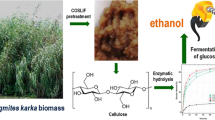
Abstract
Water hyacinth (Eichhornia crassipes) as a aquatic weed has become a source of concern for value addition. This study aimed to determine the feasibility of the weedy biomass in sustainable bioethanol production using Phanerochaete chrysosporium and Saccharomyces cerevisiae. The results indicated that P. chrysosporium significantly utilized 70.9% of cellulose and 70% of hemicellulose from raw lignocellulose of water hyacinth with significant microbial enzyme production of 1.26 IU/ml. Moreover, the microbial treatment resulted in a significant amount of soluble protein (194.30 mg/g) and reducing sugar (34.20 g/l). XRD, SEM and FTIR analyses revealed that the crystalinity of cellulose was increased with the microbial treatment and hence, the yield of sugar also. Under submerged fermentation, Saccharomyces cerevisiae produced a maximum of 20.17 g/l of ethanol. The promising results of the present study explored the microbial treatment with P. chrysosporium and fermentation with S. cerevisiae as a successful and sustainable method for ethanol production from lignocellulosic weedy biomass.





Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Code Availability
Not Applicable.
References
Tasnim F, Iqbal SA, Chowdhury AR (2017) Biogas production from anaerobic co-digestion of cow manure with kitchen waste and water hyacinth. Renew Energy 109:434–439
Ananthi V, Ramesh U, Balaji P, Kumar P, Govarthanan M, Arun A (2022) A review on the impact of various factors on biohydrogen production. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2022.08.046
Merino-Pérez O, Martínez-Palou R, Labidi J, Luque R (2015) Microwave-assisted pretreatment of lignocellulosic biomass to produce biofuels and value-added products. In: Fang Z, Smith RL, Qi X (eds) Production of biofuels and chemicals with microwave. Springer, Netherlands, Dordrecht, pp 197–224
Ganguly A, Das S, Bhattacharya A, Dey A, Chatterjee PK (2013) Enzymatic hydrolysis of water hyacinth biomass for the production of ethanol: optimization of driving parameters. Indian J Exp Biol 51:556–66
Bhuvanendran N, Ravichandran S, Narayanan M, Paulraj B, Kumarasamy S, Su H, Kandasamy S (2022) Emerging trends in biomass-derived carbon-supported metal nanostructures as efficient electrocatalysts for critical electrochemical reactions in low temperature fuel cell applications. In: Pathania D, Singh L (eds) Biorenewable nanocomposite materials, Vol 1: electrocatalysts and energy storage. American Chemical Society, Washington, pp 225–256
Hou J, Ding C, Qiu Z, Zhang Q, Xiang W-N (2017) Inhibition efficiency evaluation of lignocellulose-derived compounds for bioethanol production. J Clean Prod 165:1107–1114
Pothiraj C, Balaji P, Eyini M (2006) Enhanced production of cellulases by various fungal cultures in solid state fermentation of cassava waste. Afr J Biotechnol 5(20):1882–1885
Zhu J-Q, Li X, Qin L, Li W-C, Li H-Z, Li B-Z, Yuan Y-J (2016) In situ detoxification of dry dilute acid pretreated corn stover by co-culture of xylose-utilizing and inhibitor-tolerant Saccharomyces cerevisiae increases ethanol production. Bioresour Technol 218:380–387
de Sousa GK, Maitan-Alfenas GP, de Andrade LG, Falkoski DL, Guimarães VM, Alfenas AC, de Rezende ST (2017) Purification and characterization of xylanases from the fungus Chrysoporthe cubensis for production of xylooligosaccharides and fermentable sugars. Appl Biochem Biotechnol 182:818–830
Taha M, Foda M, Shahsavari E, Aburto-Medina A, Adetutu E, Ball A (2016) Commercial feasibility of lignocellulose biodegradation: possibilities and challenges. Curr Opin Biotechnol 38:190–197
Pothiraj C, Balaji P, Eyini M (2006) Raw starch degrading amylase production by various fungal cultures grown on cassava waste. Mycobiology 34(3):128–130
Hazeena SH, Sindhu R, Pandey A, Binod P (2020) Lignocellulosic bio-refinery approach for microbial 2, 3-Butanediol production. Bioresour Technol 302:122873
Kang Q, Appels L, Tan T, Dewil R (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. Sci World J. https://doi.org/10.1155/2014/298153
Ravindran R, Jaiswal AK (2016) A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol 199:92–102
Sambusiti C, Monlau F, Antoniou N, Zabaniotou A, Barakat A (2016) Simultaneous detoxification and bioethanol fermentation of furans-rich synthetic hydrolysate by digestate-based pyrochar. J Environ Manage 183:1026–1031
Singh R, Balagurumurthy B, Prakash A, Bhaskar T (2015) Catalytic hydrothermal liquefaction of water hyacinth. Bioresour Technol 178:157–165
Das SP, Gupta A, Das D, Goyal A (2016) Enhanced bioethanol production from water hyacinth (Eichhornia crassipes) by statistical optimization of fermentation process parameters using Taguchi orthogonal array design. Int Biodeterior Biodegrad 109:174–184
Sindhu R, Binod P, Pandey A, Madhavan A, Alphonsa JA, Vivek N, Gnansounou E, Castro E, Faraco V (2017) Water hyacinth a potential source for value addition: an overview. Bioresour Technol 230:152–162
Biswas B, Singh R, Krishna BB, Kumar J, Bhaskar T (2017) Pyrolysis of azolla, sargassum tenerrimum and water hyacinth for production of bio-oil. Bioresour Technol 242:139–145
Volynets B, Ein-Mozaffari F, Dahman Y (2017) Biomass processing into ethanol: pretreatment, enzymatic hydrolysis, fermentation, rheology, and mixing. Green Process Synth 6(1):1–22
Illuri R, Eyini M, Kumar M, Prema P, Nguyen V-H, Bukhari NA, Hatamleh AA, Balaji P (2022) Bio-prospective potential of Pleurotus djamor and Pleurotus florida mycelial extracts towards gram positive and gram negative microbial pathogens causing infectious disease. J Infect Public Health 15(2):297–306
Copa-Patiño JL, Kim YG, Broda P (1993) Production and initial characterisation of the xylan-degrading system of Phanerochaete chrysosporium. Appl Microbiol Biotechnol 40:69–76
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin-phenol reagent. J Biol Chem 193:265–275
Chesson A (1988) Lignin-polysaccharide complexes of the plant cell wall and their effect on microbial degradation in the rumen. Anim Feed Sci Technol 21(2–4):219–228
Updegraff DM (1969) Semimicro determination of cellulose inbiological materials. Anal Biochem 32(3):420–424
Deschatelets L, Yu EK (1986) A simple pentose assay for biomass conversion studies. Appl Microbiol Biotechnol 24:379–385
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Singhania RR, Sukumaran RK, Pillai A, Prema P, Szakacs G, Pandey A (2006) Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian J Biotechnol 5:332–336
Kim H, Yun S, Kweon U, Park S, Chung E, Kang W (1996) Seasonal difference in aerobic storage, ruminal degradation and chemical composition of wet brewers’ grain. RDA J Agric Sci 38:605–609
Li K, Xu F, Eriksson K-EL (1999) Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol 65(6):2654–2660
Pothiraj C, Arumugam R, Gobinath M (2014) Sustaining ethanol production from lime pretreated water hyacinth biomass using mono and co-cultures of isolated fungal strains with Pichia stipitis. Bioresour Bioprocess 1:1–10
Srinorakutara T, Subkaree Y, Boonvitthya N, Kunhanon T, Bamrungchue N (2015) Effect of commercial cellulase enzymes on ethanol production from pretreated rice straw at high solid loading. J Food Sci Eng. https://doi.org/10.17265/2159-5828/2015.02.003
Cheng C, Zhang M, Xue C, Bai F, Zhao X (2017) Development of stress tolerant Saccharomyces cerevisiae strains by metabolic engineering: new aspects from cell flocculation and zinc supplementation. J Biosci Bioeng 123(2):141–146
Caputi A, Ueda M, Brown T (1968) Spectrophotometric determination of ethanol in wine. Am J Enol Vitic 19(3):160–165
Ganguly A, Chatterjee P, Dey A (2012) Studies on ethanol production from water hyacinth—a review. Renew Sustain Energy Rev 16(1):966–972
Yan J, Wei Z, Wang Q, He M, Li S, Irbis C (2015) Bioethanol production from sodium hydroxide/hydrogen peroxide-pretreated water hyacinth via simultaneous saccharification and fermentation with a newly isolated thermotolerant Kluyveromyces marxianu strain. Bioresour Technol 193:103–109
Dagnino EP, Chamorro E, Romano SD, Felissia F, Area MC (2013) Optimization of the acid pretreatment of rice hulls to obtain fermentable sugars for bioethanol production. Ind Crops Prod 42:363–368
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37(1):19–27
Abdel-Fattah AF, Abdel-Naby MA (2012) Pretreatment and enzymic saccharification of water hyacinth cellulose. Carbohydr Polym 87(3):2109–2113
Narra M, Divecha J, Shah D, Balasubramanian V, Vyas B, Harijan M, Macwan K (2017) Cellulase production, simultaneous saccharification and fermentation in a single vessel: a new approach for production of bio-ethanol from mild alkali pre-treated water hyacinth. J Environ Chem Eng 5(3):2176–2181
Gutierrez-Rojas I, Moreno-Sarmiento N, Montoya D (2014) Mechanisms and regulation of enzymatic hydrolysis of cellulose in filamentous fungi: classical cases and new models. Rev Iberoam Micol 32(1):1–12
Gielkens MM, Dekkers E, Visser J, de Graaff LH (1999) Two cellobiohydrolase-encoding genes from Aspergillus niger require D-xylose and the xylanolytic transcriptional activator XlnR for their expression. Appl Environ Microbiol 65(10):4340–4345
de Vries RP, Visser J, de Graaff LH (1999) CreA modulates the XlnR-induced expression on xylose of Aspergillus niger genes involved in xylan degradation. Res Microbiol 150(4):281–285
Ahmad Z, Butt MS, Anjum FM, Awan MS, Rathore HA, Nadeem MT, Ahmad A, Khaliq A (2012) Effect of corn cobs concentration on xylanase biosynthesis by Aspergillus niger. Afr J Biotech 11(7):1674–1682
Aydınoğlu T, Sargın S (2013) Production of laccase from Trametes versicolor by solid-state fermentation using olive leaves as a phenolic substrate. Bioprocess Biosyst Eng 36:215–222
Annuar MSM, Murthy SS, Sabanatham V (2010) Laccase production from oil palm industry solid waste: statistical optimization of selected process parameters. Eng Life Sci 10(1):40–48
Das S, Bhattacharya A, Haldar S, Ganguly A, Gu S, Ting Y, Chatterjee P (2015) Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: comparison between artificial neural network and response surface methodology. Sustain Mater Technol 3:17–28
Das S, Gangly A, Dey A, Ting Y, Chatterjee P (2014) Characterization of water hyacinth biomass and microbial degradation of the biomass under solid state fermentation using a lignocellulolytic fungus (Alterneria spp. NITDS1). J Chem Biol Phys Sci 4:2279–2293
Wang Y, Hu L, Zhang G, Yan T, Yan L, Wei Q, Du B (2017) Removal of Pb (II) and methylene blue from aqueous solution by magnetic hydroxyapatite-immobilized oxidized multi-walled carbon nanotubes. J Colloid Interface Sci 494:380–388
Asgher M, Sharif Y, Bhatti H (2010) Enhanced production of ligninolytic enzymes by Ganoderma lucidum IBL-06 using lignocellulosic agricultural wastes. Int J Chem Reactor Eng. https://doi.org/10.2202/1542-6580.2203
Childs BC, Bohlscheid JC, Edwards CG (2015) Impact of available nitrogen and sugar concentration in musts on alcoholic fermentation and subsequent wine spoilage by Brettanomyces bruxellensis. Food Microbiol 46:604–609
Sahoo D, Ummalyma SB, Okram AK, Pandey A, Sankar M, Sukumaran RK (2018) Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour Technol 253:252–255
Zhang Q, Wei Y, Han H, Weng C (2018) Enhancing bioethanol production from water hyacinth by new combined pretreatment methods. Bioresour Technol 251:358–363
Mojović L, Nikolić S, Rakin M, Vukasinović M (2006) Production of bioethanol from corn meal hydrolyzates. Fuel 85(12–13):1750–1755
Aswathy U, Sukumaran RK, Devi GL, Rajasree K, Singhania RR, Pandey A (2010) Bio-ethanol from water hyacinth biomass: an evaluation of enzymatic saccharification strategy. Bioresour Technol 101(3):925–930
Lee S-H, Teramoto Y, Endo T (2009) Enzymatic saccharification of woody biomass micro/nanofibrillated by continuous extrusion process I-Effect of additives with cellulose affinity. Bioresour Technol 100(1):275–279
Zabed H, Sahu J, Suely A, Boyce A, Faruq G (2017) Bioethanol production from renewable sources: current perspectives and technological progress. Renew Sustain Energy Rev 71:475–501
Balat M, Balat H, Öz C (2008) Progress in bioethanol processing. Prog Energy Combust Sci 34(5):551–573
Pothiraj C, Kanmani P, Balaji P (2006) Bioconversion of lignocellulose materials. Mycobiology 34(4):159
Saratale GD, Oh M-K (2015) Improving alkaline pretreatment method for preparation of whole rice waste biomass feedstock and bioethanol production. RSC Adv 5(118):97171–97179
Zhang Q, Huang H, Han H, Qiu Z, Achal V (2017) Stimulatory effect of in-situ detoxification on bioethanol production by rice straw. Energy 135:32–39
Ko JK, Bak JS, Jung MW, Lee HJ, Choi I-G, Kim TH, Kim KH (2009) Ethanol production from rice straw using optimized aqueous-ammonia soaking pretreatment and simultaneous saccharification and fermentation processes. Bioresour Technol 100(19):4374–4380
Himmelsbach DS, Khalili S, Akin DE (2002) The use of FT-IR microspectroscopic mapping to study the effects of enzymatic retting of flax (Linum usitatissimum L) stems. J Sci Food Agric 82(7):685–696
Bronzato G, Ziegler S, Silva R, Cesarino I, Leão A (2017) Characterization of the pre-treated biomass of Eichhornia crassipes (water hyacinth) for the second generation ethanol production. Mol Cryst Liq Cryst 655(1):224–235
Singh JK, Chaurasia B, Dubey A, Faneite Noguera AM, Gupta A, Kothari R, Upadhyaya CP, Kumar A, Hashem A, Alqarawi AA (2020) Biological characterization and instrumental analytical comparison of two biorefining pretreatments for water hyacinth (Eichhornia crassipes) biomass hydrolysis. Sustainability 13(1):245
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project Number (RSP2024R165), Kind Saud University, Riyadh, Saudi Arabia. The authors thankfully recognize the Department of Botany, Government Arts and Science College, Melur and the support of the management of MGR College in Hosur, Tamilnadu, India.
Author information
Authors and Affiliations
Contributions
Conceptualization, RMG, CP and PB; methodology, RMG, RA; validation, PP, DA and SA; formal analysis, PP, DA, SA and PB; investigation, RMG, RA; data curation, VV, BVP and PB; Software: PP; writing—original draft preparation, RMG, RA and VHN; writing—review and editing, VV, VHN and PB; supervision, CP and PB.
Corresponding authors
Ethics declarations
Competing Interests
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gobinath, R.M., Pothiraj, C., Arumugam, R. et al. Biocatalytic Conversion of Lignocellulosic Water Hyacinth Biomass by Phanerochaete chrysosporium for Sustainable Ethanol Production. Top Catal (2024). https://doi.org/10.1007/s11244-024-01952-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11244-024-01952-6