20 × objective lens (Zeiss EC Epiplan), giving a 6.3 µm spot size of about 11 mW power. Note that this laser power was chosen to ensure that the tribofilm is not significantly altered by the laser beam.
The reflected light and Raman scattering from the ball wear track passed back through the dichroic, a moveable pinhole and a longpass filter before reaching a spectrometer. All optomechanical parts and beam tubes were acquired from ThorLabs, UK while the dichroic and longpass filter were acquired from Chroma, USA. The Raman scattering entered the spectrometer (Shamrock 500i, Andor) through a narrow slit and spectra were recorded on an IDUS 400i CCD camera (Andor). A grating of 1200 lines/mm with 500 nm blaze was selected. The acquisition time was 0.97 s, and 10 spectra were accumulated giving a 10 s sampling rate. The spectrometer was calibrated using the spectral lines of Ne and Hg(Ne) lamps (Oriel Instruments, USA).
Acquired spectra were background-corrected by fitting and subtracting a spline baseline. Spectra presented here are averages of five spectra obtained at different locations on a ball wear track.
3.2 Ex situ Raman Analysis
To analyse the disc surfaces from HFR tests, confocal Raman spectroscopy was carried out using an Alpha 300 RA (WITec, Germany) with a 532 nm laser source, a 600 lines/mm grating with 500 nm blaze and a Zeiss EC Epiplan 20× objective lens. Spectra were taken with an integration time of 0.5 s for 100 accumulations, giving a total acquisition time of 150 s. The laser power was about 3 mW.
3.3 Test Materials and Conditions
All HFR and MTM tests in this study employed AISI 52100 steel ball and disc specimens provided by PCS Instruments. This steel contains ca 1% C, 1.5% Cr and is a very commonly used bearing steel. All the MTM tests ball and disc were through-hardened steel. However, with the HFR and HPR, the tests in hexadecane used hardened steel discs (hard discs), but those in isooctane, which can be considered as a gasoline analogue, used annealed steel discs (soft discs), as specified in the standard diesel fuel lubricity test ASTM D6079. In the HFR, tests on hexadecane employed a longer stroke length and a lower frequency as well as a higher temperature than those on isooctane.
The HFR and HPR test conditions are listed in Table 1 while those for the MTM tests are listed in Table 2.
Two test liquids were employed, isooctane (2,2,4-trimethylpentane) (SigmaAldrich) and n-hexadecane (99%, Merch Millipore). The isooctane was used as received. Hexadecane can be considered as a low viscosity model lubricant and was purified before use by passing it through a column containing activated alumina, silica gel, and 3Å molecular sieve. The isooctane had viscosity 0.47 cP at its test temperature of 25 °C, while hexadecane had viscosity of 1.54 cP at its test temperature of 60 °C.
Four cylinder gases were used, nitrogen (99.998%), dry air (20% O2) and hydrogen (99.99%) supplied by BOC Ltd. and propane (99.5%) supplied by CK Isotopes Ltd.
4 Results
4.1 HFR in a Box
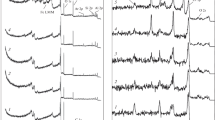
Figure 5 compares HFR friction traces for purified hexadecane in dry air and N2. The upper traces are from three repeats in dry air (20% O2) while the lower three traces (that overlap almost precisely) are from three tests in nitrogen (0% O2). The overall response is similar to that shown for isooctane in Fig. 2, with very high friction in dry air but quite low friction in N2. Friction coefficients are lower with hexadecane than for isooctane and this is believed to be because the lambda ratio (ratio of minimum EHD film thickness to composite surface roughness) in the hexadecane tests (mid stroke Λ ~ 0.15) is higher than in isooctane tests (Λ ~ 0.06). However, it may also originate in part from the difference in disc hardness. Mean ball wear scar diameters for the three repeats were 642 μm in dry air and 128 μm in nitrogen.
Figure 6 shows ex situ Raman spectra taken from the disc wear tracks. These rubbed discs were stored in a N2 atmosphere until immediately before analysis.
In tests in dry air (20% O2), the predominant peaks are those of Fe3O4 (666 cm−1) and α-Fe2O3 (1315 cm−1). However, for tests in N2 (0% O2), the D- and G-bands' characteristic of carbonaceous film are present, though there is still a peak for Fe3O4. We do not believe that these carbon-related Raman bands were significantly affected by the laser beam at the wavelength and intensity used, so that as well as indicating the presence of a carbonaceous tribofilm, they suggest that this tribofilm contains amorphous carbon. These Raman spectra are similar to those seen on discs from isooctane tests in dry air and N2 [37].
It was of interest to determine whether the carbonaceous films that formed in a nitrogen atmosphere were mechanically stable or whether they resulted from a balance of formation and removal. To explore this, tests were carried out using isooctane in N2 in which a carbonaceous film was formed and then all the isooctane removed from the lubricant bath and the test resumed in dry conditions in N2. Isooctane rather than hexadecane was studied since it is volatile and thus easy to remove completely from the test specimens and the lubricant bath. After 75 min rubbing in isooctane, the ball was halted and unloaded while maintaining the N2 atmosphere. Isooctane was then extracted from the lubricant bath using a syringe and the test rig was left for 60 min at the test temperature of 25 °C to ensure all remaining isooctane evaporated. The test was then resumed. Friction traces from two repeat tests are shown in Fig. 7. The traces around the point at which the test was halted and resumed are shown magnified in an inset. Friction started to rise ca. two minutes after rubbing resumed and rose to a high value, similar to that in dry steel/steel conditions, after 4min rubbing. Interestingly, after rubbing resumed, friction was initially lower than during rubbing in isooctane. This might be because the one hour of rest time of the carbonaceous films allowed some rearrangement of the film, perhaps with loss of friction-enhancing free radical dangling bonds.
Friction during three-stage isooctane tests in N2. Stage 1 (blue traces) show rubbing in isooctane. Stage 2 (not shown) is removal of isooctane and one hour rest time. Stage 3 (orange traces) show rubbing of pre-formed carbonaceous film in N2 in dry conditions. Inset shows enlarged view of behaviour at end of stage 1 and beginning of stage 3 (Color figure online)
4.2 HPR
The sealed HFR (HPR) was used to study the effect of switching gases since its very small internal volume made it possible to change atmosphere very rapidly.
Figure 8 shows friction response when the atmosphere was switched between dry air and N2 every 5 min during rubbing in isooctane. Three repeat tests are shown. There is some variation, but all have the same pattern of behaviour, with friction falling in N2 and then rising in dry air. By the third cycle, the response settles down and it can be seen that friction rises relatively slowly over about 2.5 min, presumably due to loss of carbonaceous film, but falls in N2 much more rapidly after about 20s rubbing. Dry air and N2 were both supplied at a flow rate of 1 L/min and the blue trace in Fig. 8 shows the O2 content measured in the outlet flow. The change in atmosphere is almost instantaneous, although it is unlikely that the O2 content within the isooctane will change as rapidly.
Figure 9 shows results from similar gas-switching tests, but now switching between dry air and H2 gas. Two repeat test results are shown. The pattern of response is broadly similar to N2, settling down after two cycles to high friction in air, low friction in H2. This suggests that the difference in friction response originates largely from the presence versus absence of O2 rather than any direct influence of N2 or H2. There are some interesting differences, with H2 continuing to give high friction during its first cycle, but this was also seen with one of the N2 switching tests. Note that the mechanisms of tribofilm formation may depend on the gaseous environment and hence the formation rate and nature of the resulting films in N2 and H2 may differ. Further work would be needed to investigate the specific influence of H2. No O2 trace is shown in Fig. 9 since the fast-response zirconia O2 sensor available was not compatible with H2. However, the flow rates employed were the same as those in dry air/N2 switching tests.
The above two figures show the effect of switching atmospheres above the liquid lubricant isooctane. One limitation of this approach for determining the kinetics of friction change and thus tribofilm formation and removal is that although the change in atmosphere is very fast and quantifiable, it is unlikely that the O2 content of the isooctane reaches equilibrium with the atmosphere above it as rapidly. To obtain a better assessment of the rate of tribofilm formation and removal, some switching tests were therefore carried out using gases.
Figure 10 shows friction variation when a steel ball is rubbed against a hard disc at 25 °C while switching atmospheres between dry air and propane. Three repeats are shown and, while there is considerable variation between tests, it is evident that propane gives lower friction than dry air. The fall in friction when dry air is replaced by propane gas takes typically 1–2 min but the increase in friction when air is introduced is almost instantaneous.
4.3 MTM with Raman
The effect of the atmosphere on the formation of the carbonaceous tribofilm on steel-steel contacts from isooctane was examined in an MTM using Raman spectroscopy in situ to minimise the perturbation of the tribofilm. In these experiments, rubbing took place in nitrogen (0% O2) (Stage 1), after which rubbing was halted and the unloaded, rubbed surfaces rested in 0% O2 (Stage 2), followed by in dry air (20% O2) (Stage 3). Finally, rubbing was resumed in 20% O2 (Stage 4). The duration of each stage is listed in Table 3.
During rubbing in N2 in Stage 1, friction coefficient fell to a low value of 0.15, suggesting the formation of carbonaceous tribofilm. This was confirmed by Raman spectra of the wear tracks obtained in situ at the end of Stage 1 as shown in Fig. 11, where the characteristic D- and G-bands are visible (black trace). Note that the intensity of G-band is stronger than that of D-band in most repeats. While this tribofilm is patchy and its relatively noisy Raman spectrum suggests that it is quite thin, it is clearly adequate to provide low friction.
Resting the wear track in 0% oxygen without rubbing for 20 min (Stage 2) affected the Raman spectra only marginally, with the intensities of the carbon-related spectral peaks dropping slightly (red trace). This suggests that the top-most surface of the tribofilm may be unstable and have different chemistry to the subsurface. Note that in HFR tests, resting in 0% O2 resulted in a reduction in friction of the tribofilm (Fig. 6), also indicative of some change in surface chemistry.
Exposing the stationary tribofilm to 20% O2 (Stage 3) had little further effect of the film (green trace), implying that in the absence of rubbing it was now stable to oxygen. This may also be due to O2 diffusion being limited to only the very top of the tribofilm surface. In some cases, a small peak at around 670 cm−1, which is assigned to Fe3O4, could be observed at this stage. This was attributed to the exposure of pristine steel surface to oxygen due to the patchy nature of the tribofilm.
Finally, in Stage 4 the ball and disc were rubbed together in dry air (20% O2). The friction coefficient rose sharply to a value of ca 0.7, much higher than seen in a N2 atmosphere. Figure 12 shows an in situ Raman spectrum of the ball track at the end of Stage 4. The tribofilm has changed in two ways. Firstly, there are strong peaks at ~ 660 cm−1 and 1315 cm−1, and a shoulder around 609 cm−1, indicating the formation of Fe3O4 and α-Fe2O3. Secondly, the carbon-based film has been largely removed so that it is overwhelmed by the very strong signals from iron oxides. This is illustrated by a lack of a clearly distinguishable D-band, while a peak at the location commonly assigned to G-band may also be assigned to iron oxide. By contrast, the spectra of carbon-rich tribofilms in Stages 1, 2 and 3 have D- and G-bands, with the former slightly weaker than the latter.
In situ Raman spectrum of wear tracks on steel ball taken at the end of Stage 4, where the tribofilm was rubbed in 20% O2. The spectrum is an average of spectra from five locations on a wear track. The two peaks for CaF2 are from the observation window. All spectra are normalised against the highest of the two CaF2 peaks (Color figure online)
Another set of experiments were conducted where Stage 1 was set at 10min. With this reduced rubbing time, the carbon D and G peaks had slightly lower intensities than in the longer Stage 1 tests but were still clearly distinguishable in the Raman spectra of the wear tracks. The two tribofilms behaved similarly upon resting in 0% and 20% O2, which, together with the relatively constant friction coefficient, suggests that apart from the top-most layer, the chemistry of the remaining tribofilm is probably quite uniform.
5 Discussion
5.1 Kinetics of Formation/Removal and Durability of Carbonaceous Films
It is not straightforward to determine rate of formation of carbonaceous film using tests starting in N2 since an oxide layer that may have to be removed by rubbing prior to any tribofilm formation will be present on the surfaces. However, the atmosphere switching tests in propane/air shown in Fig. 10 suggest that the friction drop takes 1 to 2 min once propane is introduced. In Fig. 2, tests with isooctane in a 5% O2 atmosphere showed friction that jumped between a low and high value, believed to represent, respectively, the presence and absence of a carbonaceous film. Figure 13 shows part of one test trace extracted from this figure, chosen to include two such friction excursions. The rate of friction drop is quite variable but occurs over about 50 to 100s.
Magnified view of variation of friction over rubbing time for isooctane in 5% O2, extracted from Fig. 2
This rate of carbonaceous film formation may still represent the net result of film formation and removal. The N2 atmosphere test shown in Fig. 7, where isooctane is removed after carbonaceous film formation and then rubbing resumed, suggests that the film is relatively weak and, unless able to be replenished, can only withstand about two minutes rubbing in HFR conditions.
Both the MTM Raman and the HPR switching tests illustrate the deleterious effect of O2 on a pre-formed carbonaceous film. The propane/air switching tests show that replacement of propane by air causes almost instantaneous increase in friction, suggesting that the carbonaceous film is removed extremely rapidly in reciprocating sliding conditions. The rate of loss of film when isooctane is present is slower, but this may reflect the time needed for oxygen molecules to dissolve in the lubricant. From all the results described above, it is evident that carbonaceous films form much more readily in an inert nitrogen atmosphere than in dry air. It is also evident from the MTM Raman work that the carbonaceous film is not affected greatly by exposure to O2 but is very rapidly removed by rubbing in a 20% O2 environment. The most likely explanation for this is that the tribofilm is weak to rubbing action, so that when the presence of O2 prevents it being replenished, it is rapidly and permanently removed during rubbing, resulting in high friction and wear.
5.2 Mechanism of Formation of Carbonaceous Film
Previous researchers have suggested two main mechanisms for the formation of carbonaceous films on surfaces from organic liquid or vapour during rubbing.
-
(i)
C–C scission of adsorbed molecules to form hydrocarbyl free radicals, driven by mechanical and/or thermal stresses from rubbing, followed by free radical rearrangement and oligomerisation [1, 4, 6, 10, 15, 26]
-
(ii)
Catalytic dehydrogenation, i.e. catalysed cleavage of C-H bonds of adsorbed molecules to form hydrogen and olefin, followed by olefin polymerisation [15, 19, 38].
Most researchers suggest that the metallic substrate plays an important role in catalysing these processes, although some also propose that strong adsorption on this substrate is necessary to immobilise molecules so that the latter can experience high mechanical forces needed to promote C–C cleavage. Lu et al. suggest that alkanes adsorb and react on ferrous metal surfaces only after oxide layers have been removed by rubbing [12, 13].
The predominant mechanism favoured by the authors of this paper to explain the observed results is (i) above, i.e. that hydrocarbon and related organic molecules are sufficiently immobilised on rubbing steel surfaces to experience very high mechanical forces generated due to asperity interaction and that these forces cleave C–C bonds mechanochemically to form hydrocarbyl free radicals. The fact that strained carbon ring molecules preferentially form carbonaceous films [21] supports a free radical process. Note that the flexibility of acyclic hydrocarbons, such as isooctane and hexadecane, may make their C–C bonds less prone to being mechanochemically cleaved than cyclic hydrocarbons. This may affect the concentration of radicals and thus will have implications on the formation rate of the carbon film.
A few researchers have suggested that C–C cleavage might be caused by flash temperatures due to rubbing, and even that such temperatures might exceed 1000 °C [15]. We consider that this is unlikely; such high flash temperatures can only occur at much higher sliding speeds and loads than are present in any of the research to date on carbonaceous films. However, it is now generally accepted that nanoscale mechanical forces acting directly on individual molecules can promote covalent bond breaking and subsequent molecular reactions so that such reactions to occur at much lower temperatures than would otherwise be the case. For example, polymer molecules in melt or solution are well known to undergo homolytic fission at near-central C–C bonds to form hydrocarbyl radicals at room temperature when subjected to elongational stress [39] or even from stresses created during adsorption [40].
What follows then depends on the environment. When the lubricant is in an atmosphere of N2 (or argon [37]), the free radicals will have time to rearrange and interact – for example undergoing intra- and inter-molecular hydrogen atom transfer, isomerism and addition to generate carbon-rich species in a process quite similar to that occurring in low temperature pyrolysis [41]. However, in air, or indeed in an atmosphere with more than about 5% O2, most hydrocarbyl free radicals will react almost immediately with O2 molecules to form peroxy radicals (Eq. 1), and so initiate the hydroperoxide oxidation cycle, rather than rearranging.
Maillard et al. [42] have shown that this reaction has second-order kinetics and is extremely rapid, with a rate constant between 1 and 5×109 M−1s−1 depending on the structure of the free radical. The solubility of oxygen in hydrocarbons is such that in air at atmospheric pressure and 25 °C the oxygen concentration is typically 0.002 M [42, 43]. This means that for a rate constant of 4×109 M−1s−1 (typical of an ethyl free radical), the half-life of the free radical in solution is only ca 100 ns. In consequence, Eq. 1 will pre-empt most other possible radical reactions [44].
Thus, in this scenario, friction-generated hydrocarbyl free radicals can only proceed to form a carbonaceous film if; (i) very low or no oxygen is present; (ii) the rate of radical formation is so fast that local oxygen depletion takes place, which is quite possible within a thin film rubbing contact, or (iii) there is an alternative reaction pathway that does not involve formation of hydrocarbyl free radicals. The last is discussed further below.
The above by no means rules out a catalytic contribution from the substrate and indeed this appears almost certainly the case with the Pt metals as outlined earlier in the Background. The question that arises is whether this is an adjunct to stress-driven C–C bond cleavage, helping to break C–C bonds homolytically to form free radicals, or whether such catalysis provides an alternative, radical-free degradation route such as dehydrogenation [45] or, even heterolytic C–C bond fission to form hydrocarbyl ions [46]. Pt and Pd are also powerful passive autocatalytic recombiners [47], promoting the room temperature reaction of hydrogen with oxygen, and in this role might well help promote carbonaceous tribofilm formation in air by promptly sequestering the hydrogen noted by researchers to be generated during rubbing [12,13,14].
The Pt metals, and the oxides of metals such as Cr, Mo, V and Ga, are well known as dehydrogenation catalysts in which role they promote cleavage of C–H bonds in alkanes to form alkenes and hydrogen [46]. This catalysis normally occurs only at relatively high temperatures, but Pd has recently been shown to dehydrogenate alkanes at room temperature under high applied pressure [48]. It is thus quite possible that the first stage in the formation of carbonaceous films observed with Pt [6, 36] and Cu/metal oxide [15] is dehydrogenation of alkane to form a much more reactive alkene. One question that arises in the context of the current study is whether the presence of oxygen, as studied in the current work, might be expected to hinder such catalysis. This seems unlikely to be the case for metal oxides but might be so for the Pt metals since Sattler et al. report that noble metals are active for dehydrogenation in the metallic state, and in some cases a reduction step is necessary prior to reaction [45].
In addition to inhibiting carbonaceous tribofilm formation, it is also evident from the gas-switching and Raman results outlined above that molecular oxygen promotes removal of such films. Berman et al. have also suggested that very thin graphene films retain their ability to reduce friction only when they are rubbed in a nitrogen atmosphere as opposed to air [49]. This may originate from a direct, stress-promoted oxidation of the carbonaceous film, but based on the current work it may simply be that oxygen prevents carbonaceous film formation by trapping hydrocarbyl free radicals so that any pre-existing film is not replaced as it is removed by rubbing.
The precise mechanism of carbonaceous tribofilm formation is not fully resolved. It has become commonplace to append some reactive MD simulation work to published experimental work on carbonaceous film formation, but unfortunately such modelling simply counts bonds breaking under stress; it does not reveal whether such bond cleavage is homolytic to form free radicals, or heterolytic to produce ions. Nor is the subsequent reactions of these species, whatever their nature, revealed. Evidently, there is scope for more sophisticated modelling studies.
6 Practical Significance
Several previous studies have suggested that the formation of carbonaceous films from organic hydrocarbons during rubbing may be an effective strategy to reduce friction and wear (e.g. [15,16,17, 19, 21]). In the current work, while friction and wear are very greatly reduced in N2 as compared to air for base oils (as evident for hexadecane in Fig. 5), it is important to note that the actual values are not exceptionally low by formulated lubricant standards; they simply appear so compared to the base oil performance in air. Indeed, this is a limitation of much previous work where comparison is often made only between contacts that form carbonaceous tribofilms from hydrocarbons and those that form no tribofilms whatsoever. It is also important to note that for liquid lubricated systems, the actual friction and wear measured will depend on the lambda ratio and so the proportion of the load supported by solid–solid contact, as opposed to fluid film pressure. Thus, the higher friction coefficient seen for isooctane versus hexadecane tests in a N2 atmosphere in Figs. 2 and 5, respectively, does not reflect a higher boundary friction coefficient of the carbonaceous film formed from isooctane but rather a lower lambda ratio for this very low viscosity fluid.
In terms of lubrication performance, it is perhaps relevant to compare friction and wear behaviour of a system that forms a carbonaceous tribofilm with one that forms a conventional lubricant additive tribofilm in air. This is shown in Fig. 14 where three friction traces are shown, one of hexadecane in N2 (taken from Fig. 5) and two for solutions of friction modifier additives, glyceryl monooleate (GMO) and molybdenum dialkyldithiocarbamate (MoDTC), in hexadecane under the same condition except that the atmosphere is now dry air. It is evident that a well-chosen friction modifier additive film in air is more effective than a carbonaceous tribofilm formed in nitrogen. Similarly, it was found that most phosphate-based antiwear additive solutions gave lower wear in dry air than did carbonaceous tribofilms formed from hexadecane alone in nitrogen.
Of course, this does not negate the potential value of carbonaceous tribofilms in reducing friction and wear in systems where formulated lubricants cannot be used, and also, possibly, to augment the tribological performance of such lubricants, especially if these interact favourably with carbon-based surfaces. The current work also suggests that the carbonaceous films formed are relatively weak and need to be continuously replenished but this may not be the case in all such carbonaceous tribofilms forms, especially those on strongly catalytic surfaces.
7 Conclusions
It has been found that the presence of oxygen gas has a profound effect on the ability of organic liquid and gaseous lubricants to form carbonaceous films on rubbing steel surfaces. When lubricated steel surfaces are rubbed together in an oxygen-free environment in both pure sliding and rolling-sliding conditions, a carbonaceous tribofilm forms rapidly on the rubbed tracks, as evidenced by both in situ and ex situ Raman surface analysis. This tribofilm results in quite low friction and wear. However, when rubbing takes place in dry air (which contains 20% O2), no such film is formed, and very high friction and wear are observed.
The carbonaceous tribofilm formed in zero or at low O2 levels appears to be quite weak and is removed rapidly when the organic lubricant is no longer available or when oxygen is introduced into the surrounding atmosphere, with a consequent increase in friction and wear. The tribofilm is thus probably being continuously removed and replenished in zero or low O2 environment.
We consider that the most likely mechanism of this carbonaceous tribofilm formation is mechanochemical in origin, with C–C bonds in surface-adsorbed organic molecules being homolytically cleaved by the high stresses present in asperity rubbing contact to form pairs of hydrocarbyl free radicals. In the absence of oxygen, these free radicals then rearrange, dehydrogenate and recombine in a fashion akin to low temperature pyrolysis, eventually to form amorphous carbonaceous material. However, when oxygen is present, the hydrocarbyl free radicals react very rapidly with oxygen molecules to form hydroperoxy radicals, the classical first stage in hydrocarbon oxidation chemistry. They are thus no longer available to form a carbonaceous tribofilm.
These findings provide important new insight into carbonaceous film formation and also into tribo-oxidation and the tribological behaviour of inerted, sustainable lubricant systems.
Data Availability
Data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Hermance, H.W., Egan, T.F.: Organic deposits on precious metal contacts. Bell Syst. Tech. J. 37(3), 739–776 (1958)
Dietrich, I., Honrath-Barkhansen, M.: The formation of organic insulating films on electrical contacts. Z. Angewandte Physik. 11, 399–403 (1959)
Campbell, W.E., Lee, R.E., Jr.: Polymer formation on sliding metals in air saturated with organic vapors. ASLE Trans. 5, 91–104 (1962)
Chaikin, S.W.: On frictional polymer. Wear 10(1), 49–60 (1967)
Crossland, W., Murphy, P.: The formation of insulating organic films on palladium-silver contact alloys. IEEE Trans Parts, Hybrids Packaging 10(1), 64–73 (1974)
Argibay, N., Babuska, T.F., Curry, J.F., Dugger, M.T., Lu, P., Adams, D.P., Chandross, M.: In-situ tribochemical formation of self-lubricating diamond-like carbon films. Carbon 138, 61–68 (2018)
DelRio, F.W., Mangolini, F., Edwards, C.E., Babuska, T.F., Adams, D.P., Ping, Lu., Curry, J.F.: Revealing the structure-property relationships of amorphous carbon tribofilms on platinum-gold surfaces. Wear 522, 204690 (2023)
Vinogradov, G.V., Arkharova, V.V., Petrov, A.A.: Anti-wear and anti-friction properties of hydrocarbons under heavy loads. Wear 4(4), 274–291 (1961)
Fein, R.S., Kreuz, K.L.: Chemistry of boundary lubrication of steel by hydrocarbons. ASLE Trans. 8(1), 29–38 (1965)
John, P.J., Cutler, J.N., Sanders, J.H.: Tribological behavior of a multialkylated cyclopentane oil under ultrahigh vacuum conditions. Tribol. Lett. 9, 167–173 (2001)
Kohara, M., Kawamura, T., Egami, M.: Study on mechanism of hydrogen generation from lubricants. Tribol. Trans. 49(1), 53–60 (2006)
Lu, R., Minami, I., Nanao, H., Mori, S.: Investigation of decomposition of hydrocarbon oil on the nascent surface of steel. Tribol. Lett. 27, 25–30 (2007)
Lu, R., Nanao, H., Kobayashi, K., Kubo, T., Mori, S.: Effect of lubricant additives on tribochemical decomposition of hydrocarbon oil on nascent steel surfaces. J. Jpn. Petrol. Inst. 53(1), 55–60 (2010)
Kuerten, D., Winzer, N., Kailer, A., Pfeiffer, W., Spallek, R., Scherge, M.: In-situ detection of hydrogen evolution in a lubricated sliding pin on disk test under high vacuum. Tribol. Int. 93, 324–331 (2016)
Erdemir, A., Ramirez, G., Eryilmaz, O.L., Narayanan, B., Liao, Y., Kamath, G., Sankaranarayanan, S.K.: Carbon-based tribofilms from lubricating oils. Nature 536(7614), 67–71 (2016)
Ramirez, G., Eryilmaz, O.L., Fatti, G., Righi, M.C., Wen, J., Erdemir, A.: Tribochemical conversion of methane to graphene and other carbon nanostructures: implications for friction and wear. ACS Appl. Nano Mater. 3(8), 8060–8067 (2020)
Shirani, A., Li, Y., Smith, J., Curry, J.F., Lu, P., Wilson, M., Chandross, M., Argibay, N., Berman, D.: Mechanochemically driven formation of protective carbon films from ethanol environment. Mater. Today Chem. 26, 101112 (2022)
Shirani, A., Li, Y., Eryilmaz, O.L., Berman, D.: Tribocatalytically-activated formation of protective friction and wear reducing carbon coatings from alkane environment. Sci. Rep. 11(1), 20643 (2021)
Xu, X., Xu, Z., Sun, J., Tang, G., Su, F.: In situ synthesizing carbon-based film by tribo-induced catalytic degradation of poly-α-olefin oil for reducing friction and wear. Langmuir 36(35), 10555–10564 (2020)
Sattler, J.J.H.B., Ruiz-Martinez, J., Santillan-Jimenez, E., Weckhuysen, B.M.: Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014)
Johnson, B., Wu, H., Desanker, M., Pickens, D., Chung, Y.W., Wang, Q.J.: Direct formation of lubricious and wear-protective carbon films from phosphorus-and sulfur-free oil-soluble additives. Tribol. Lett. 66, 1–13 (2018)
Wu, H., Khan, A.M., Johnson, B., Sasikumar, K., Chung, Y.W., Wang, Q.J.: Formation and nature of carbon-containing tribofilms. ACS Appl. Mater. Interf. 11(17), 16139–16146 (2019)
Khan, A.M., Wu, H., Ma, Q., Chung, Y.W., Wang, Q.J.: Relating tribological performance and tribofilm formation to the adsorption strength of surface-active precursors. Tribol. Lett. 68, 1–9 (2020)
Ma, Q., Khan, A.M., Wang, Q.J.: Dependence of tribological performance and tribopolymerization on the surface binding strength of selected cycloalkane-carboxylic acid additives. Tribol. Lett. 68, 1–10 (2020)
Fuadi, Z., Adachi, K., Muhammad, T.: Formation of carbon-based tribofilm under palm methyl ester. Tribol. Lett. 66, 1–11 (2018)
Yu, H., Chen, H., Zheng, Z., Ba, Z., Qiao, D., Feng, D., Gong, Z., Dong, G.: Transformation mechanism between the frictional interface under dioctyl sebacate lubrication. Tribol. Int. 155, 106745 (2021)
Rouhani, M., Hobley, J., Hsin-Hung, O., Lee, J.-T., Metla, S.B.S., Jeng, Y.-R.: A new gateway to ecofriendly self-healing amorphous carbon tribofilms from ancient oils.”. Appl. Mater. Today 29, 101616 (2022)
Liao, Y., Pourzal, R., Wimmer, M.A., Jacobs, J.J., Fischer, A., Marks, L.D.: Graphitic tribological layers in metal-on-metal hip replacements. Science 334(6063), 1687–1690 (2011)
Khaemba, D.N., Neville, A., Morina, A.: A methodology for Raman characterisation of MoDTC tribofilms and its application in investigating the influence of surface chemistry on friction performance of MoDTC lubricants. Tribol. Lett. 59, 1–17 (2015)
Garcia, G.C., Ueda, M., Spikes, H.A., Wong, J.: Temperature dependence of molybdenum dialkyl dithiocarbamate (MoDTC) tribofilms via time-resolved Raman spectroscopy. Sci. Rep. 11(1), 3621 (2021)
Wimmer, M.A., Laurent, M.P., Mathew, M.T., Nagelli, C., Liao, Y., Marks, L.D., Fischer, A.: The effect of contact load on CoCrMo wear and the formation and retention of tribofilms. Wear 332, 643–649 (2015)
Khan, A.M., Ahmed, J., Liu, S., Martin, T., Berkebile, S., Chung, Y.W., Wang, Q.J.: Formation of wear-protective tribofilms on different steel surfaces during lubricated sliding. Tribol. Lett. 71(2), 63 (2023)
Cheng, U.C., Stair, P.C.: In situ study of multialkylated cyclopentane and perfluoropolyalkyl ether chemistry in concentrated contacts using ultraviolet Raman spectroscopy. Tribol. Lett. 4(2), 163–170 (1998)
Merlen, A., Buijnsters, J.G., Pardanaud, C.: A guide to and review of the use of multiwavelength Raman spectroscopy for characterizing defective aromatic carbon solids: from graphene to amorphous carbons. Coatings 7(10), 153 (2017)
Li, Y.-S., Seokhoon, J., Arman, M.K., Martin, T.V., Ogrinc, A.L., Jane Wang, Q., Martini, A., Chung, Y.-W., Kim, S.H.: Possible origin of D-and G-band features in Raman spectra of tribofilms. Tribol. Lett. 71(2), 57 (2023)
Jones, M.R., DelRio, F.W., Beechem, T.E., McDonald, A.E., Babuska, T.F., Dugger, M.T., Chandross, M., Argibay, N., Curry, J.F.: Stress-and time-dependent formation of self-lubricating In situ carbon (SLIC) films on catalytically-active noble alloys.". JOM 73, 3658–3667 (2021)
Zhang, J., Campen, S., Wong, J.S.S., Spikes, H.A.: Oxidational wear in lubricated contacts – or is it? Trib. Intern. 165, 107287 (2022)
Li, Y.S., Jang, S., Bhuiyan, F.H., Martini, A., Kim, S.H.: Molecular structure and environment dependence of shear-driven chemical reactions: Tribopolymerization of methylcyclopentane, cyclohexane and cyclohexene on stainless steel. Tribol. Lett. 71(2), 49 (2023)
Caruso, M.M., Davis, D.A., Shen, Q., Odom, S.A., Sottos, N.R., White, S.R., Moore, J.S.: Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 109(11), 5755–5798 (2009)
Sheiko, S.S., Sun, F.C., Randall, A., Shirvanyants, D., Rubinstein, M., Lee, H.I., Matyjaszewski, K.: Adsorption-induced scission of carbon-carbon bonds. Nature 440(7081), 191–194 (2006)
Bounaceur, R., Warth, V., Marquaire, P.M., Scacchi, G., Dominé, F., Dessort, D., Pradier, B., Brevart, O.: Modeling of hydrocarbons pyrolysis at low temperature. Automatic generation of free radicals mechanisms. J. Analyt. Appl. Pyrol. 64(1), 103–122 (2002)
Maillard, B., Ingold, K.U., Scaiano, J.C.: Rate constants for the reactions of free radicals with oxygen in solution. J. Am. Chem. Soc. 105(15), 5095–5099 (1983)
Battino, R., Rettich, T.R., Tominaga, T.: The solubility of oxygen and ozone in liquids. J. Phys. Chem. Ref. Data 12(2), 163–178 (1983)
Tsang, W.: The stability of alkyl radicals. J. Am. Chem. Soc. 107(10), 2872–2880 (1985)
Sattler, J.J., Ruiz-Martinez, J., Santillan-Jimenez, E., Weckhuysen, B.M.: Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114(20), 10613–10653 (2014)
Sakaguchi, M., Miwa, Y., Hara, S., Sugino, Y., Yamamoto, K., Shimada, S.: Triboelectricity in polymers: effects of the ionic nature of carbon–carbon bonds in the polymer main chain on charge due to yield of mechano-anions produced by heterogeneous scission of the carbon–carbon bond by mechanical fracture. J. Electrostat. 62(1), 35–50 (2004)
Bachellerie, E., Arnould, F., Auglaire, M., De Boeck, B., Braillard, O., Eckardt, B., Ferroni, F., Moffett, R.: Generic approach for designing and implementing a passive autocatalytic recombiner PAR-system in nuclear power plant containments. Nucl. Eng. Des. 221(1–3), 151–165 (2003)
Frost, M., McBride, E.E., Smith, D., Smith, J.S., Glenzer, S.H.: Pressure driven dehydrogenation by palladium metal. Adv. Mater. Interf. 10(9), 2202081 (2023)
Berman, D., Erdemir, A., Sumant, A.V.: Reduced wear and friction enabled by graphene layers on sliding steel surfaces in dry nitrogen. Carbon 59(2013), 167–175 (2013)
Acknowledgements
The authors wish to acknowledge the contribution of Mr C.H. Leung who carried out some of the HFR tests and Raman analysis for this study and PCS Instruments for supplying the HPR. B.B. is supported by the Shell University Technology Centre (UTC) for Fuels and Lubricants. For the purpose of open access, the author has applied for a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising.
Funding
JZ and BB are supported by the Shell University Technology Centre (UTC) for Mobility and Lubricants.
Author information
Authors and Affiliations
Contributions
J.Z. and B.B. acquired and analysed the data. H.S. and J.W. conceptualised the work and interpreted the data. H.S. drafted the work. H.S. and J.W. revised the work. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Bolle, B., Wong, J.S.S. et al. Influence of Atmosphere on Carbonaceous Film Formation in Rubbing, Metallic Contacts. Tribol Lett 72, 4 (2024). https://doi.org/10.1007/s11249-023-01801-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11249-023-01801-9