Abstract
Cereal crops such as maize and rice are considered attractive for vaccine production and oral delivery. Here, we evaluated the rice Oryza sativa for production of As16—an antigen protective against the roundworm Ascaris suum. The antigen was produced as a chimeric protein fused with cholera toxin B subunit (CTB), and its expression level in the endosperm reached 50 μg/g seed. Feeding the transgenic (Tg) rice seeds to mice elicited an As16-specific serum antibody response when administered in combination with cholera toxin (CT) as the mucosal adjuvant. Although omitting the adjuvant from the vaccine formulation resulted in failure to develop the specific immune response, subcutaneous booster immunization with bacterially expressed As16 induced the antibody response, indicating priming capability of the Tg rice. Tg rice/CT-fed mice orally administered A. suum eggs had a lower lung worm burden than control mice. This suggests that the rice-delivered antigen functions as a prophylactic edible vaccine for controlling parasitic infection in animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following early studies on transgenic (Tg) plant-based vaccines in the 1990s (Arakawa et al. 1998; Carrillo et al. 1998; Haq et al. 1995; Mason et al. 1996; Thanavala et al. 1995), numerous species of higher plants have been genetically modified for production of prophylactic vaccines against infections in humans and animals, allergies, and autoimmune diseases (e.g., Daniell 2006; Dus Santos and Wigdorovitz 2005; Floss et al. 2007; Ma et al. 2005; Streatfield 2005; Tacket 2005; Takaiwa 2007). Rapidly improved knowledge about gene-expression technologies in plants, combined with mucosal immunology, has contributed to advances in Tg plant edible vaccine technologies. The advantages of using plant expression systems to produce vaccines include:
-
1.
conventional agricultural technology is sufficient in driving the expression of vaccine antigens and, thus, high-cost fermentation processes can be avoided;
-
2.
expressed vaccine antigens, particularly in grains, can be stably stored in plant tissues for extended periods of time at an ambient temperature, so refrigeration is not required during storage and transportation (no need for cold-chain);
-
3.
because of the direct consumption of plant materials, the antigen purification process can be avoided;
-
4.
needle-free immunization regimes are stress-free and contribute toward preventing the spread of infectious agents;
-
5.
the vaccine antigens contained within the natural capsule of plant tissues are resistant to enzymatic degradation during passage through the gastrointestinal environment and, thus, efficient induction of mucosal immune response is expected; and
-
6.
mass immunization programs appear feasible for humans and livestock.
These features essentially provide major cost advantages for practical applications.
Tg plant technologies have recently shown rapid advancement, thereby enabling genetic engineering of numerous plant species. However, cereal crops such as maize (Chikwamba et al. 2003; Guerrero-Andrade et al. 2006; Tacket et al. 2004), barley (Joensuu et al. 2006), and rice (Gu et al. 2006; Nochi et al. 2007; Oszvald et al. 2007; Qian B et al. 2008; Takagi et al. 2005; Wu et al. 2007; Yang et al. 2008; Yang et al. 2007) are considered to be the most suitable plant species for vaccine production and oral delivery because they can accumulate high levels of recombinant proteins in the seed endosperm (Chikwamba et al. 2003; Takagi et al. 2005). Unlike vegetative tissues of plants, this natural storage organ compartmentalizes foreign proteins in the protein bodies—specialized organelle in mature seeds—where the proteins are stored stably for more than one year at an ambient temperature without degradation. Rice, for example, synthesizes a large amount of glutelin—a major storage protein in its seeds—constituting up to 80% of the endosperm proteins (Katsube et al. 1999; Okita et al. 1989).
Ascaris roundworms are gastrointestinal nematodes infecting both humans and animals, and the infection is endemic in many parts of the world (Crompton 2001). Ascaris suum has been identified as a ubiquitously occurring swine roundworm. It initiates infection when embryonated eggs encapsulating third-stage larvae (L3) are ingested by the host animals. In the infected host, the L3 hatch in the small intestine and migrate to the liver and lungs via the portal vein and finally reach the cecum and/or proximal colon, where they develop into adult worms. Recent studies have shown that A. suum can infect humans. Further, they have emphasized its importance as a zoonotic parasite (Maruyama et al. 1996; Nakamura-Uchiyama et al. 2006). Previous studies have shown that animals can be protected from A. suum infection by immunization with L3 or their cuticle component (Hill et al. 1994). This led us to discover a protective 16-kDa antigen (As16) of A. suum L3. This antigen was found to be expressed in the intestine, hypodermis, and cuticles of the larvae and adults (Tsuji et al. 2003). We recently demonstrated that the serum antibodies elicited by intranasal immunization with Escherichia coli-produced As16 administered in combination with cholera toxin (CT) directly kills A. suum L3 (Tsuji et al. 2004; Tsuji et al. 2003). This indicated that it is feasible to use the mucosal vaccination approach against this parasite.
In this study, we expressed As16 as a chimeric fusion protein with the B subunit of CT (CTB) in the rice endosperm under the control of the endosperm-specific glutelin-GluB-1 promoter and found that the Tg rice accumulated the chimeric fusion protein in seeds at up to 50 μg per gram grains. When the Tg rice seeds were fed, a weak immunogenic response occurred. Therefore, an extraneous addition of CT to the vaccine formulation was required to confer protection from parasite challenge infection in mice. There is considerable scope for efficacy improvement of the Tg rice plants constructed in this study. However, the results of this study demonstrate that the Tg rice-delivered antigen functions as a prophylactic edible vaccine for the control of parasitic infections in animals. Thus, a new oral vaccine platform is now available.
Materials and methods
Generation of Tg rice plants
A fusion gene—CTB-As16—was placed downstream of the 2.3-kb GluB-1 promoter. The original signal peptide sequence of CTB was replaced with the GluB-1 signal. A spacer hinge sequence was inserted between the CTB and As16 sequences, and the endoplasmic reticulum (ER)-retention signal encoding sequence (SEKDEL) was placed 3′ to the As16 sequence. The binary vector pGTV-GluBsigCTB-As16 was introduced into the rice Oryza sativa (japonica cv. Kitaake) genome by the Agrobacterium tumefaciens EH105-mediated transformation method, as described by Goto et al. 1999. The shoots that regenerated from the Tg calli were transplanted to soil and grown to maturity in a greenhouse for 6 months (designated as T0 plants). The seeds collected from the T0 plants (designated as T1 seeds) were evaluated for the production of recombinant protein in the endosperm. The embryos derived from seeds whose endosperm were positive for the recombinant protein production were germinated and grown to maturity in the greenhouse to harvest T2 seeds.
Protein extraction from rice seeds for immunoblot and GM1 ganglioside-enzyme-linked immunosorbent assay (GM1-ELISA)
Rice seeds were grounded to a fine powder with a Multi-Beads Shocker (Yasui Kikai, Osaka, Japan) and protein extraction buffer (20 mM Tris-HCl (pH 6.8), 8 M urea, 4% sodium dodecyl sulfate (SDS), 5% β-mercaptoethanol, 0.5% bromophenol blue, and 20% glycerol) was added to these seeds (500 μl/seed). The mixture was vortex mixed for 30 min and the supernatant collected by centrifugation (15,000 rpm for 20 min; 10 μl) was separated by SDS-polyacrylamide gel electrophoresis (PAGE) in Tris-glycine buffer. The separated proteins were blotted on to an immobilon-P membrane (Millipore, Bedford, MA, USA), blocked with 3% skimmed milk in phosphate-buffered saline (PBS), and the membrane was incubated with anti-As16 antibody developed in mice (1:400). The membrane was washed three times in PBS with 0.05% Tween-20 (PBS-T) and incubated with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (1:3,000) and washed again in the same manner. The membrane was then incubated with enhanced chemiluminescence (ECL) western blot detection reagents (Amersham Biosciences UK, Buckinghamshire, UK) for 1 min and exposed to a X-OMAT radiographic film (Eastman Kodak, Rochester, NY, USA) for 1–15 min for the detection of chemiluminescence. The extracted protein was analyzed by GM1-ELISA for the detection of the multimeric forms of the chimeric protein. In brief, three seeds from each Tg line were ground using a mortar and pestle and vortexed in 300 μl PBS for 30 min. After centrifugation at 15,000 rpm for 20 min, the supernatants were collected and applied to a 96-well microtiter plate that was coated with 0.1 ng/μl of GM1-ganglioside (Sigma–Aldrich, St Louis, MO, USA) in bicarbonate buffer (74 mM NaHCO3 and 26 mM Na2CO3; pH 9.5). Rabbit anti-CT antibody (1:5,000), and then HRP-conjugated goat anti-rabbit IgG (1:3,000)—both diluted with 3% skimmed milk—were added and incubated for 1 h at 37°C. After washing three times with PBS-T, 0.004% o-phenylanediamine and 0.003% H2O2 in phosphate–citrate buffer (103 mM Na2HPO4 and 52 mM citric acid; pH 5.0) were added, and incubation was performed for 30 min in the dark at room temperature (RT). The reaction was stopped by adding 20 μl 6 M H2SO4, and OD490 was measured with a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Oral immunization with Tg rice producing CTB-As16
Six-week-old female BALB/c mice were purchased from CLEA Japan (Tokyo, Japan) and maintained in the Animal Care Facility at the University of Tokyo. All animal experiment protocols were evaluated and permission was obtained from the University’s Animal Care Committee. To prepare rice seeds with absorbed CT adjuvant, the Tg or non-Tg seeds were soaked with CT solution for 5 h at RT, and approximately 8 μg CT was found to be absorbed per gram of seeds. The mice were fed ad libitum 1 g of the Tg or non-Tg seeds with or without CT per day for the indicated period of time.
Challenge infection with A. suum
One week after the final feeding, the mice were orally infected with 2,500 A. suum mature eggs suspended in PBS. One week after the infection, the mice were sacrificed to count the number of larvae that migrated to the lungs. In brief, the lungs were minced with a surgical knife and placed in a small mesh bag containing 30 ml PBS supplemented with ampicillin (100 mg/l) and kanamycin (100 mg/l) and incubated overnight at 37°C. The larvae were recovered by the method of Baermann, as described by Slotved et al. (1996) and counted under a microscope. The statistical significance of differences in the recovered larvae was determined by the Mann-Whitney U-test.
Results
CTB-As16 fusion protein production in the endosperm of Tg rice plants
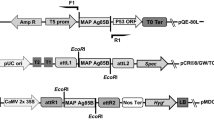
A plant transformation binary vector was engineered to generate Tg rice plants by the A. tumefaciens-mediated transformation method (Fig. 1). The homozygosity for the introduced gene was evaluated by detecting the recombinant protein in at least ten seeds from a single Tg line. Further, the plants were considered to be homozygous if all the seeds derived from the plants in the second generation were positive for the recombinant protein expression, with the assumption that the introduced gene was inherited according to Mendelian laws. Western blot analysis of the seed extracts from several independent Tg lines using anti-CT antiserum revealed that a pentameric CTB fusion protein was produced, while protein bands reactive with the anti-CT antiserum were not found in non-Tg wild-type rice seeds (Fig. 2a). Following heat treatment, the pentamer dissociated into monomers with an apparent molecular mass of approximately 30 kDa—this value corresponds well with the expected molecular mass for the chimeric protein (12 kDa CTB + 16 kDa As16). In addition, the chimeric protein was found to retain the specific binding capacity for GM1-ganglioside—a natural receptor for the CTB multimer (Fig. 2a). Western blot analysis of the Tg rice seed extract using anti-As16-specific immuneserum revealed the presence of the As16 protein moiety within the fusion chimera, and its expression was maintained stably for further two consecutive generations (T1 and T2; Fig. 2b). The expression level reached 53 μg/g seeds (approximately 1.5 μg/seed)—approximately a factor of 20 less than that for the unfused rice-derived CTB reported by Nochi et al. (2007). These results indicated that the Tg rice produced a CTB-As16 chimeric protein that retained the pentamer configuration with the specific lectin-like character of the wild-type CTB.
Transformation of rice plants. (a) Schematic representation of rice transformation vector pGTV-GluBsigCTB-As16. The gene expression is driven by the 2.3-kb GluB-1 promoter and 0.6-kb GluB-1 terminator. Sigctb, rice-plant codon-optimized mature CTB gene fused with the signal peptide of the GluB-1 gene; As16, A. suum 16-kDa antigen gene; h, flexible hinge (GPGP) coding sequence; ER, endoplasmic reticulum retention signal (SEKDEL); 35S, cauliflower mosaic virus 35S promoter; hpt, hygromycin phosphotransferase gene; NOS Ter, nopaline synthase transcription terminator; LB, left border; RB, right border. (b) A brief overview of the Agrobacterium tumefaciens EH105-mediated rice Oriza sativa (japonica cv. Kitaake) transformation procedure. Steps 1 and 2: Callus formation induction from sterile seeds at days 3 and 30, respectively; Steps 3 and 4: A. tumefaciens infection of induced calli; Step 5: Transformant selection on hygromycin-containing medium; Steps 6 and 7: Regeneration induction of transgenic plantlets from transformed calli; Step 8: Transfer of regenerated plantlets to soil pot; Step 9: Maturation of Tg rice plants
Expression analysis of the CTB-As16 chimeric fusion protein extracted from Tg rice-plant lines. (a) Four independent Tg lines (#1–4 at T0 generation) were analyzed for expression of the chimeric protein in the rice seed endosperm by anti-CTB antiserum in the western blot (upper panel). Seed extracts were either boiled or unboiled prior to SDS-PAGE. Putative protein bands for the pentamer and monomer have been indicated. Chimeric protein from a Tg line (T0 generation) was analyzed for specific binding affinity for GM1-ganglioside by GM1-ELISA (lower panel). Protein extract from non-Tg wild-type rice was used as a negative control. Extracted protein samples were serially diluted eightfold. (b) Chimeric protein extracted from a Tg line (T0 generation) was analyzed for specific reactivity with As16 antiserum in the western blot (left panel). Samples were boiled prior to SDS-PAGE. Protein extract from non-Tg wild-type rice was used as a negative control. Stable expression of the chimeric protein was determined for two consecutive generations (T1 and T2) by using anti-As16 antiserum (right panel). Protein band for monomeric chimeric protein has been indicated
Oral immunogenicity and the protective effect of Tg rice
To evaluate whether the Tg rice that produced the chimeric protein was orally immunogenic, BALB/c mice were fed ad libitum Tg or control non-Tg rice seeds for 14 consecutive days. However, in the first set of immunization experiments, all animals failed to develop any specific antibody response (data not shown). Therefore, to confirm whether ingestion of the Tg rice seeds had induced a priming response that was specific for As16, the mice were administered a subcutaneous booster immunization with the recombinant As16. We found that two out of five mice responded to the antigen (data not shown). This indicates that the Tg rice has weak oral immunogenicity; however, it can induce a priming response specific to the parasite antigen.
From the first set of experiments, we concluded that the Tg rice seeds probably induced a weak but sufficiently strong antigen-specific recall response. Based on these experiments, we conducted a second set of immunization experiments in which the mice were fed the Tg rice seeds either with or without CT for longer periods of time, i.e., every week for seven consecutive weeks, and the serum antibody was analyzed a week after the last feeding. The mice that were fed the Tg rice with CT, but not the Tg rice alone or non-Tg rice, developed specific serum IgG against As16 (Fig. 3a). Based on the success of the induction of As16-specific IgG response, the immunized mice were challenge infected with 2,500 A. suum embryonated eggs by oral inoculation, and a week after the infection, the number of larvae recovered from the lungs was counted. We found that the mice that were fed the Tg rice with CT exhibited significantly reduced number of larvae compared with the mice that were fed the Tg rice without CT or mice that were given regular food (P < 0.05; Fig. 3b).
Oral immunogenicity and protective effect of Tg rice. (a) Induction of As16-specific serum IgG by Tg rice seed feeding. BALB/c mice were fed Tg rice with or without CT weekly (1 g/day) for seven weeks, and immunesera of five mice obtained a week after the final feeding were reacted with bacterially expressed As16 protein. (b) Parasite load in the lungs of immunized mice was analyzed. One week after the final feeding, mice were orally infected with 2,500 A. suum infectious eggs, and the L3 larvae that migrated to the lungs were counted. * P < 0.05 vs. untreated
Discussion
Numerous studies have reported the potential benefits of using Tg plants as prophylactic oral vaccines for infectious diseases. However, few studies have successfully demonstrated that rice-based Tg edible vaccines provide protection against infectious agents in animal models (Wu et al. 2007). Rice is the staple food of the people of Asia and is the most widely consumed cereal grain throughout the world. Thus, it is the largest source of food calories for humans. Although ground raw rice is used to prepare beverages such as rice milk and sake, rice is generally boiled or steamed to make it edible. Therefore, rice-based oral vaccines are probably more beneficial when rice is eaten raw, unless heat-resistant vaccine antigens are engineered (e.g., linear T- or B-cell epitopes; Takagi et al. 2005). However, unlike humans, domestic animals such as chickens and pigs can be fed raw or finely ground rice without cooking it. Therefore, this system is more suitable for designing veterinary vaccines.
In this study, we generated Tg rice plants that produced a swine roundworm A. suum-protective antigen (As16) fused with the mucosal carrier molecule CTB and evaluated its expression patterns and oral immunogenicity in mice. The production levels of the CTB-As16 chimeric fusion protein reached the value of 50 μg/g seeds, which is approximately equal to 1.5 μg/seed. It has been reported that unfused CTB is expressed in rice seeds at up to 30 μg/seed (Nochi et al. 2007). The level of expression in our system is lower by a factor of twenty, probably because of the burden imposed on CTB at the molecular level. This markedly reduces its pentamerization and thus often results in low production levels (Harakuni et al. 2005). Non-use of codon optimization for As16 antigen gene could be another factor.
A previous study has shown that the As16-specific serum IgG provides protection by killing A. suum L3 (Tsuji et al. 2004). The study suggests that a mucosal vaccine formulation is effective because the mucosal administration of soluble antigens induces a systemic antibody response if it is appropriately combined with a mucosal delivery vehicle and/or adjuvant. In this study, we exploited a mucosal delivery function of CTB. However, concurrent use of a CT adjuvant was essential for efficient induction of an immune response. Further studies need to be conducted in the future to make the application of this system more practical by generating Tg rice plants that produce higher concentrations of receptor-binding competent chimeric CTB pentamers or that are equipped with a targeting molecule that is capable of more precise pin-pointed delivery to the mucosal immune inductive sites for an enhanced immune response. Although there is considerable scope for efficacy improvement of the Tg rice plants constructed in this study, this is the first study that demonstrates how a rice-based oral vaccine can provide protection against a parasite, in an animal model. Thus, it provides a basis for the edible vaccine development strategies against parasitic infections.
References
Arakawa T, Chong DK, Langridge WH (1998) Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat Biotechnol 16:292–297.
Carrillo C, Wigdorovitz A, Oliveros JC, Zamorano PI, Sadir AM, Gomez N et al (1998) Protective immune response to foot-and-mouth disease virus with VP1 expressed in transgenic plants. J Virol 72:1688–1690
Chikwamba RK, Scott MP, Mejia LB, Mason HS, Wang K (2003) Localization of a bacterial protein in starch granules of transgenic maize kernels. Proc Natl Acad Sci USA 100:11127–11132.
Crompton DW (2001) Ascaris and ascariasis. Adv Parasitol 48:285–375.
Daniell H (2006) Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol J 1:1071–1079.
Dus Santos MJ, Wigdorovitz A (2005) Transgenic plants for the production of veterinary vaccines. Immunol Cell Biol 83:229–238.
Floss DM, Falkenburg D, Conrad U (2007) Production of vaccines and therapeutic antibodies for veterinary applications in transgenic plants: an overview. Transgenic Res 16:315–332.
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17:282–286.
Gu Q, Han N, Liu J, Zhu M (2006) Expression of Helicobacter pylori urease subunit B gene in transgenic rice. Biotechnol Lett 28:1661–1666.
Guerrero-Andrade O, Loza-Rubio E, Olivera-Flores T, Fehervari-Bone T, Gomez-Lim MA (2006) Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Res 15:455–463.
Haq TA, Mason HS, Clements JD, Arntzen CJ (1995) Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science 268:714–716.
Harakuni T, Sugawa H, Komesu A, Tadano M, Arakawa T (2005) Heteropentameric cholera toxin B subunit chimeric molecules genetically fused to a vaccine antigen induce systemic and mucosal immune responses: a potential new strategy to target recombinant vaccine antigens to mucosal immune systems. Infect Immun 73:5654–5665.
Hill DE, Fetterer RH, Romanowski RD, Urban JF Jr (1994) The effect of immunization of pigs with Ascaris suum cuticle components on the development of resistance to parenteral migration during a challenge infection. Vet Immunol Immunopathol 42:161–169.
Joensuu JJ, Kotiaho M, Teeri TH, Valmu L, Nuutila AM, Oksman-Caldentey KM et al (2006) Glycosylated F4 (K88) fimbrial adhesin FaeG expressed in barley endosperm induces ETEC-neutralizing antibodies in mice. Transgenic Res 15:359–373.
Katsube T, Kurisaka N, Ogawa M, Maruyama N, Ohtsuka R, Utsumi S et al (1999) Accumulation of soybean glycinin and its assembly with the glutelins in rice. Plant Physiol 120:1063–1074.
Ma JK, Drake PM, Chargelegue D, Obregon P, Prada A (2005) Antibody processing and engineering in plants, and new strategies for vaccine production. Vaccine 23:1814–1818.
Maruyama H, Nawa Y, Noda S, Mimori T, Choi WY (1996) An outbreak of visceral larva migrans due to Ascaris suum in Kyushu, Japan. Lancet 347:1766–1767.
Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ (1996) Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc Natl Acad Sci USA 93:5335–5340.
Nakamura-Uchiyama F, Tokunaga Y, Suzuki A, Akao N, Hiromatsu K, Hitomi S et al (2006) A case of Ascaris suum visceral larva migrans diagnosed by using A. suum larval excretory-secretory (ES) antigen. Scand J Infect Dis 38:221–224.
Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M et al (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci USA 104:10986–10991.
Okita TW, Hwang YS, Hnilo J, Kim WT, Aryan AP, Larson R et al (1989) Structure and expression of the rice glutelin multigene family. J Biol Chem 264:12573–12581
Oszvald M, Kang TJ, Tomoskozi S, Tamas C, Tamas L, Kim TG et al (2007) Expression of a synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat labile enterotoxin in rice endosperm. Mol Biotechnol 35:215–223.
Qian B, Shen H, Liang W, Guo X, Zhang C, Wang Y, Li G, Wu A, Cao K, Zhang D (2008) Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res 17:621–631
Slotved HC, Roepstorff A, Barnes EH, Eriksen L, Nansen P (1996) Comparison of two methods for recovering migrating Ascaris suum larvae from the liver and lungs of pigs. J Parasitol 82:612–615.
Streatfield SJ (2005) Delivery of plant-derived vaccines. Expert Opin Drug Deliv 2:719–728.
Tacket CO (2005) Plant-derived vaccines against diarrheal diseases. Vaccine 23:1866–1869.
Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S (2004) Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine 22:4385–4389.
Takagi H, Hiroi T, Yang L, Tada Y, Yuki Y, Takamura K et al (2005) A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc Natl Acad Sci USA 102:17525–17530.
Takaiwa F (2007) A rice-based edible vaccine expressing multiple T-cell epitopes to induce oral tolerance and inhibit allergy. Immunol Allergy Clin North Am 27:129–139.
Thanavala Y, Yang YF, Lyons P, Mason HS, Arntzen C (1995) Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc Natl Acad Sci USA 92:3358–3361.
Tsuji N, Suzuki K, Kasuga-Aoki H, Isobe T, Arakawa T, Matsumoto Y (2003) Mice intranasally immunized with a recombinant 16-kilodalton antigen from roundworm Ascaris parasites are protected against larval migration of Ascaris suum. Infect Immun 71:5314–5323.
Tsuji N, Miyoshi T, Islam MK, Isobe T, Yoshihara S, Arakawa T et al (2004) Recombinant Ascaris 16-kilodalton protein-induced protection against Ascaris suum larval migration after intranasal vaccination in pigs. J Infect Dis 190:1812–1820.
Wu J, Yu L, Li L, Hu J, Zhou J, Zhou X (2007) Oral immunization with transgenic rice seeds expressing VP2 protein of infectious bursal disease virus induces protective immune responses in chickens. Plant Biotechnol J 5:570–578.
Yang L, Kajiura H, Suzuki K, Hirose S, Fujiyama K, Takaiwa F (2008) Generation of a transgenic rice seed-based edible vaccine against house dust mite allergy. Biochem Biophys Res Commun 365:334–339
Yang ZQ, Liu QQ, Pan ZM, Yu HX, Jiao XA (2007) Expression of the fusion glycoprotein of Newcastle disease virus in transgenic rice and its immunogenicity in mice. Vaccine 25:591–598.
Acknowledgments
This study was supported in part by a Grant-in-aid for Scientific Research (B) (No. 16380198) and Exploratory Research (No. 19658113) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This study also received financial support from the Bio-oriented Technology Research Advancement Institution (BRAIN) and the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, Y., Suzuki, S., Nozoye, T. et al. Oral immunogenicity and protective efficacy in mice of transgenic rice plants producing a vaccine candidate antigen (As16) of Ascaris suum fused with cholera toxin B subunit. Transgenic Res 18, 185–192 (2009). https://doi.org/10.1007/s11248-008-9205-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-008-9205-4