Abstract
Two phyllosilicates (montmorillonite and saponite) have been selected as starting materials to synthesize ZrO2- and Al2O3-pillared clays by the insertion of polyoxocations and subsequent calcination. These pillared clays display higher surface area, porosity and available acid sites in comparison to their respective raw clays. These samples were tested in the one-pot process to transform furfural into obtain valuable products. The incorporation of ZrO2 allows to reach the highest furfural conversion values, with high yields towards furfuryl alcohol (FOL) at shorter reaction times, whereas the formation of i-propyl furfuryl ether (iPFE) is favored at longer times, attaining iPFE yields of about 50% after 24 h at 170 ºC, using isopropanol as sacrificing alcohol.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Introduction
The depletion of the fossil fuels has led to the search of alternative and sustainable resources to obtain chemicals and fuels, which are currently synthesized from fossil-based feedstocks [1, 2]. In this context, biomass has emerged as the most abundant and economical resource, and, in particular, lignocellulosic biomass is being the most studied for the production of high value-added chemicals [3]. Lignocellulose is mainly formed by three fractions: cellulose, hemicellulose and lignin, which can be isolated and then be subjected to biological and/or thermochemical processes to obtain their respective monomers, which can be transformed into valuable chemicals.
Focusing on the hemicellulose, this can be depolymerized by a hydrothermal treatment in acid medium to obtain liquors of its respective monomers, mainly xylose, which can be dehydrated to give rise to furfural (FUR) [4, 5]. FUR is considered as a building block molecule due to it is possible to produce a large variety of products from hydrogenation, oxidation, condensation, amination reactions, among others [6]. Nevertheless, about 60–70% of the total production of FUR is used to obtain furfuryl alcohol (FOL) [7, 8] due to its high interest in the plastic and polymer fields by its high thermal resistance [9].
The hydrogenation reaction is been carried out industrially using copper chromite [6]. However, the high toxicity of Cr has driven research towards the development of alternative Cr-free catalysts, mainly based on Cu [10,11,12,13] or Ni as active phase [14,15,16,17]. Another alternative to reduce FUR into FOL is through the Meerwein-Ponndorf-Verley (MPV) reduction [18,19,20]. This process has been well known for more than 100 years; however, in the last decade, it has attracted the interest of the scientific community for the synthesis of FOL under mild conditions [21]. MPV process takes place through a catalytic transfer hydrogenation (CTH), where usually a secondary alcohol is used as sacrificing alcohol, which donates a hydrogen through a six-membered intermediate to reduce a carbonyl group of an aldehyde or ketone to the corresponding hydroxyl group, while the sacrificing secondary alcohol is oxidized to a ketone [21].
Generally, the CTH reaction has been carried out in the presence of Lewis acid catalysts or basic catalysts [21, although the coexistence of Lewis and Brönsted acid sites can lead to consecutive reactions yielding valuable products, such as i-propyl levulinate (iPL) or γ-valerolactone GVL from FUR [21]. The first studies were performed using aluminum alkoxide [24, 25], and, more recently, heterogeneous catalysts have been tested for this reaction, which facilitate isolation of products and catalyst reuse. Among the heterogeneous catalysts studied in the CTH reaction, Al- [26, 27] and Zr-based [28,29,30] materials have been the most reported catalysts. In this sense, those catalysts where a small proportion of Zr has been structurally incorporated into porous silicas [31,32,33], zeolites [35,36,37,38,39,40], metal organic frameworks (MOFs) [41,42,43] or carbon [44,45,46] have demonstrated to be very active in CTH of FUR. Considering these results, it is necessary to synthesize more cheap catalysts for achieving a sustainable furfural hydrogenation process.
Clay minerals are very abundant in nature, being used in adsorption and catalytic processes. In addition, some phyllosilicates, like smectites, can host small molecules or cations in their interlayer region, even allowing the insertion of polyoxocations, whose thermal treatment gives rise to pillared clays (PILCs) [47, 48]. Thus, many studies have reported that smectites intercalated with inorganic species maintain meso- and microporosity after a thermal treatment above 300 ºC [47]. In the present work, Al2O3 or ZrO2 have been inserted in the form of polyoxocations into two smectites (montmorillonite and saponite). The presence of these metal oxides could improve the textural properties and the amount of acid sites, thus promoting the hydrogenation of FUR through CTH to obtain high value-added chemicals.
2 Materials and Methods
2.1 Reagents
Two smectites (montmorillonite and saponite), supplied by TOLSA, were used as starting materials for the preparation of the corresponding pillared clays. The Al-pillars were synthesized using aluminum nitrate nonahydrate, Al(NO3)3·9H2O (Sigma-Aldrich, > 99%), while the Zr-pillars were formed using zirconium oxychloride octahydrate, ZrOCl2·8H2O (Sigma-Aldrich, 99%). Sodium hydroxide, NaOH > 99%, was supplied by VWR.
The catalytic process was carried out using furfural (Aldrich, > 98%), o-xylene (Aldrich, 99%), as the internal standard, and the solvent and sacrificing alcohol was 2-propanol (VWR, 99%).
The gases involved in the reaction, quantification of reagent and products and the characterization of the catalyst were He (Air Liquide, 99.999%), H2 (Air Liquide, 99.999%), N2 (Air Liquide, 99.9999%) and N2:O2 (80:20% vol.) (Air Liquide 99.99%).
2.2 Synthesis of the Pillared Clays
The synthesis of Al-pillared clays was carried out following the methodology previously described in the literature [49, 50]. Briefly, a solution of NaOH 0.5 M was added dropwise to another solution of Al(NO3)3 0.2 M, maintaining a OH/Al molar ratio of 2, obtaining the cation [AlO4Al12(OH)24(H2O)12]7+. After 24 h stirring at room temperature, the pillaring solution was added dropwise to a 2 wt.% suspension of smectite. Then, the obtained suspension was aged at 60 ºC for 6 h. The solid obtained after centrifugation was washed three times with distilled water. Finally, the solid was dried at 60 ºC overnight and calcined at 400 ºC for 2 h, with a heating rate of 2 ºC/min.
Zr-pillared clays were obtained according to the methodology proposed previously [49, 51]. In this, a solution of ZrOCl2 0.1 M was added to a suspension of the smectite with a ratio of 1 mmol of Zr/g of smectite. The suspension was stirred at 60 ºC for 24 h. Then, the solid was obtained after centrifugation and washed three times with distilled water. The final thermal treatment was similar to that of Al-pillared clays.
2.3 Characterization of the Catalysts
The starting clays and the pillared materials have been characterized by X-ray diffraction (XRD), attenuated total reflectance (ATR) FTIR spectroscopy, transmission electron microscopy (TEM), N2 adsorption–desorption at −196 ºC, NH3 thermoprogrammed desorption (NH3-TPD), pyridine thermoprogrammed desorption (Pyr-TPD), 2,6-dimethylpyridine desorption (2,6-DMPyr-TPD) and X-ray photoelectron spectroscopy (XPS).
The detailed information of the apparatus and the methodologies used for the analysis of the samples are compiled in the Supplementary Information.
2.4 Catalytic Tests
The hydrogenation of FUR was performed in Ace glass reactors, 15 mL of volume (Aldrich). Each test was carried out with 100 mg of catalyst, 0.1 mmol of FUR and 2-propanol, which was used as hydrogen donor, with a 2-propanol/FUR molar ratio of 50. In addition, 0.02 mL of o-xylene was added to the reaction medium as internal standard.
Before each test, the glass reactors were purged with He to minimize collateral reactions. The reaction was performed until a maximum time of 24 h with a temperature range between 130 and 170 ºC, under magnetic stirring of 300 rpm. The temperature was controlled using a thermocouple, directly in contact with an aluminum block, where the glass reactors were placed, which is directly heated with a heating plate with stirring.
The reuse studies were carried out under conditions like those described previously. Between each cycle, the catalyst was filtered and washed with the solvent (2-propanol). After that, the catalyst was dried at 100 ºC overnight for later use in the next cycle.
Once the reaction is finished, the reactors were taken out of the aluminum block and cooled in water. Then, the liquid was microfiltered and analyzed by gas chromatography (GC), which was equipped a flame ionization detector (TCD) and a CP-Wax 52 CB capillary column using a heating rate of 2 ºC/min from 40 ºC to 200 ºC, maintaining this temperature to 15 min. The analyzes were carried out in split mode.
The FUR conversion and yields of the products were determined as follows (Eq. 1 and Eq. 2):
3 Characterization of the Catalysts
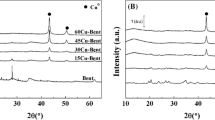
The XRD patterns of the raw clays (Mont and Sap), compiled in Fig. 1, reveal that both phyllosilicates exhibit a broad diffraction peak, ascribed to the d001 reflection, with a maximum of 2θ of 7.0º, which corresponds to a layered structure with a basal spacing of 12.3 Å. This value is higher than that of a dry smectite (10 Å), which can be explained by the presence of cations, generally alkaline, in the interlayer region to compensate the excess of negative charge in the phyllosilicate sheets. Furthermore, these cations are generally hydrated. In the same way, other typical diffraction peaks of smectites (d110, d020, d130, d200 and d060) can also be observed. Among them, the d060 reflection is very useful, since it allows to discern between dioctahedral and trioctahedral smectites. Thus, a dioctahedral smectite, like montmorillonite, displays a d060 reflection of 2θ = 62.04º (1.49 Å), while for trioctahedral smectites, like saponite, appears at 2θ = 60.78º (1.52 Å) [52]. In addition, minor amounts of crystalline quartz, gypsum and feldspars are also detected [52].
After the pillaring process, the basal diffraction (d001) disappears in all cases, suggesting that the formation of a pillared structure causes the formation of a disordered, or delaminated phyllosilicates in the form of a house of cards, as previously described in the literature [53]. Moreover, it is also noteworthy that some impurities, mainly quartz, are still observed after the pillaring treatment. On the other hand, no segregation phases of crystalline Al2O3 or ZrO2 were detected by XRD after the pillaring process.
The morphology of the raw and modified clays was evaluated by TEM (Fig. 2). These micrographs evidence the lamellar structures of both Mont and Sap. However, these sheets have an anisotropic structure due to the genesis of these materials are subject to environmental effects. After the pillaring process, as previously noted, disordered and delaminated structures can be observed, mainly in those materials where Zr species has been incorporated. The study of the dispersion of Al and Zr species, after the pillaring step, by EDX mapping (Supplementary Information, Figures S1 and S2), would confirm the suitable dispersion of these species, discarding a segregation of Al2O3 or ZrO2 phases, as was inferred by XRD (Fig. 1).
The structural characterization of the raw Mont and Sap was also carried out by ATR FTIR spectroscopy (Fig. 3). The OH stretching region, between 3750 and 3000 cm−1, shows, for Mont, an absorption band located at 3625 cm−1, which is attributed to stretching vibrations of OH forming Al(OH)Al linkages [54], confirming the presence of an Al-rich smectite, as montmorillonite. In the case of Sap, its spectrum in the OH stretching region shows a less defined signal with a maximum located above 3680 cm−1. This signal is assigned to OH stretching vibrations of Mg(OH)Mg- groups, which are typical of Mg-rich smectites, like saponite [55]. The Si–O stretching vibration appears about 980 cm−1 for both raw clays. In addition, other absorption bands are detected at lower wavenumber values. Thus, it has been previously reported that the bands located between 950 and 800 cm−1 can be attributed to Al-rich smectites, while the bands between 700 and 600 cm−1 are assigned to Mg-rich smectites [56]. Finally, a small and broad band is observed between 1300–1600 cm−1 in the Sap sample [57], which is ascribed to low proportion of carbonate species, although crystalline metal carbonate were not detected by XRD.
Once the polyoxocations of Al and Zr have been incorporated and subsequently calcined to form nanoparticles of the corresponding metal oxides, acting as pillars in the interlayer region, the OH stretching region is modified, since the bands located between 3600 and 3700 cm−1, which are ascribed to silanol groups, disappear. In this sense, the thermal treatment must cause a dehydroxylation in both phyllosilicates. On the other hand, the incorporation of Al and Zr species also provokes a shift of the Si–O stretching vibration at higher wavenumber values.
The determination of the textural properties was estimated from their N2 adsorption–desorption isotherms at −196 ºC (Fig. 4). According to the IUPAC classification, the isotherms of the raw clay are between Types II and IV, which would imply that these solids display macroporosity ascribed to the voids between adjacent particles, but they also present a certain micro and mesoporosity [58], mainly in the case of the Sap sample where higher amount of N2 is adsorbed at lower relative pressure. Moreover, the hysteresis loops can be considered as H3, which is found in non-rigid aggregates of plate-like particles, as those of Mont or Sap [58]. When Al or Zr species are incorporated in the interlayer region of these phyllosilicates, the isotherms evidence an increase in the N2 adsorbed at low relative pressures, which results in an increase in microporosity, mainly in those materials where Zr species were incorporated.
The specific surface area of the materials has been calculated by the BET equation (Table 1) [59]. Raw Mont has a SBET value of 47 m2/g, while for Sap is 128 m2/g. In this sense, the higher surface of Sap could be ascribed to its lower crystallinity, since this phyllosilicate displays less intense and broader diffraction peaks, as observed in Fig. 1. After the incorporation of Al and Zr species, SBET values increase, although this improvement is more notable for Zr-based materials, reaching the maximum value for Sap-Zr sample (237 m2/g). This improvement of the textural properties is assigned to an increase in the meso- and, mainly, the microporosity by the formation of a pillared structure, as well as a partial delamination of the starting clays.
The study of the pore size distribution was carried out by DFT calculations (Fig. 5). Both raw phyllosilicates display a pore width with a maximum about 12 Å. After the incorporation of Al and Zr species in the phyllosilicates, a shift in the maximum of the pore width distribution at higher values is observed. This could confirm the formation of pillars in the interlayer region, although its deposition on the external surface cannot be ruled out. In the same way, the size distribution in the micropore region has also been studied for all samples (Fig. 6). The obtained data reveal that both raw clays (Mont and Sap) hardly display microporosity; however, the incorporation of Al and Zr species causes a different size pattern in both phyllosilicates. Thus, for Mont, a clear increase in the microporosity is observed, whereas, on the contrary, hardly affects the narrow microporosity of Sap.
Active sites for the CTH of FUR are associated to acid centers, so the determination of total acidity was evaluated by NH3-TPD (Table 2 and Supplementary Information, Figure S3). This study reveals that raw clays displays an acidity of 68 and 145 µmol/g for Mont and Sap, respectively, being ascribed to the presence of silanol groups, but also to the presence of Al3+ or Mg2+ species located in the octahedral sheets. The insertion of Al and Zr species in Mont or Sap causes a notable increase in the amount of acid sites in all cases, achieving values in the range of 159 and 435 µmol/g, respectively. The NH3-TPD data reveal that Sap-based catalysts display a higher acidity than those based on Mont, reaching the maximum value for the Sap-Zr catalyst (435 µmol/g).
In order to identify the nature (Lewis and/or Brönsted) of acid sites involved in the one-pot process of FUR conversion into valuable products, Pyr- and 2,6-DMPyr-TPD experiments were carried out (Table 2). In all cases, these values are below those obtained from NH3-TPD, which is expected, since Pyr and 2.6-DMPyr are bigger molecules than NH3, in such a way that these molecules can suffer steric hindrance. The TPD of 2,6-DMPyr quantifies the amount of available Brönsted acid sites [60]. In all cases, the concentration of Brönsted acid sites is very low, reaching the highest value (20 µmol/g) for Sap-Zr. The Pyr-TPD provides information related to the sum of Brönsted and Lewis acid sites, so the difference between Pyr- and 2.6-DMPyr-TPD data provides the amount of Lewis acid sites. Lewis acidity predominates in all catalysts (Table 2). The incorporation of ZrO2 and Al2O3 in both phyllosilicates increases the amount of Lewis acid sites compared to raw clays. Among them, Zr-based catalysts display a higher concentration of Lewis acid sites, achieving the highest value for Sap-Zr (140 µmol/g).
The analysis of the surface chemical composition of catalysts was performed by XPS (Table 3). As expected, Mont has a high Al content, while Mg is higher in Sap. These data confirm that Mont is a dioctahedral smectite, while Sap is trioctahedral, as was deduced by XRD (Fig. 1) and ATR FTIR spectroscopy (Fig. 3). The Si content is similar in both cases, with a surface content between 14.9 and 16.8%. On the other hand, the XPS results suggest that Na+ is the main cation located in the interlayer region to counterbalance the charge deficiency of sheets. In all cases, the binding energy values are typical of phyllosilicates, such as montmorillonite and saponite.
After the formation of pillars, a decrease in carbon content is observed in all cases. This could suggest the presence of carbonate species in the raw clays, which are removed during the pillaring process. On the other hand, the incorporation of Al and Zr species causes an increase in the surface content of these cations of around 5% compared to raw smectites (Fig. 7). This confirms the incorporation of Zr or Al species after the pillaring process. Furthermore, the XPS data confirm that both Al and Zr appear in their oxide form. Finally, it is also noteworthy the decrease in the Na content, confirming that the Na+ ions are generally replaced by the Zr and Al polyoxocations, which are calcined to form nanoparticles of Al2O3 and ZrO2, acting as pillars in the interlayer region of phyllosilicates.
4 Catalytic Results
Raw clays and pillared samples were evaluated as catalysts in the one-pot process of FUR conversion to valuable products. In this sense, it has been previously claimed that this one-pot process starts with the CTH reaction of FUR to obtain FOL. This reaction requires Lewis acid sites, and the mechanism takes place through a six-membered intermediate where a sacrificing alcohol, generally a secondary alcohol, transferred a hydrogen to an aldehyde or ketone, promoting its reduction. In the next step, FOL can be etherified with the sacrificing alcohol to yield iPFE, on Lewis or Brönsted acid sites. Then, both FOL and iPFE could suffer ring opening on Brönsted acid sites to produce alkyl levulinate, which is quickly reduced by a CTH reaction on Lewis acid sites via a lactonization process, forming GVL in the last step [34, 35].
In the first study, the catalytic activity of the raw clays was evaluated at 170 ºC, which was selected according to previous works, since it is required for some steps involved in the one-pot process [21, 61,62,63] (Fig. 8). In both cases, FUR conversion increases with the reaction time, reaching a maximum conversion of 66% after 24 h with Sap, while it was only 52% for Mont. In spite of both catalysts possess a similar concentration of acid sites, as determined by Pyr-TPD, Sap has better textural (SBET of 143 versus 47 m2/g) and acid (145 versus 60 mol NH3 desorbed/g) properties, which could explain its better catalytic performance.
Regarding the reaction products, FOL is preferably formed at short reaction times with Mont, with a maximum yield of 15% after 6 h. At longer reaction times, a progressive decay in FOL yield is observed, which is accompanied by a rise of the iPFE and iPL yields, the last being the main product after 24 h, with a yield of 17%. It is also noteworthy that a yield of about 15% is associated to non-detected products. In the case of Sap, a similar trend is observed, since FOL is also obtained at short reaction times, decreasing with the reaction time. However, the yield pattern at longer reaction times differs from Mont, since Sap shows a lower proportion of iPL, the main product being iPFE, attaining a maximum value of 24% after 24 h. Moreover, the formation of non-detected products is also high, with a yield of 20%.
Next, the catalytic study of pillared clays (Fig. 9) revealed that, in all cases, FUR conversion was improved compared to raw clays. The incorporation of Al2O3 pillars in Mont or Sap led to lower FUR conversion values (Fig. 9A). These differences are even more pronounced at shorter reactions times. Thus, Mont-Al only reaches a FUR conversion of 13% after 1 h at 170 ºC, while Sap-Al achieves a conversion of 33%, under similar experimental conditions. Longer reaction time causes an improvement in FUR conversion, attaining a value of 75% for Mont-Al and 86% for Sap-Al after 24 h. In the case of the Zr-based catalysts, the conversion values increase notably at shorter reaction time, since Mont-Zr reaches a value of 40% after 1 h, while Sap-Zr provides a conversion of 82%, with full conversion after about 6 h.
FUR conversion (A), furfuryl alcohol, FOL (B), i-propyl furfuryl ether, iPFE (C), i-propyl levulinate, iPL (D), γ-valerolactone, GVL (E) and non-detected products (F) yields in the one-pot reaction of FUR into valuable products using Mont-Al, Mont-Zr, Sap-Al and Sap-Zr. (Experimental conditions: 0.1 g of catalyst, temperature reaction: 170 °C, reaction time: 0–24 h, iPOH/FUR molar ratio: 50, FUR/catalyst weight ratio: 1)
The higher FUR conversion attained with pillared clays could be ascribed to the incorporation of these metal oxides increases the amount of acid sites, which are involved in the consecutive reactions to obtain valuable products (Table 2). Between Al2O3 and ZrO2, it has been previously reported that Zr(IV) species give rise to more active sites involved in the one-pot process of FUR [32, 34, 35, 42, 46].
Concerning the reaction products (Fig. 9B), FOL is again obtained at shorter reaction times. The incorporation of ZrO2 to smectites leads to higher FOL yield, achieving a maximum value of 42% for Sap-Zr after 1 h, which agrees with the high activity in the MPV reaction of Zr-based catalysts [37]. In this sense, the presence of Lewis acid sites in these catalysts promotes the catalytic transfer of hydrogen, as several authors have stated [21, 34, 35].
The use of longer reaction times again causes a progressive decrease in FOL yield, which is associated to the formation of iPFE (Fig. 9C). It has been previously reported that this step can be performed with the participation of Lewis or Brönsted acid sites [62]. The formation of iPFE is faster when ZrO2 has been incorporated in the phyllosilicates. In both cases, yields close to 50% are reached for Mont-Zr and Sap-Zr after 12 h. However, those catalysts where Al2O3 has been incorporated, Mont-Al and Sap-Al, display a more progressive increase in the iPFE yield, a maximum yield of 43% being attained with Sap-Al after 24 h. These data indicate that Zr-based catalysts, which are more active in the reaction, are also the catalysts most likely to favor etherification reactions with the sacrificial alcohol to form iPFE. Thus, several papers have reported that Al2O3-based catalysts are less prone to promote etherification reaction than ZrO2-based ones [26, 34, 35].
On the other hand, the formation of iPL (Fig. 9D) is more progressive throughout the 24 h, although the maximum iPL yield is 24% with Mont-Al, while the rest of catalysts display yields below 16%. From these results, the one pot process seems to be retained in the formation of FOL or iPFE. This fact could be ascribed to the low proportion of Brönsted acid sites (Table 2), which are required for the opening of the furan ring to form alkyl levulinates [34]. Finally, small proportions of GVL are also observed (Fig. 9E), which is expected, since the one-pot process does not progress to more advanced consecutive reactions. In any case, it has been reported that the formation of GVL is the limiting step in the one-pot process, due to long reaction time and high temperature are required to promote the final lactonization to form GVL, through Lewis or Brönsted acid sites [34]. Thus, the maximum GVL yield is obtained for Sap-Zr, with 10% after 24 h.
In addition, the analysis of the carbon balance has revealed the formation of non-detected products (Fig. 9F), whose yields increase at longer reaction times, with values between 25 and 33% after 24 h.
Once the catalysts were tested in the CTH of FUR to obtain valuable products, the used catalysts were recovered to identify modifications of physico-chemical properties of the catalysts along the reaction. The analysis of the textural properties of the used catalysts, reported in Table 4, shows a drastic decrease in both mesoporosity and microporosity in all catalysts. A detailed study of the pore size distribution, determined by the DFT method (Supplementary Information, Figure S4), has confirmed a blockage of the pore with dimensions in the range of 1–2 nm. In this sense, previous studies have reported that FUR and FOL are prone to suffer polymerization reactions leading to humins or resins [64,65,66], which can be deposited in the micro- and mesopores, worsening textural properties of catalysts.
The characterization of the catalysts after the reaction by XPS showed that the atomic concentration data differ compared to the fresh catalysts (Table 5). Thus, the surface C content increases markedly after the reaction, with percentages between 38.2 and 58.6%, which agrees with studies reported in other reactions of hemicellulose valorization [67].
However, the Si, Al, Mg and Zr contents decreases after the reaction (Supplementary Information, Figure S5). These data would point out the deposition of carbonaceous species on the catalyst surface, which causes a progressive blockage of the active sites involved in the reaction. In fact, the detailed analysis of the C 1 s core level spectra (Fig. 10A) reveal the presence of C–O–C or C–O–H bonds (at a binding energy of 286.0 eV), which could confirm the deposition of furfural or furfuryl alcohol derivatives in the form of humins. In the same way, the O 1 s core level spectra also display a new contribution located about 533 eV (Fig. 10B), which is ascribed to organic C–O bonds, also agreeing with the deposition of carbonaceous species.
In the next study, the influence of the reaction temperature on the CTH of FUR has been evaluated (Fig. 11). In the entire temperature range studied, the insertion of ZrO2 nanoparticles in smectites led to a higher activity at lower reaction times. The highest FUR conversion was obtained for Sap-Zr, which achieves a FUR conversion of 81% after only 6 h at 130 ºC, while it was almost complete after 6 h at 170 ºC. Regarding the obtained products, FOL and iPFE are generally formed at lower temperature [31]. Among them, in most of cases, FOL yields decay when the temperature increases, except in the case of Mont-Zr, where FOL and iPFE grew similarly. For the other catalysts, a drop in FOL yield and a concomitant increase in iPFE yield was observed due to the etherification reaction of FOL with iPOH, in such a way that iPFE is the main product at higher reaction temperature. In the same way, the formation of iPL is also favored at higher temperature [68]. However, these yields are relatively low compared to those of iPFE, confirming that the reaction is retained in the furan ring opening regardless the reaction temperature due to the low proportion of Brönsted acid sites in the catalysts (Table 2). Finally, GVL is only observed in Sap-Zr catalyst, being expected due to the poor iPL yield attained in previous studies (Fig. 9). In addition, as previously noted, the lactonization reaction to form GVL requires high temperatures and longer reaction times. Moreover, the formation of non-detected products increases with the reaction temperature in all cases, confirming that the polymerization and undesired condensation are favored at higher temperatures.
In a final study, the reuse of the most active catalysts (Sap-Zr and Mont-Zr) was also evaluated (Fig. 12). In both catalysts, FUR conversion decays with the number of cycles due to the formation of carbonaceous deposits on the catalyst surface, because of polymerization or condensation reactions of FUR or FOL. This implies an impoverish of the textural properties (Table 4 and Supplementary Information Figures S4 and S5), as well as a blockage of the acid sites involved in the one-pot process causing a progressive decay in the catalytic activity. Thus, Mont-Zr shows a decrease of FUR conversion from 78% after the 1st cycle to 40% after the 4th cycle, after 6 h at 170 ºC in each cycle. In the case of Sap-Zr, a similar trend is observed, since the FUR yield decays from 98% after the 1st cycle to 62% after the 4th cycle. The regeneration of the catalyst by a thermal treatment at 550 ºC for 6 h with air in static conditions causes a notable improvement of the catalytic performance, probably due to the removal of the carbonaceous deposits, reaching values close to those obtained after the 1st cycle. In this sense, the FUR conversion values are slightly below those obtained after the 1st cycle due to the loss of acid sites after the thermal treatment at 550 ºC.
The profile of the obtained products also differs after each cycle. Thus, both iPL and iPFE yields progressively decrease after each cycle; however, the FOL yield enhances. In this sense, the blockage of acid sites retains the one-pot process in earlier steps, in such a way that the CTH reaction to form FOL is the main reaction. In contrast, the etherification and ring opening reactions are more limited due to the worsening of textural properties and blocking of active sites. The regeneration of the catalyst by calcination leads to a similar pattern of products in comparison to the 1st cycle, although it is not possible to recover the initial values.
5 Conclusions
Two smectites, montmorillonite and saponite, have been used as starting materials for the synthesis of pillared clays by the insertion of polyoxocations in the interlayer region and subsequent calcination. The presence of these polyoxocations causes a partial delamination of the clays, as well as an increase in the surface area and pore volume. In the same way, the insertion of Al2O3 and ZrO2 promotes the formation of acid sites, generally of Lewis type, which are involved in the catalytic transfer hydrogenation to reduce aldehydes into alcohols. In the same way, the presence of a small proportion of Brönsted acid sites also promotes other reactions as the opening of the furanic ring, in such a way that consecutive reactions take place in this process.
The study of these samples in the one-pot process to convert FUR into valuable products reveals that Sap sample displays a higher conversion in comparison to Mont, probably due to its better textural properties (larger surface area and pore volume). The study of the pillared clays points out an improvement in the catalytic behavior, reaching full conversion at shorter reaction times, mainly in the case of the Zr-based catalysts. Regarding the obtained products, the most active catalyst is Sap-Zr, showing the formation of a high proportion of FOL at short reaction times,with a maximum FOL yield of 42% after 1 h of reaction at 170 ºC. However, longer reaction times provoke a decrease in FOL yield, while the formation of iPFE is favored notably, achieving iPFE yields close to 50% for Mont-Zr and Sap-Zr samples, after 12 h of reaction at 170 ºC. On the other hand, it seems that the reactions are retained in the formation of iPFE, probably due to the low proportion of Brönsted acid sites involved in the one-pot process.
The reuse evaluation shows a notable decrease in the FUR conversion due to the formation of carbonaceous deposits along the reaction, causing a decrease in the surface area, pore volume, as well as the blockage of the acid sites. However, these catalysts can be regenerated by calcination, achieving conversion and yields close to those of the fresh catalysts.
Data availability
Raw data are available upon request.
References
Kohli K, Prajapati R, Sharma BK (2019) Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 12:233. https://doi.org/10.3390/en12020233
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem Rev 106:4044–4098. https://doi.org/10.1021/cr068360d
Ashokkumar V, Venkatkarthick R, Jayashree S, Chuetor S, Dharmaraj S, Kumar G, Chen WH, Ngamcharussrivichai C (2022) Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—a critical review. Bioresource Technol 344:126195. https://doi.org/10.1016/j.biortech.2021.126195
Mäki-Arvela P, Salmi T, Holmbom B, Willför S, Murzin DY (2011) Synthesis of sugars by hydrolysis of hemicelluloses—a review. Chem Rev 111:5638–5666. https://doi.org/10.1021/cr2000042
Delbecq F, Wang Y, Muralidhara A, El Ouardi K, Marlair G, Len C (2018) Hydrolysis of hemicellulose and derivatives—a review of recent advances in the production of furfural. Front Chem 6:146. https://doi.org/10.3389/fchem.2018.00146
Mariscal R, Maireles-Torres P, Ojeda M, Sádaba I, López-Granados M (2016) Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ Sci 9:1144–1189. https://doi.org/10.1039/C5EE02666K
Lange JP, van der Heide E, van Buijtenen J, Price R (2012) Furfural - A promising platform for lignocellulosic biofuels. Chemsuschem 5:150–166. https://doi.org/10.1002/cssc.201100648
Hoydonckx HE, van Rhijn WM, van Rhijn W, de Vos DE, Jacobs PA, Hoydonckx HE, van Rhijn WM, van Rhijn W, de Vos DE, Jacobs PA (2007) Furfural and Derivatives. Ullmann’s Encycl. Ind. Chem., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany. https://doi.org/10.1002/14356007.a12_119.pub2
Yan K, Wu G, Lafleur T, Jarvis C (2014) Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew Sustain Energy Rev 38:663–676. https://doi.org/10.1016/j.rser.2014.07.003
García-Sancho C, Mérida-Robles JM, Cecilia-Buenestado JA, Moreno-Tost R, Maireles-Torres PJ (2023) The role of copper in the hydrogenation of furfural and levulinic acid. Int J Mol Sci 24:2443. https://doi.org/10.3390/ijms24032443
Wang Y, Zhao D, Rodríguez-Padrón D, Len C (2019) Recent advances in catalytic hydrogenation of furfural. Catalysts 9:796. https://doi.org/10.3390/catal9100796
Jiménez-Gómez CP, Cecilia JA, Moreno-Tost R, Maireles-Torres P (2017) Selective production of 2-methylfuran by gas-phase hydrogenation of furfural on copper incorporated by complexation in mesoporous silica catalysts. Chemsuschem 10:1448–1459. https://doi.org/10.1002/cssc.201700086
Jiménez-Gómez CP, Cecilia JA, Alba-Rubio AC, Cassidy A, Moreno-Tost R, García-Sancho C, Maireles-Torres P (2022) Tailoring the selectivity of Cu-based catalysts in the furfural hydrogenation reaction: Influence of the morphology of the silica support. Fuel 319:123827. https://doi.org/10.1016/j.fuel.2022.123827
Nakagawa Y, Nakazawa H, Watanabe H, Tomishige K (2012) Total hydrogenation of furfural over a silica-supported nickel catalyst prepared by the reduction of a nickel nitrate precursor. ChemCatChem 4:1791–1797. https://doi.org/10.1002/cctc.201200218
Meng X, Yang Y, Chen L, Xu M, Zhang X, Wei M (2019) A control over hydrogenation selectivity of furfural via tuning exposed facet of Ni catalysts. ACS Catal 9:4226–4235. https://doi.org/10.1021/acscatal.9b00238
Jiménez-Gómez CP, Cecilia JA, Moreno-Tost R, Maireles-Torres P (2017) Nickel phosphide/silica catalysts for the gas-phase hydrogenation of furfural to high-added-value chemicals. ChemCatChem 9:2881–2889. https://doi.org/10.1002/cctc.201700312
Guerrero-Torres A, Jiménez-Gómez CP, Cecilia JA, García-Sancho C, Franco F, Quirante-Sánchez JJ, Maireles-Torres P (2019) Ni supported on sepiolite catalysts for the hydrogenation of furfural to value-added chemicals: influence of the synthesis method on the catalytic performance. Top Catal 62:535–550. https://doi.org/10.1007/s11244-019-01168-z
Meerwein H, Schmidt R (1925) Ein neues verfahren zur reduktion von aldehyden und ketonen. Liebigs Ann Chem 444:221–238. https://doi.org/10.1002/jlac.19254440112
Ponndorf W (1926) Der reversible austausch der oxydationsstufen zwischen aldehyden oder ketonen einerseits und primären oder sekundären alkoholen anderseits. Angew Chem 39:138–143. https://doi.org/10.1002/ange.19260390504
Verley M (1925) The Exchange of functional groups between two molecules. The passage of ketones to alcohols and the reverse. Bull Soc Chim Fr 37:871–874
Gilkey MJ, Xu B (2016) Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal 6:1420–1436. https://doi.org/10.1021/acscatal.5b02171
Hidalgo-Carrillo J, Parejas A, Cuesta-Rioboo MJ, Marinas A, Urbano FJ (2018) MPV reduction of furfural to furfuryl alcohol on Mg, Zr, Ti, Zr-Ti, and Mg-Ti solids: Influence of acid-base properties. Catalysts 8:539. https://doi.org/10.3390/catal8110539
Maderuelo-Solera R, López-Asensio R, Cecilia JA, Jiménez-Gómez CP, García-Sancho C, Moreno-Tost R, Maireles-Torres P (2019) Catalytic transfer hydrogenation of furfural to furfuryl alcohol over calcined MgFe hydrotalcites. Appl Clay Sci 183:105351. https://doi.org/10.1016/j.clay.2019.105351
Ooi T, Miura T, Itagaki Y, Ichikawa H, Maruoka K (2002) Catalytic Meerwein-Ponndorf-Verley (MPV) and Oppenauer (OPP) reactions: Remarkable acceleration of the hydride transfer by powerful bidentate aluminum alkoxides. Synthesis 2:279–291. https://doi.org/10.1055/s-2002-19802
Liu YC, Ko BT, Huang BH, Lin CC (2002) Reduction of aldehydes and ketones catalyzed by a novel aluminum alkoxide: mechanistic studies of Meerwein−Ponndorf−Verley reaction. Organometallics 21:2066–2069. https://doi.org/10.1021/om0200454
López-Asensio R, Cecilia JA, Jiménez-Gómez CP, García-Sancho C, Moreno-Tost R, Maireles-Torres P (2018) Selective production of furfuryl alcohol from furfural by catalytic transfer hydrogenation over commercial aluminas. Appl Catal A 556:1–9. https://doi.org/10.1016/j.apcata.2018.02.022
García-Sancho C, Jiménez-Gómez CP, Viar-Antuñano N, Cecilia JA, Moreno-Tost R, Mérida-Robles JM, Requies J, Maireles-Torres P (2021) Evaluation of the ZrO2/Al2O3 system as catalysts in the catalytic transfer hydrogenation of furfural to obtain furfuryl alcohol. Appl Catal A 609:117905. https://doi.org/10.1016/j.apcata.2020.117905
Montes V, Miñambres JF, Khalilov AN, Boutonnet M, Marinas JM, Urbano FJ, Maharramov AM, Marinas A (2018) Chemoselective hydrogenation of furfural to furfuryl alcohol on ZrO2 systems synthesized through the microemulsion method. Catal Today 306:89–95. https://doi.org/10.1016/j.cattod.2017.05.022
Komanoya T, Nakajima K, Kitano M, Hara M (2015) Synergistic catalysis by Lewis acid and base sites on ZrO2 for Meerwein–Ponndorf–Verley reduction. J Phys Chem C 119:26540–26546. https://doi.org/10.1021/acs.jpcc.5b08355
Akinnawo CA, Bingwa N, Meijboom R (2021) Surface properties vs activity of meso-ZrO2 catalyst in chemoselective Meerwein-Ponndorf-Verley reduction of citral: effect of calcination temperature. Micropor Mesopor Mater 311:110693. https://doi.org/10.1016/j.micromeso.2020.110693
López-Asensio R, Jiménez-Gómez CP, García-Sancho C, Moreno-Tost R, Cecilia JA, Maireles-Torres P (2019) Influence of structure-modifying agents in the synthesis of Zr-doped SBA-15 silica and their use as catalysts in the furfural hydrogenation to obtain high value-added products through the Meerwein-Ponndorf-Verley reduction. Int J Mol Sci 20:828. https://doi.org/10.3390/ijms20040828
Maderuelo-Solera R, Richter S, Jiménez-Gómez CP, García-Sancho C, García-Mateos FJ, Rosas JM, Moreno-Tost R, Cecilia JA, Maireles-Torres P (2021) Porous SiO2 nanospheres modified with ZrO2 and their use in one-pot catalytic processes to obtain value-added chemicals from furfural. Ind Eng Chem Res 60:18791–18805. https://doi.org/10.1021/acs.iecr.1c02848
Iglesias J, Melero JA, Morales G, Moreno J, Segura Y, Paniagua M, Cambra A, Hernández B (2015) Zr-SBA-15 Lewis acid catalyst: activity in Meerwein Ponndorf Verley reduction. Catalysts 5:1911–1927. https://doi.org/10.3390/catal5041911
Iglesias J, Melero JA, Morales G, Paniagua M, Hernández B, Osatiashtiani A, Lee AF, Wilson K (2018) ZrO2-SBA-15 catalysts for the one-pot cascade synthesis of GVL from furfural. Catal Sci Technol 8:4485–4493. https://doi.org/10.1039/C8CY01121D
Melero JA, Morales G, Iglesias J, Paniagua M, López-Aguado C (2018) Rational optimization of reaction conditions for the one-pot transformation of furfural to γ-valerolactone over Zr–Al-beta zeolite: Toward the efficient utilization of biomass. Ind Eng Chem Res 57:11592–11599. https://doi.org/10.1021/acs.iecr.8b02475
Boronat M, Corma A, Renz M (2006) Mechanism of the Meerwein−Ponndorf−Verley−Oppenauer (MPVO) redox equilibrium on Sn- and Zr-Beta zeolite catalysts. J Phys Chem B 110:21168–21174. https://doi.org/10.1021/jp063249x
Zhu Y, Chuah GK, Jaenicke S (2006) Selective Meerwein–Ponndorf–Verley reduction of α, β-unsaturated aldehydes over Zr-zeolite beta. J Catal 241:25–33. https://doi.org/10.1016/j.jcat.2006.04.008
Gonell F, Boronat M, Corma A (2017) Structure–reactivity relationship in isolated Zr sites present in Zr-zeolite and ZrO2 for the Meerwein–Ponndorf–Verley reaction. Catal Sci Technol 7:2865–2873. https://doi.org/10.1039/C7CY00567A
Wang J, Okamura K, Jaenicke S, Chuah GK (2015) Post-synthesized zirconium-containing Beta zeolite in Meerwein–Ponndorf–Verley reduction: Pros and cons. Appl Catal A 493:112–120. https://doi.org/10.1016/j.apcata.2015.01.001
Khoele M, Lobo RF (2016) Lewis acidic zeolite Beta catalyst for the Meerwein–Ponndorf–Verley reduction of furfural. Catal Sci Technol 6:3018–3026. https://doi.org/10.1039/C5CY01501D
Valekar AH, Lee M, Yoon JW, Kwak J, Hong DY, Oh KR, Cha GY, Kwon YU, Jung J, Chang JS, Hwang YK (2020) Catalytic transfer hydrogenation of furfural to furfuryl alcohol under mild conditions over Zr-MOFs: Exploring the role of metal node coordination and modification. ACS Catal 10:3720–3732. https://doi.org/10.1021/acscatal.9b05085
Zhang P, Hou P, Ma M, Bu K, Guo Q, Yue H, Tian G, Feng S (2023) Bifunctional zirconium-based metal-organic frameworks as chemoselective catalysts for the synthesis of γ-valerolactone from furfural via a one-pot cascade reaction. Appl Catal A 653:119064. https://doi.org/10.1016/j.apcata.2023.119064
Wang Y, Huang J, Lu S, Lei P, Xia X, Li C, Li F (2020) Phosphorus-modified zirconium metal organic frameworks for catalytic transfer hydrogenation of furfural. New J Chem 44:20308–20315. https://doi.org/10.1039/D0NJ04285D
Yang J, Zhang Y, Shen F, Qi X (2023) Glucose-derived zirconium-containing mesoporous composite for efficient catalytic transfer hydrogenation of furfural to furfuryl alcohol. Biomass Bioenergy 170:106723. https://doi.org/10.1016/j.biombioe.2023.106723
Liu Y, Huang R, Zhang J, He L, Peng L (2021) Design of a multifunctional zirconium-based hybrid with natural lignocellulose for transfer hydrogenation of furfural to furfuryl alcohol. Energy Fuels 35:20231–20242. https://doi.org/10.1021/acs.energyfuels.1c03109
Zhang T, Lu Y, Li W, Su M, Yang T, Agunbiyi A, Jin Y (2019) One-pot production of γ-valerolactone from furfural using Zr-graphitic carbon nitride/H-β composite. Int J Hydrogen Energy 44:14527–14353. https://doi.org/10.1016/j.ijhydene.2019.04.059
Bergaya F, Aouad A, Mandalia T (2006) Pillared clays and clay minerals. Develop Clay Miner 1:393–421. https://doi.org/10.1016/S1572-4352(05)01012-3
Brindley GW, Sempels RE (1977) Preparation and properties of some hydroxy-aluminium beidellites. Clay Miner 12:229–237. https://doi.org/10.1180/claymin.1977.012.3.05
Issaadi R, Garin F, Chitour CE, Maire G (2001) Catalytic behaviour of combined palladium-acid catalysts: use of Al and Zr-pillared montmorillonite as supports: part I. Reactivity of linear, branched and cyclic hexane hydrocarbons. Appl Catal A 207:323–332. https://doi.org/10.1016/S0926-860X(00)00664-5
Martínez-Ortiz MJ, Fetter G, Domínguez JM, Melo-Banda JA, Ramos-Gómez R (2003) Catalytic hydrotreating of heavy vacuum gas oil on Al- and Ti-pillared clays prepared by conventional and microwave irradiation methods. Micropor Mesopor Mater 58:73–80. https://doi.org/10.1016/S1387-1811(02)00592-9
Bartley GJJ (1988) Zirconium pillared clays. Catal Today 2:233–241. https://doi.org/10.1016/0920-5861(88)85006-5
Cecilia JA, Pardo L, Pozo M, Bellido E, Franco F (2018) Microwave-assisted acid activation of clays composed of 2:1 clay minerals: a comparative study. Minerals 8:376. https://doi.org/10.3390/min8090376
Occelli ML (1988) Surface properties and cracking activity of delaminated clay catalysts. Catal Today 2:339–355. https://doi.org/10.1016/0920-5861(88)85014-4
Zviagina BB, McCarty DK, Srodon J, Drits VA (2004) Interpretation of infrared spectra of dioctahedral smectites in the region of OH-stretching vibrations. Clays Clay Miner 52:399–410. https://doi.org/10.1346/CCMN.2004.0520401
Franco F, Pozo M, Cecilia JA, Benítez-Guerrero M, Lorente M (2016) Effectiveness of microwave assisted acid treatment on dioctahedral and trioctahedral smectites. The influence of octahedral composition. Appl Clay Sci 120:70–80. https://doi.org/10.1016/j.clay.2015.11.021
Madejová J (2003) FTIR techniques in clay mineral studies. Vib Spectrosc 31:1–10. https://doi.org/10.1016/S0924-2031(02)00065-6
Marques Correia L, Cecilia JA, Rodríguez-Castellón E, Cavalcante CL (2017) Silveira Vieira (2017) Relevance of the physicochemical properties of calcined quail eggshell (CaO) as a catalyst for biodiesel production. J Chem 1:1–12. https://doi.org/10.1155/2017/5679512
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodríguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. https://doi.org/10.1021/ja01269a023
Corma A, Rodellas C, Fornes V (1984) Characterization of acid surfaces by adsorption of 2,6-dimethylpyridine. J Catal 88:374–381. https://doi.org/10.1016/0021-9517(84)90014-9
Peng Q, Wang H, Xia Y, Liu X (2021) One-pot conversion of furfural to gamma-valerolactone in the presence of multifunctional zirconium alizarin red S hybrid. Appl Catal A 621:118203. https://doi.org/10.1016/j.apcata.2021.118203
Kim KD, Kim J, Teoh WY, Kim JC, Huang J, Ryoo R (2020) Cascade reaction engineering on zirconia-supported mesoporous MFI zeolites with tunable Lewis-Brønsted acid sites: a case of the one-pot conversion of furfural to γ-valerolactone. RSC Adv 10:35318–35238. https://doi.org/10.1039/D0RA06915A
Miñambres JF, Cejka J (2023) Meerwein-Ponndorf-Verley reduction in current heterogeneous catalysis research: a review. Catal Rev. https://doi.org/10.1080/01614940.2023.2197716
Filiciotto L, Balu AM, Van der Waal J, Luque R (2018) Catalytic insights into the production of biomass-derived side products methyl levulinate, furfural and humins. Catal Today 302:2–15. https://doi.org/10.1016/j.cattod.2017.03.008
Liu S, Zhu Y, Liao Y, Wang H, Liu Q, Ma L, Wang C (2022) Advances in understanding the humins: formation, prevention and application. Appl Energy Combust Sci 10:100062. https://doi.org/10.1016/j.jaecs.2022.100062
Di Carmine G, Leonardi C, Forster L, Hu M, Lee D, Parlett CMA, Bortoloni O, Isaacs MA, Massi A, D’Agostino C (2023) Humin formation on SBA-15-pr-SO3H catalysts during the alcoholysis of furfuryl alcohol to ethyl levulinate: effect of pore size on catalyst stability, transport, and adsorption. ACS Appl Mater Interfaces 15:24528–24540. https://doi.org/10.1021/acsami.3c04613
Chatterjee A, Hu X, Lam FLY (2019) Modified coal fly ash waste as an efficient heterogeneous catalyst for dehydration of xylose to furfural in biphasic medium. Fuel 239:726–736. https://doi.org/10.1016/j.fuel.2018.10.138
Gómez-Bernal H, Benito P, Rodríguez-Castellón E, Raspolli Galletti AM, Funaioli T (2019) Synthesis of isopropyl levulinate from furfural: Insights on a cascade production perspective. Appl Catal A 575:111–119. https://doi.org/10.1016/j.apcata.2019.02.018
Acknowledgements
This research was funded by Spanish Ministry of Science and Innovation (PID2021–122736OB-C42), FEDER (European Union) funds (PID2021–122736OB-C42, FQM-155, UMA20-FEDERJA-088). Moreover, F. Franco thanks Cátedra Ciencias del Litoral Costa del Sol (807/41.5223) for the financial support.
Funding
Funding for open access publishing: Universidad Málaga/CBUA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sih, S.E., Franco-Duro, F., García-Sancho, C. et al. Valorization of Furfural to Obtain High Value-Added Products with ZrO2- and Al2O3-Pillared Clays. Top Catal (2024). https://doi.org/10.1007/s11244-024-01971-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-024-01971-3