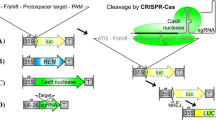
Abstract
The advent of genome editing platforms such as the CRISPR/Cas9 system ushers an unprecedented speed in the development of new crop varieties that can withstand the agricultural challenges of the 21st century. The CRISPR/Cas9 system depends on the specificity of engineered single guide RNAs (sgRNAs). However, sgRNA design in plants can be challenging due to the multitude of design tools to choose from, many of which use guidelines that are based on animal experiments yet allow the use of plant genomes. Upon choosing sgRNAs, it is also unclear whether an in vitro assay is needed to validate the targeting efficiency of a particular sgRNA before in vivo delivery of the CRISPR/Cas9 system. Here, we demonstrate the in vitro and in vivo activity of four different sgRNAs that we selected based on their ability to target multiple members of the eggplant polyphenol oxidase gene family. Some sgRNAs that have high in vitro cleavage activity did not produce edits in vivo, suggesting that an in vitro assay may not be a reliable basis to predict sgRNAs with highly efficient in vivo cleavage activity. Further analysis of our sgRNAs using other design algorithms suggest that plant-validated criteria such as the presence of necessary secondary structures and appropriate base-pairing may be the reason for the discrepancy between our observed in vitro and in vivo cleavage efficiencies. However, recent reports and our data suggests that there is no guaranteed way to ensure the in vivo cleavage of chosen sgRNAs.





Similar content being viewed by others
References
Anderson EM, Haupt A, Schiel JA et al (2015) Systematic analysis of CRISPR–Cas9 mismatch tolerance reveals low levels of off-target activity. J Biotechnol 211:56–65. https://doi.org/10.1016/J.JBIOTEC.2015.06.427
Assem SK, El-itriby H, Hussein EH et al (2002) Comparison of the efficiency of some novel maize promoters in monocot and dicot plants. Arab J Biotechnol 5:57–66
Barchi L, Rabanus-Wallace MT, Prohens J et al (2021) Improved genome assembly and pan-genome provide key insights into eggplant domestication and breeding. Plant J. https://doi.org/10.1111/tpj.15313
Bell CC, Magor GW, Gillinder KR, Perkins AC (2014) A high-throughput screening strategy for detecting CRISPR–Cas9 induced mutations using next-generation sequencing. BMC Genomics 15:1–7. https://doi.org/10.1186/1471-2164-15-1002/FIGURES/3
Bente H, Mittelsten Scheid O, Donà M (2020) Versatile in vitro assay to recognize Cas9-induced mutations. Plant Direct 4:e00269. https://doi.org/10.1002/PLD3.269
Campenhout C, Van, Cabochette P, Veillard A-C et al (2019) Guidelines for optimized gene knockout using CRISPR/Cas9. Biotechniques 66:295–302. https://doi.org/10.2144/btn-2018-0187
Chen Y, Wang X (2022) Evaluation of efficiency prediction algorithms and development of ensemble model for CRISPR/Cas9 gRNA selection. Bioinformatics 38:5175–5181. https://doi.org/10.1093/BIOINFORMATICS/BTAC681
Chung CH, Allen AG, Sullivan NT et al (2020) Computational analysis concerning the impact of DNA accessibility on CRISPR–Cas9 cleavage efficiency. Mol Ther 28:19–28. https://doi.org/10.1016/j.ymthe.2019.10.008
Concordet JP, Haeussler M (2018) CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res 46:W242–W245. https://doi.org/10.1093/NAR/GKY354
Doench JG, Fusi N, Sullender M et al (2016) Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR–Cas9. Nat Biotechnol 34:184–191. https://doi.org/10.1038/nbt.3437
Doench JG, Hartenian E, Graham DB et al (2014) Rational design of highly active sgRNAs for CRISPR-Cas9–mediated gene inactivation. Nat Biotechnol 32:1262–1267. https://doi.org/10.1038/nbt.3026
Foster SD, Glover SR, Turner AN et al (2019) A mixing heteroduplex mobility assay (mHMA) to genotype homozygous mutants with small indels generated by CRISPR-Cas9 nucleases. MethodsX 6:1–5. https://doi.org/10.1016/j.mex.2018.11.017
Gagnon JA, Valen E, Thyme SB et al (2014) Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 9:5–12. https://doi.org/10.1371/journal.pone.0098186
Grainger S, Lonquich B, Oon CH et al (2017) CRISPR guide RNA validation in vitro. Zebrafish 14:383–386. https://doi.org/10.1089/zeb.2016.1358
Gudeta D (2019) Genome editing: tools and application in plants. Open Access J Microbiol Biotechnol. https://doi.org/10.23880/oajmb-16000135
Haeussler M, Concordet J-P (2023) CRISPOR manual. http://crispor.tefor.net/manual/. Accessed 16 Feb 2023
Holalu S, Fang A, Blackman B (2019) In vitro testing of guide RNA efficiency for CRISPR-mediated genome editing. In Protocols.io. https://doi.org/10.17504/PROTOCOLS.IO.8ZSHX6E
Impens L, Jacobs TB, Nelissen H et al (2022) Mini-Review: transgenerational CRISPR/Cas9 gene editing in plants. Front Genome Ed 4:825042. https://doi.org/10.3389/fgeed.2022.825042
Jinek M, Chylinski K, Fonfara I et al (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science (1979) 337:816–821. https://doi.org/10.1126/science.1225829
Karmakar S, Behera D, Baig MJ, Molla KA (2021) In vitro cas9 cleavage assay to check guide RNA efficiency. In: Islam MT, Molla KA (eds) CRISPR-Cas methods, 1st edn, vol 2. Springer Protocols Handbooks. Humana, New York, NY, pp. 23–39. https://doi.org/10.1007/978-1-0716-1657-4_3
Kaur M, Manchanda P, Kalia A et al (2021) Agroinfiltration mediated scalable transient gene expression in genome edited crop plants. Int J Mol Sci 22:10882. https://doi.org/10.3390/ijms221910882
Khan MA, Makhdoom R, Husnain T et al (2001) Expression of bt gene in a dicot plant under promoter derived from a monocot plant. Pak J Biol Sci 4:1518–1522. https://doi.org/10.3923/pjbs.2001.1518.1522
Kong X, Pan W, Sun N et al (2021) GLABRA2-based selection efficiently enriches Cas9-generated nonchimeric mutants in the T1 generation. Plant Physiol 187:758–768. https://doi.org/10.1093/plphys/kiab356
Lazar I, Lazar I (2022) Gel Analyzer 19.1: freeware 1D gel electrophoresis image analysis software. http://www.gelanalyzer.com/index.html
Liang Z, Chen K, Li T et al (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8:1–5. https://doi.org/10.1038/ncomms14261
Liang G, Zhang H, Lou D, Yu D (2016) Selection of highly efficient sgRNAs for CRISPR/Cas9-based plant genome editing. Sci Rep 6:21451. https://doi.org/10.1038/srep21451
Liu H, Ding Y, Zhou Y et al (2017) CRISPR-P 2.0: an improved CRISPR-Cas9 tool for genome editing in plants. Mol Plant 10:530–532. https://doi.org/10.1016/J.MOLP.2017.01.003
Liu X, Yang J, Song Y et al (2022) Effects of sgRNA length and number on gene editing efficiency and predicted mutations generated in rice. Crop J 10:577–581. https://doi.org/10.1016/j.cj.2021.05.015
Maioli A, Gianoglio S, Moglia A et al (2020) Simultaneous CRISPR/Cas9 editing of three PPO genes reduces fruit flesh browning in Solanum melongena L. Front Plant Sci 11:607161. https://doi.org/10.3389/fpls.2020.607161
Malnoy M, Viola R, Jung M-H et al (2016) DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front Plant Sci 7:1–9. https://doi.org/10.3389/fpls.2016.01904
Matson AW, Hosny N, Swanson ZA et al (2019) Optimizing sgRNA length to improve target specificity and efficiency for the GGTA1 gene using the CRISPR/Cas9 gene editing system. PLoS ONE 14:e0226107. https://doi.org/10.1371/JOURNAL.PONE.0226107
Mikami M, Toki S, Endo M (2015) Comparison of CRISPR/Cas9 expression constructs for efficient targeted mutagenesis in rice. Plant Mol Biol 88:561–572. https://doi.org/10.1007/s11103-015-0342-x
Mohammadhassan R, Tutunchi S, Nasehi N et al (2022) The prominent characteristics of the effective sgRNA for a precise CRISPR genome editing. In: Chen Y-C (ed) CRISPR Technology—Recent Advances. IntechOpen, London. https://doi.org/10.5772/INTECHOPEN.106711
Naim F, Shand K, Hayashi S et al (2020) Are the current gRNA ranking prediction algorithms useful for genome editing in plants? PLoS ONE 15:e0227994. https://doi.org/10.1371/journal.pone.0227994
Ordon J, Gantner J, Kemna J et al (2016) Generation of chromosomal deletions in dicotyledonous plants employing a user-friendly genome editing toolkit. Plant J. https://doi.org/10.1111/tpj.13319
Papikian A, Liu W, Gallego-Bartolomé J, Jacobsen SE (2019) Site-specific manipulation of Arabidopsis loci using CRISPR-Cas9 SunTag systems. Nat Commun 10:729. https://doi.org/10.1038/s41467-019-08736-7
Park J, Bae S, Kim JS (2015) Cas-Designer: a web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 31:4014–4016. https://doi.org/10.1093/BIOINFORMATICS/BTV537
Paterson AH, Brubaker CL, Wendel JF (1993) A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol Biol Report 11:122–127
Sagarbarria MGS, Caraan JAM, Lipio PG et al (2023) Agrobacterium-mediated genetic transformation and plant regeneration from cotyledons in Philippine Eggplant (Solanum melongena L.) Acc. PH 11424.’ bioRxiv 07. https://doi.org/10.1101/2023.07.12.548781
Schindele A, Dorn A, Puchta H (2020) CRISPR/Cas brings plant biology and breeding into the fast lane. Curr Opin Biotechnol 61:7–14. https://doi.org/10.1016/J.COPBIO.2019.08.006
Sentmanat MF, Peters ST, Florian CP et al (2018) A survey of validation strategies for CRISPR-Cas9 Editing. Sci Rep 8:1–8. https://doi.org/10.1038/s41598-018-19441-8
Shetty SM, Chandrashekar A, Venkatesh YP (2011) Eggplant polyphenol oxidase multigene family: Cloning, phylogeny, expression analyses and immunolocalization in response to wounding. Phytochemistry 72:2275–2287. https://doi.org/10.1016/j.phytochem.2011.08.028
Stemmer M, Thumberger T, Del Sol Keyer M et al (2015) CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS ONE 10:e0124633. https://doi.org/10.1371/JOURNAL.PONE.0124633
Tang J, Chen L, Liu Y-G (2019) Off-target effects and the solution. Nat Plants 5:341–342. https://doi.org/10.1038/s41477-019-0406-z
Thyme SB, Akhmetova L, Montague TG et al (2016) Internal guide RNA interactions interfere with Cas9-mediated cleavage. Nat Commun 7:1–7. https://doi.org/10.1038/ncomms11750
Uusi-Mäkelä MIE, Barker HR, Bäuerlein CA et al (2018) Chromatin accessibility is associated with CRISPR-Cas9 efficiency in the zebrafish (Danio rerio). PLoS ONE 13:e0196238. https://doi.org/10.1371/journal.pone.0196238
Xiao A, Cheng Z (2014) CasOT: a genome-wide Cas9/gRNA off-target searching tool. Bioinformatics 30:1180–1182. https://doi.org/10.1093/bioinformatics/btt764
Zhang D, Zhang Z, Unver T, Zhang B (2021) CRISPR/Cas: a powerful tool for gene function study and crop improvement. J Adv Res 29:207–221. https://doi.org/10.1016/j.jare.2020.10.003
Zischewski J, Fischer R, Bortesi L (2017) Detection of on-target and off-target mutations generated by CRISPR/Cas9 and other sequence-specific nucleases. Biotechnol Adv 35:95–104. https://doi.org/10.1016/J.BIOTECHADV.2016.12.003
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415. https://doi.org/10.1093/nar/gkg595
Acknowledgements
This study was made possible through the support provided by the following institutions: the Philippine Department of Science and Technology – Science Education Institute (DOST-SEI) for the M.Sc. thesis grants of MSSagarbarria under the Accelerated Science and Technology Human Resources Development Program (ASTHDRP) and JMCaraan under the Graduate Research and Education Assistantship for Technology (GREAT) Program; the Philippine Council for Agriculture, Aquatic, and Natural Resources Research and Development (PCAARRD) project grant to the University of the Philippines Los Baños (UPLB) led by Dr. Lourdes D. Taylo (Project Code: N91832A) for the research funds. The research was also supported in part by the Plant Transgenic Design Initiative (PTraD) in the Gene Research Center at Tsukuba Plant Innovation Research Center, University of Tsukuba, Japan. We thank Dr. Kazuo N. Watanabe, who provided the sgRNA cloning vector and all-in-one Cas9/sgRNA vector. We thank Dr. Desiree M. Hautea for her research supervision in the early stages of this work. We also thank Ms. Rowena B. Frankie for her assistance with tissue culture, DNA preparation, PCR and PAGE.
Author information
Authors and Affiliations
Contributions
MGSS conceptualized the study and all authors contributed to the study design. JAMC performed the in vitro experiments and construct assembly. All authors performed the T7E1 assay and HMA. Transformation and regeneration were performed by MGSS and JAMC. All authors performed material preparation, data collection and analysis. The first draft of the manuscript was written by MGSS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Biosafety approval
All experiments were conducted in accordance with the Joint Department Circular No. 1 Series of 2016 for contained use. The Department of Science and Technology Biotechnology Committee (DOST-BC) issued the corresponding Biosafety Permit No. 2020-0324. The biosafety permit conditions were complied with throughout the conduct of every experiment and associated greenhouse and laboratory activities under the supervision of the UPLB Institutional Biosafety Committee and the DA-BPI Central Post-Entry Quarantine Station.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sagarbarria, M.G.S., Caraan, J.A.M. & Layos, A.J.G. Usefulness of current sgRNA design guidelines and in vitro cleavage assays for plant CRISPR/Cas genome editing: a case targeting the polyphenol oxidase gene family in eggplant (Solanum melongena L.). Transgenic Res 32, 561–573 (2023). https://doi.org/10.1007/s11248-023-00371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-023-00371-9